Abstract
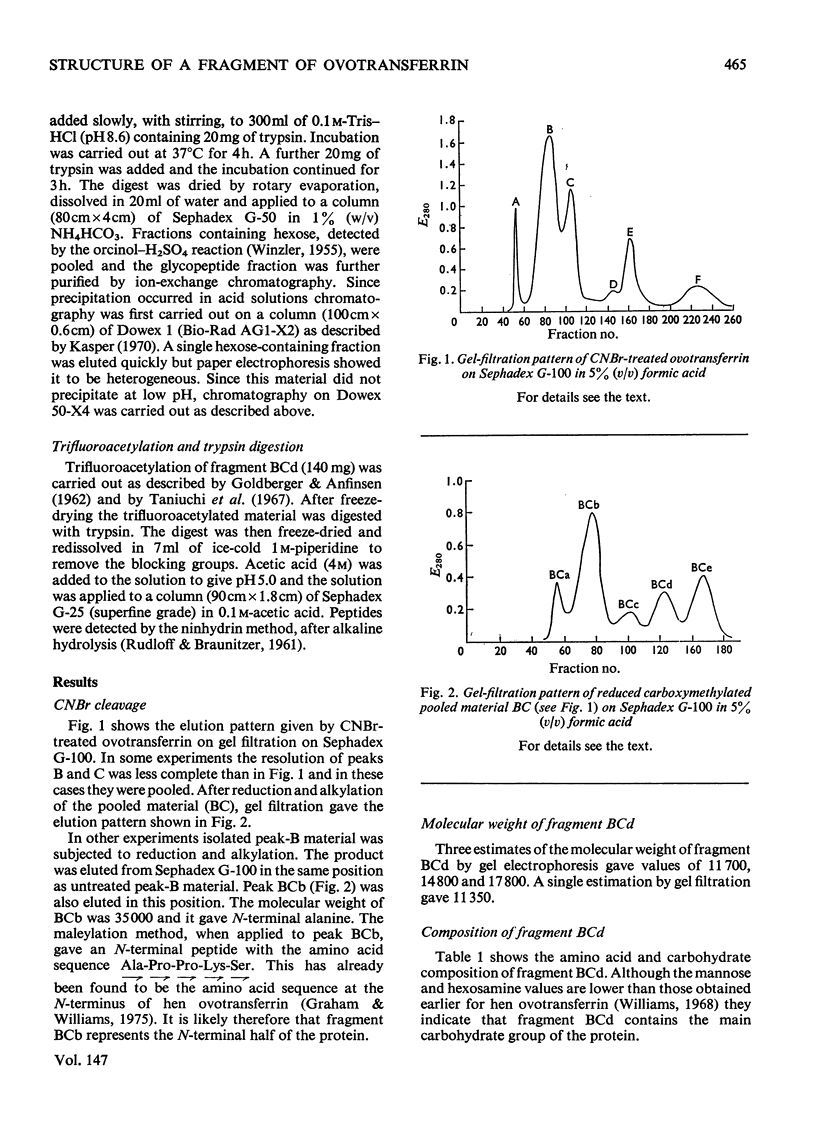
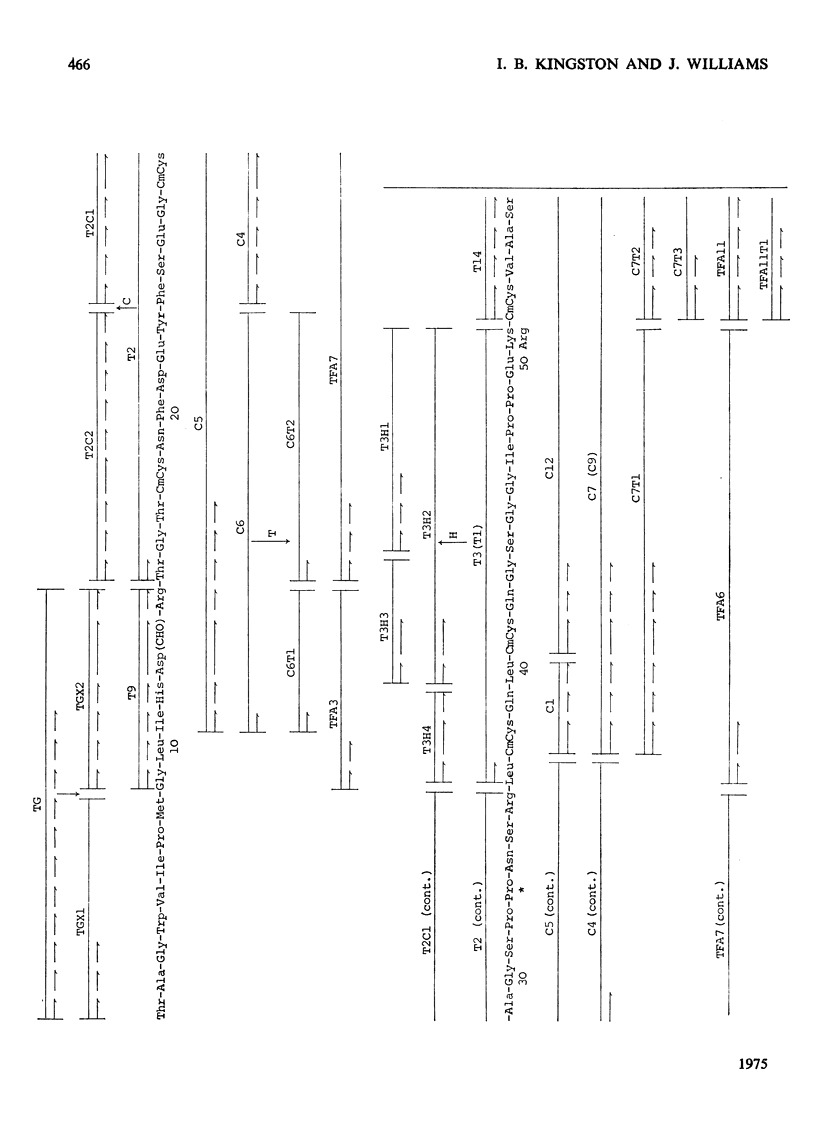
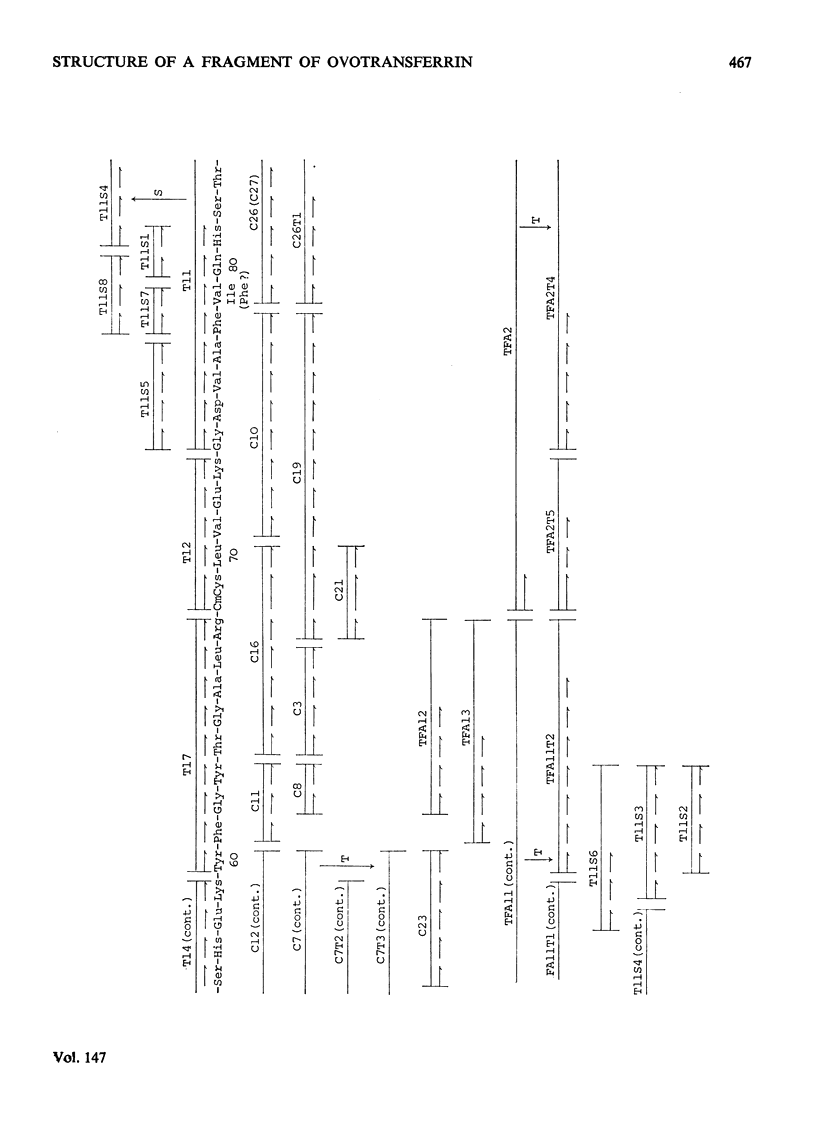
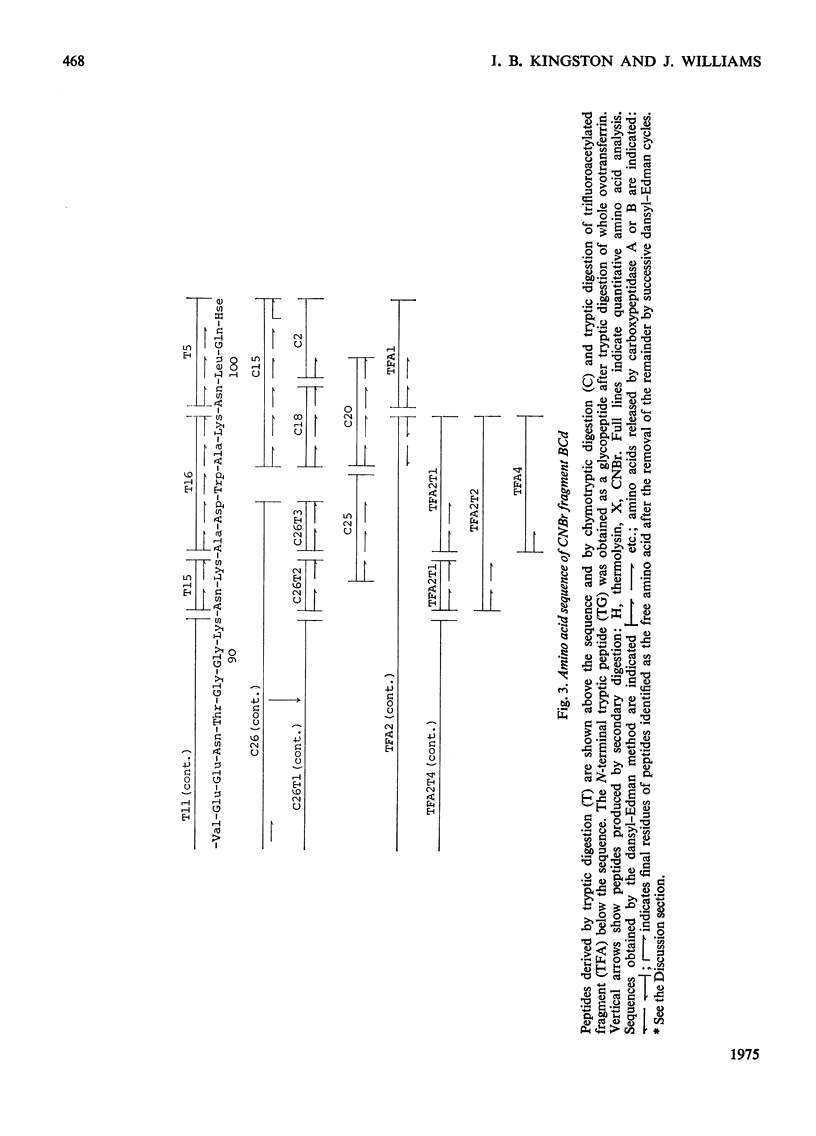
1. Hen ovotransferrin was treated with CNBr and fractionated by gel filtration. 2. After further treatment by reduction and carboxymethylation a carbohydrate-containing fragment of molecular weight 11990 was obtained (fragment BCd). 3. The amino acid sequence of this fragment was determined. It consists of a single chain of 94 residues. 4. The structure of a tryptic glycopeptide derived from whole ovotransferrin permitted a further eight residues to be assigned at the N-terminus of fragment BCd. 5. Heterogeneity was found at two positions. 6. Further evidence has been deposited as Supplementary Publication SUP 50045 (19 pages) at the British Library (Lending Division), Boston Spa, Wetherby, W. Yorkshire LS23 7BQ, U.K., from whom copies may be obtained on the terms indicated in Biochem. J. (1975), 145, 5.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. M., Manwell C., Labisky R. F., Harper J. A. Molecular genetics of avian proteins--V. Egg, blood and tissue proteins of the ring-necked pheasant, Phasianus colchicus L. Comp Biochem Physiol. 1966 Feb;17(2):467–499. doi: 10.1016/0010-406x(66)90581-0. [DOI] [PubMed] [Google Scholar]
- Baker E., Shaw D. C., Morgan E. H. Isolation and characterization of rabbit serum and milk transferrins. Evidence for difference in sialic acid content only. Biochemistry. 1968 Apr;7(4):1371–1378. doi: 10.1021/bi00844a019. [DOI] [PubMed] [Google Scholar]
