Abstract
The System L amino acid transporter, particularly the isoform Large Neutral Amino Acid Transporter Small Subunit 1 (LAT1) encoded by SLC7A5, is believed to mediate the transfer of essential amino acids in the human placenta. Placental System L amino acid transporter expression and activity is decreased in pregnancies complicated by IUGR and increased in fetal overgrowth. However, it remains unknown if changes in the expression of LAT1 are mechanistically linked to System L amino acid transport activity. Here we combined overexpression approaches with protein analysis and functional studies in cultured primary human trophoblast (PHT) cells to test the hypothesis that SLC7A5 overexpression increases the uptake of essential amino acids and activates mTOR signaling in PHT cells. Overexpression of SLC7A5 resulted in a marked increase in protein expression of LAT1 in the PHT cells microvillous plasma membrane and System L amino acid transporter activity. Moreover, mTOR signaling was activated, and System A amino acid transporter activity increased following SLC7A5 overexpression, suggesting coordination of trophoblast amino transporter expression and activity to ensure balanced nutrient flux to the fetus. This is the first report showing that overexpression of LAT1 is sufficient to increase the uptake of essential amino acids in PHT cells, which activates mTOR, a master regulator of placental function. The decreased placental System L activity in human IUGR and the increased placental activity of this transporter system in some cases of fetal overgrowth may directly contribute to changes in fetal amino acid availability and altered fetal growth in these pregnancy complications.
Keywords: Placenta, Maternal-Fetal Exchange, Fetal Growth, Fetal Development, Pregnancy Complications
Introduction:
Pregnancies complicated by intrauterine growth restriction (IUGR) or fetal overgrowth are associated with increased perinatal morbidity and mortality and increase the risk for the child of developing cardiovascular and metabolic disease later in life (1–3). However, the mechanisms causing abnormal fetal growth are still not fully understood, and no specific treatment is currently available. Numerous studies show that changes in placental amino acid transport capacity are strongly associated with altered birth weight (4–7), suggesting that changes in placental amino acid transport directly contribute to changes in the fetal growth trajectory. However, the mechanistic roles of specific transporters in placental amino acid transfer remains to be fully established.
The system L amino acid transporter is a key transport system mediating the sodium-independent transfer of essential amino acids, including leucine, across the placental barrier. This heterodimeric transporter forms a protein complex consisting of a light chain, typically LAT1 (large neutral amino acid transporter 1) (Solute Carrier Family 7 Member 5, SLC7A5) or LAT2 (SLC7A8), and a heavy chain, 4F2hc/CD98 (4F2 cell-surface antigen heavy chain/cluster of differentiation 98) (SLC3A2). LAT1 has been colocalized with 4F2hc at the syncytiotrophoblast polarized plasma membrane domains, i.e., the maternally facing microvillous membrane (MVM) and the basal plasma membrane (BM), adjacent to the fetal capillary (8–11). LAT1 protein is expressed in the human placental syncytiotrophoblast plasma membranes across gestation (12). Specifically, LAT1 and LAT2 are present and functional in the syncytiotrophoblast MVM, whereas LAT2 is also expressed in the BM and the fetal capillary endothelium (9).
Using primary human trophoblast (PHT) and BeWo cells cultured in a Trans well system, Zaugg and coworker (13) demonstrated that System L transporter inhibitors reduce leucine transfer across the placental barrier in vitro. Placental System L amino acid transporter activity is decreased in human IUGR (14, 15) and increased in fetal overgrowth in women with gestational diabetes (16). In addition, we recently reported that MVM LAT1 expression is increased and correlates with birth weight and neonatal fat mass in women with Type 2 diabetes (17). Ample indirect evidence suggests that System L is critical in mediating trophoblast uptake and placental transfer of essential amino acids and that placental System L activity is linked to fetal growth. For example, siRNA-mediated inhibition of mTOR signaling, a positive regulator of System L, in cultured PHT cells decreased the abundance of LAT1 protein in the microvillus plasma membrane and reduced the uptake of leucine (18). Moreover, reduced placental system L amino acid transport activity precedes the development of decreased fetal growth in undernourished rats (4) and non-human primates (19). However, it is largely unknown if changes in the expression/activity of placental LAT1 are mechanistically linked to the placental transport of essential amino acids.
The mechanistic target of rapamycin (mTOR) signaling pathway is a conserved master regulator of placental function, including amino acid transport (18). mTOR responds to an array of diverse nutritional and metabolic signals, particularly essential amino acids, including those transported by LAT1(20). Furthermore, altered placental mTOR signaling has been recognized as a central integral mechanism linked to altered fetal growth (6, 21). It has been reported that leucine is the essential amino acid that most effectively activates the mTOR signaling in various cancer cells(22). We recently demonstrated that silencing of trophoblast DEPTOR, an endogenous inhibitor of mTOR signaling, resulted in activation of mTOR signaling and System L amino acid transport (23). The AMP-activated protein kinase (AMPK) is highly conserved multimeric kinase complex. The activation of AMPK results in the suppression of mTORC1 through a mechanism that is either dependent on or independent of tuberous sclerosis complex 2 (TSC2). However, if the function of trophoblast LAT1 is mechanistically linked to placental AMPK/mTOR signaling is unknown.
System A mediates sodium-dependent uptake of non-essential neutral amino acids into the cell (24). All three isoforms of System A (SNAT1, SLC38A1; SNAT2, SLC38A2 and SNAT4, SLC38A4) are expressed in the human placenta. Trophoblast-specific SNAT2 knock-down and SNAT4 gene knockout studies indicate that placental system A amino acid transport activity is necessary for normal placental and fetal growth in mice (25, 26). In addition, placental system A amino acid transport activity is decreased in human IUGR, suggesting that changes in placental amino acid transport activity may directly contribute to fetal growth restriction (27, 28). System L transport activity is believed to depend on the intracellular concentration of non-essential amino acids accumulated by System A (29). Moreover, the activity of placental amino acid transporters system L and system A activity are decreased in IUGR and have been shown to be upregulated in fetal overgrowth (4, 7, 19, 30–33). However, the mechanistic link between trophoblast System A and System L amino acid transport is remains elusive.
We hypothesized that SLC7A5 overexpression increases the uptake of essential amino acids and activates mTOR signaling in PHT cells. Using gene targeting approaches in cultured PHT cells allowed us to generate mechanistic information in an experimental system believed to represent the in vivo syncytium in women, contributing to the physiological and clinical relevance of our findings.
Materials and Methods
Human subjects
Cytotrophoblast cells were isolated from placentas collected from uncomplicated term C-section deliveries and cultured in vitro. The Institutional Review Board of the University of Colorado Anschutz Medical Campus approved the placental collection (COMIRB 14–1073). The research protocol was conducted out in accordance with the World Medical Association Declaration of Helsinki, and all recruited participants provided written informed consent. The exclusion criteria were smoking, use of illicit drugs, concurrent diseases, such as diabetes and hypertension, and the development of pregnancy complications, including gestational diabetes, pregnancy-induced hypertension, and preeclampsia, as well as fetal anomalies, preterm birth, and birth-related complications.
Cytotrophoblast isolation and culture from term placentas
Following collection of the placenta, primary human trophoblast (PHT) cells were isolated using a well-established protocol involving sequential trypsin digestion and Percoll purification(34). After the isolation, cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St. Louis, MO) and Ham’s F-12 nutrient mixture (Life Technologies, Carlsbad, CA) containing 10 % of fetal bovine serum (FBS, Atlanta Biological, Atlanta, GA), 50 μg/ml gentamicin, 60 μg/ml benzyl penicillin and 100 μg/ml streptomycin (Sigma-Aldrich). Cells were plated in either 60-mm culture dishes (~7.5 × 106 cells/dish for Western blot analysis) or six-well plates (for amino acid uptake experiments; ~2.75 × 106 cells/well for LAT1 gene overexpression) and cultured in 5% CO2, 95% atmosphere air at 37°C for 90 h with daily change of the culture media. All studies were repeated in PHT cells isolated from 3 to 6 different placentas.
Overexpression of LAT1
The SLC7A5 cDNA (NM_003486, Origene Rockville, MD) was cloned into a pENTR vector (Thermo Fisher Scientific, Waltham, MA) so that the expression of SLC7A5 was driven by the trophoblast specific promoter Cyp19I.1. Both pENTR Cy19I.1- SLC7A5 and vector EGFP plasmid (control) from Origene Rockville, MD were transfected (at 18 h in culture) into Primary Human Trophoblast (PHT) cells using X-treme GENE HP DNA transfection reagent (Sigma-Aldrich, St Louis, MO, USA) (35). After 90 h in culture, the efficiency of target gene over expression was determined at the protein level using Western blot.