- Bezkorovainy A., Grohlich D. Comparative study of several proteins of the transferrin class. Comp Biochem Physiol B. 1974 Apr 15;47(4):787–797. doi: 10.1016/0305-0491(74)90024-8. [DOI] [PubMed] [Google Scholar]
- Bruton C. J., Hartley B. S. Chemical studies on methionyl-tRNA synthetase from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):165–178. doi: 10.1016/0022-2836(70)90023-9. [DOI] [PubMed] [Google Scholar]
- Crowshaw K., Jessup S. J., Ramwell P. W. Thin-layer chromatography of 1-dimethylaminonaphthalene-5-sulphonyl derivatives of amino acids present in superfusates of cat cerebral cortex. Biochem J. 1967 Apr;103(1):79–85. doi: 10.1042/bj1030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C., Williams J. The amino acid sequences of cysteic acid-containing peptides from performic acid-oxidized ovotransferrin. Biochem J. 1970 Feb;116(3):515–532. doi: 10.1042/bj1160515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Graham I., Williams J. A comparsion of glycopeptides from the transferrins of several species. Biochem J. 1975 Feb;145(2):263–279. doi: 10.1042/bj1450263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene F. C., Feeney R. E. Physical evidence for transferrins as single polypeptide chains. Biochemistry. 1968 Apr;7(4):1366–1371. doi: 10.1021/bi00844a018. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Jeppsson J. O. Subunits of human transferrin. Acta Chem Scand. 1967;21(7):1686–1694. doi: 10.3891/acta.chem.scand.21-1686. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W., Cox A. C., Tanford C. Single-chain nature of human serum transferrin. Biochemistry. 1970 Mar 17;9(6):1348–1354. doi: 10.1021/bi00808a008. [DOI] [PubMed] [Google Scholar]
- Mueller J O, Smithies O, Irwin M R. Transferrin Variation in Columbidae. Genetics. 1962 Oct;47(10):1385–1392. doi: 10.1093/genetics/47.10.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGDEN A. L., MORTON J. R., GILMOUR D. G., McDERMID E. M. Inherited variants in the transferrins and conalbumins of the chicken. Nature. 1962 Sep 8;195:1026–1028. doi: 10.1038/1951026b0. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDLOFF V., BRAUNITZER G. [On hemoglobin. VI. A method for the preparative production of natural peptides. The isolation of tryptic cleavage products of human hemoglobin A with Dowex 1X2 using a ninhydrin-negative volatile buffer]. Hoppe Seylers Z Physiol Chem. 1961 May 3;323:129–144. doi: 10.1515/bchm2.1961.323.1.129. [DOI] [PubMed] [Google Scholar]
- SMITH I. Colour reactions on paper chromatograms by a dipping technique. Nature. 1953 Jan 3;171(4340):43–44. doi: 10.1038/171043a0. [DOI] [PubMed] [Google Scholar]