System A and System L amino acid uptake assay
At 90 hours in culture, the amino acid uptake of primary human trophoblast cells was determined. The activity of System A and System L amino acid transporters was determined by measuring the Na+-dependent uptake of [14C] methyl-aminoisobutyric acid (MeAIB; 20 μM) and the 2-amino-2-norbornane-carboxylic acid (BCH; 64 μM)-inhibitable uptake of [3H] leucine (0.0125 μM), respectively, as described in detail previously (23).
Isolation of microvillous plasma membranes from trophoblast cells
Microvillous plasma membranes (MVMs) were isolated from total homogenates of cultured primary human trophoblast cells using differential centrifugation and Mg2+ precipitation as described previously (23, 36). Briefly, centrifugation stages were performed out at 4°C, where as all other steps were performed on ice. Cells were lysed, scraped off the plate and subsequently homogenized using buffer D (10 mm Tris-Hepes, 250 mm sucrose, 1 mm EDTA). Next, the cell homogenate was centrifuged at 10,000 g for 15 min, the supernatant was collected, and the pellet was resuspended, homogenized in 1 ml of buffer D, and centrifuged again at 10,000 g for 10 min. The two resulting supernatants were combined and centrifuged at 125,000 g for 30 min. The pelleted crude membrane fraction was resuspended in 2 ml of buffer, and 12 mM MgCl2 was added. After 20 minutes of steady stirring on ice, the mixture was centrifuged at 2500 g for 10 minutes. The supernatant containing MVMs was subsequently centrifuged at 125,000 g for 30 min and the final pellet was resuspended in buffer D. Protein concentration was determined using the Bradford assay. Using the MVM/homogenate ratio of alkaline phosphatase activity, MVM enrichment was evaluated.
Western Blotting
For immunoblotting, cells were lysed in 1x RIPA buffer containing phosphatase and protease inhibitors. Subsequently, cells were scraped, collected, and sonicated. Proteins in cell homogenate and MVMs (isolated from PHT cells) were separated by electrophoresis. Western blotting was performed out as described previously (18). Total protein from cell homogenate (5μg), used to determine total protein expression and phosphorylation of cellular signaling proteins, were separated and probed using primary antibodies (Total and phosphorylated S6 ribosomal protein, 4EBP-1 and AMPK) and beta actin or total protein was used as a protein loading control to calculate relative abundance. Total and phosphorylated S6 ribosomal protein, 4EBP-1 and AMPK antibodies were validated as described previously (4, 6, 30, 36). Secondary anti-rabbit HRP antibody conjugate was used to target the primary antibody. Protein expression of the System L amino acid transporter isoform LAT1 was analyzed in MVM preparations. Antibodies targeting the LAT1 were produced in rabbits (37). The specificity of LAT1 antibodies has previously been validated using gene-silencing techniques (9). Target protein expression was normalized to β-actin or total protein expression. The mean density of the control sample bands was arbitrarily set to 1 for each protein target. All densitometry values were expressed relative to this mean value.
Data presentation and statistics
Data are presented as mean ± SEM or mean + SEM. For PHT cells, the number of experiments (n) represents the number of placentas studied. In the amino acid uptake experiments, each condition was studied in triplicate, and data were averaged to represent trophoblast cells isolated from one placenta. The statistical significance of differences between control and experimental groups was assessed using Student’s t test. A P value <0.05 was considered significant.