- Sutton M. R., Brew K. Purification and characterization of the seven cyanogen bromide fragments of human serum transferrin. Biochem J. 1974 Apr;139(1):163–168. doi: 10.1042/bj1390163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi H., Anfinsen C. B., Sodja A. The amino acid sequence of an extracellular nuclease of Staphylococcus aureus. 3. Complete amino acid sequence. J Biol Chem. 1967 Oct 25;242(20):4752–4758. [PubMed] [Google Scholar]
- Tsao D., Azari P., Phillips J. L. On the structure of ovotransferrin. I. Isolation and characterization of cyanogen bromide fragments. Reevaluation of the primary structure. Biochemistry. 1974 Jan 29;13(3):397–403. doi: 10.1021/bi00700a001. [DOI] [PubMed] [Google Scholar]
- UPHAUS R. A., GROSSWEINER L. I., KATZ J. J., KOPPLE K. D. Fluorescence of tryptophan derivatives in trifluoroacetic acid. Science. 1959 Mar 6;129(3349):641–643. doi: 10.1126/science.129.3349.641. [DOI] [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]
- Wenn R. V. The electrophoretic mobilities of 5-dimethylaminoaphthalene-1-suphonyl-glycopeptides and their relation molecular weight. Biochem J. 1975 Feb;145(2):281–285. doi: 10.1042/bj1450281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A comparison of glycopeptides from the ovotransferrin and serum transferrin of the hen. Biochem J. 1968 Jun;108(1):57–67. doi: 10.1042/bj1080057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. The formation of iron-binding fragments of hen ovotransferrin by limited proteolysis. Biochem J. 1974 Sep;141(3):745–752. doi: 10.1042/bj1410745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