Results
Validation of overexpression efficiency of SLC7A5 in PHT cells
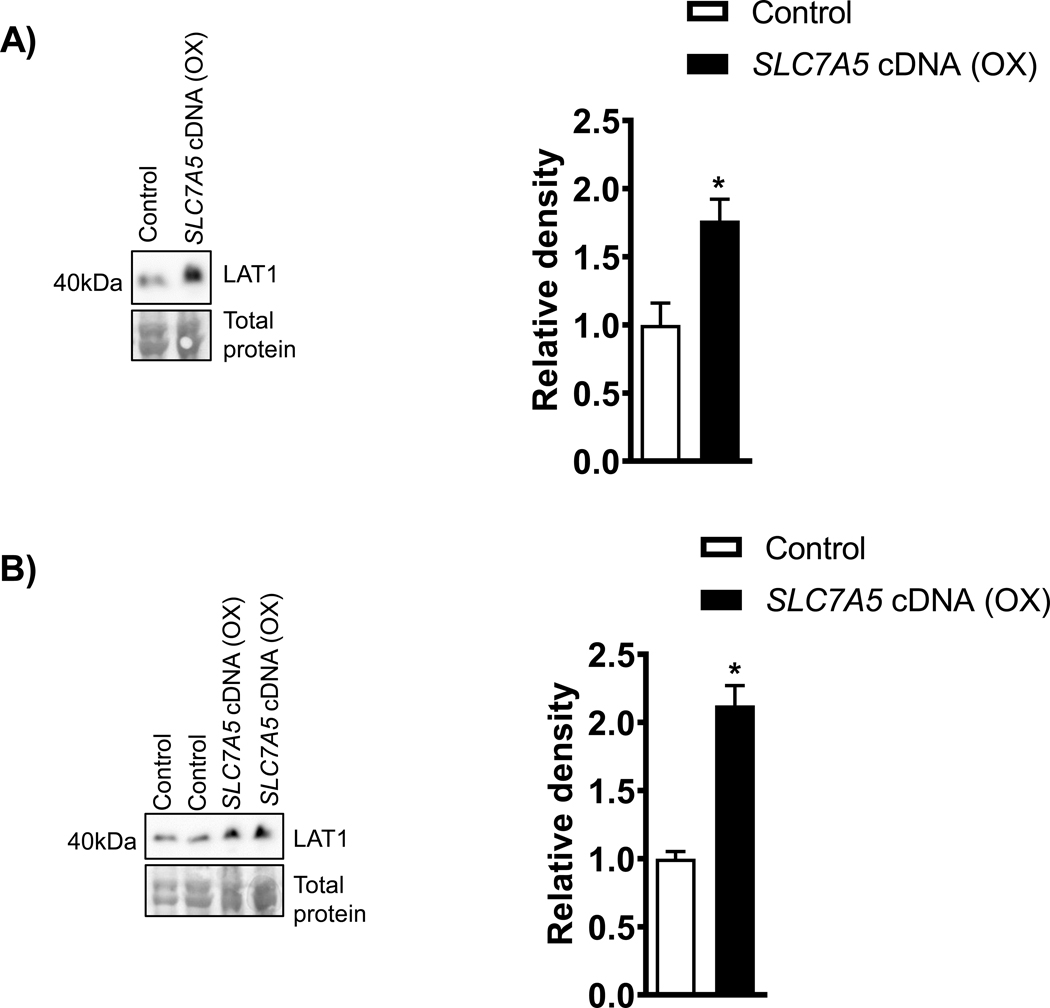
Cultured cytotrophoblasts form syncytial islands, which serve as a model for the syncytiotrophoblast, the transporting epithelium of the human placenta. After 66 h in culture, there was an increase in hCG production by trophoblast cells, and the levels remained elevated until at least 90 h after plating (data not shown). Because hCG is produced primarily by syncytiotrophoblast, these data provide evidence of syncytialization. In addition, the hCG secretion profile of PHT cells in which SLC7A5 was overexpressed was comparable to those of cells cultured with the vector control (data not shown). These findings suggest that critical components of the System L amino acid transport can be overexpressed without compromising trophoblast cell survival and differentiation. To confirm that SLC7A5 overexpression resulted in increased LAT1 expression in PHT cells, we performed Western blots on SLC7A5 transfected PHT cells at 90 h of culture. Overexpression of SLC7A5 increased the protein expression of LAT1 by 76 % (p= 0.02, Figure 1 A). In addition, we found that overexpression of SLC7A5 resulted a marked increase in the expression of LAT1 (+112%, P<0.0001; n=5, Figure 1B) in the MVM fraction.
Figure 1: Effect of SLC7A5 overexpression on trophoblast LAT1 protein expression in PHT cells cell lysates and microvillus plasma membrane.
A) Representative western blots of LAT1 (n= 5) in cell lysates of PHT cells transfected with vector control (Control, C) or SLC7A5 cDNA (OX). Equal loading was performed. Summary of the Western blot data. After normalization to total protein, the mean density of vector control (Control) samples was assigned an arbitrary value of 1. Subsequently, individual control and SLC7A5 group density values were expressed relative to this mean. Values are given as means + SEM. *P < 0.05 versus control, Paired Student’s t test.
B) Representative western blots of LAT1 (n= 5) in microvillus plasma membrane isolated from PHT cells transfected with vector control (Control, C) or SLC7A5 cDNA (OX). Equal loading was performed. Summary of the Western blot data. After normalization to total protein, the mean density of vector control (Control) samples was assigned an arbitrary value of 1. Subsequently, individual control and SLC7A5 group density values were expressed relative to this mean. Values are given as means + SEM. *P < 0.05 versus control, Paired Student’s t test.
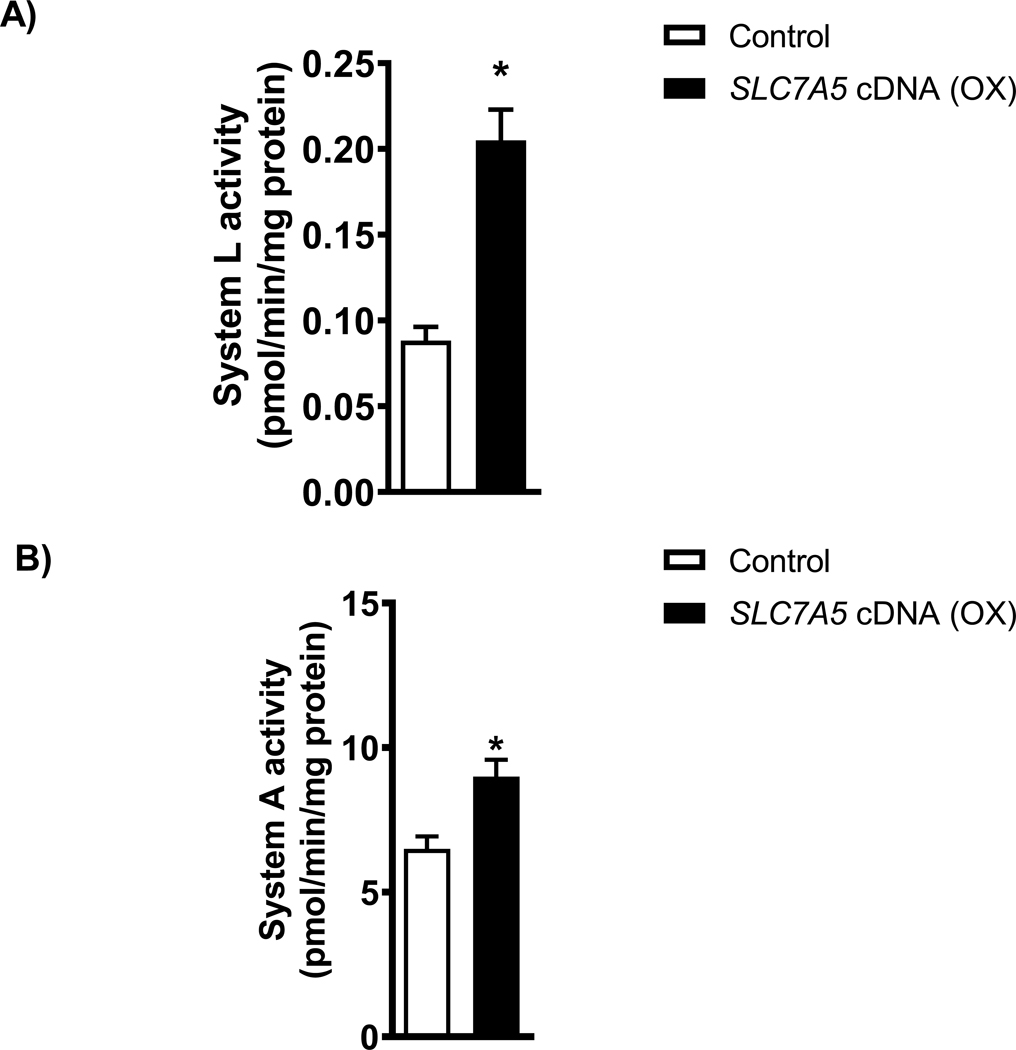
Overexpression of SLC7A5 increases System L and System A amino acid transport in PHT cells.
We measured the System L amino acid transport activity in PHT cells transfected with SLC7A5. Overexpression of SLC7A5 in PHT cells did increase the System L amino acid transport activity (+156 %, p=0.0003, Figure 2A) as compared to control. In addition, System A amino acid transport activity increased by 38 % (p=0.0007, Figure 2B) in PHT cells with SLC7A5 overexpression.
Figure 2: Trophoblast System L and System A amino acid transport in PHT cells with SLC7A5 overexpression.
(A-B) System L activity was measured as the BCH-inhibitable uptake of [3H] leucine, and System A activity was determined as the Na+-dependent uptake of [14C] MeAIB. Overexpression of SLC7A5 increased the System L (A) and System A (B) transporters in cultured human primary trophoblast cells. Values are given as means + SEM. *P < 0.05 versus control, Paired Student’s t test.
Overexpression of SLC7A5 activates mTORC1 signaling in PHT cells
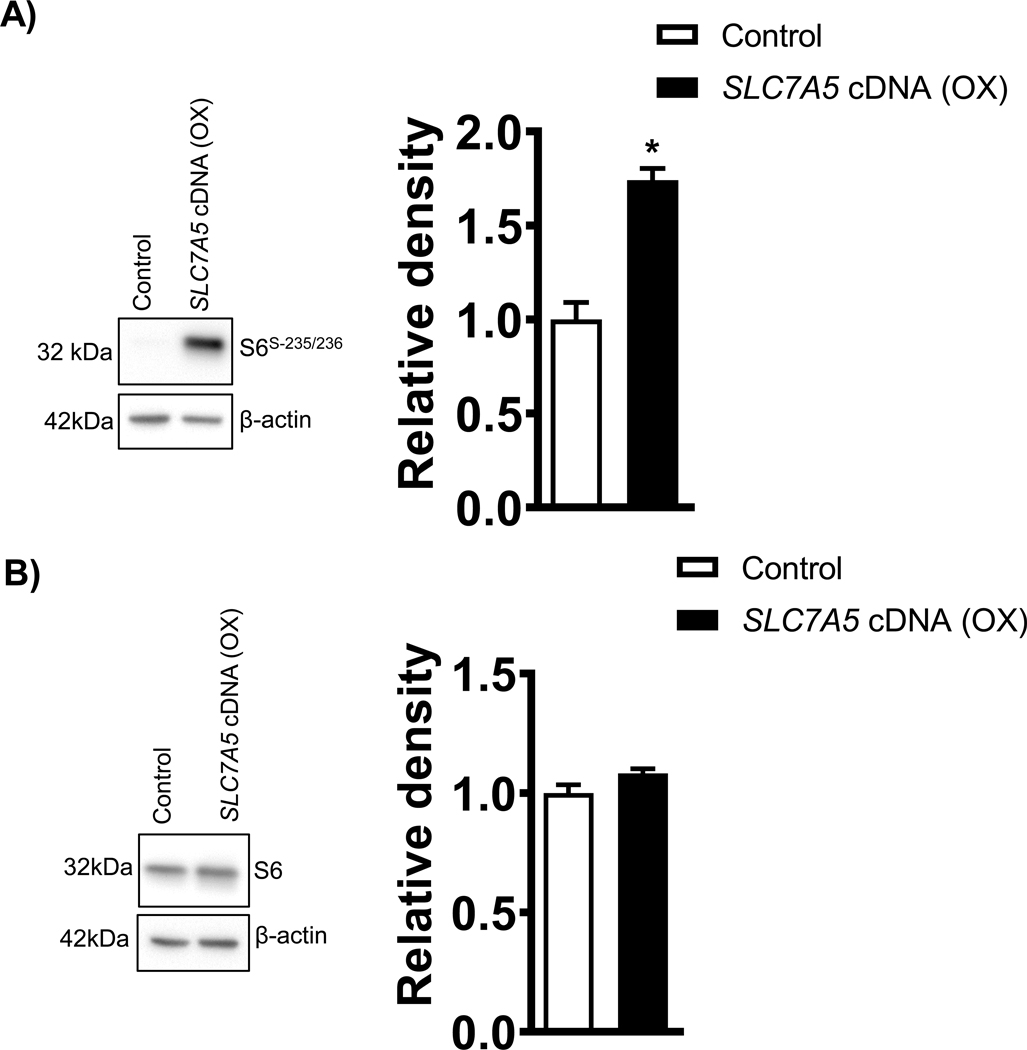
As shown in the Figure 3A, the phosphorylation of ribosomal protein S6 (Ser-235/236), a component of the 40S ribosome and a physiologically relevant S6K1 substrate, was increased by 73% (p=0.007, Figure 3A) in PHT cells transfected with SLC7A5 as compared to control. However, there was no significant difference in the total expression of S6 ribosomal protein between SLC7A5 overexpressing and control PHT cells (Figure 3B).
Figure 3: Overexpression of SLC7A5 activates ribosomal S6 phosphorylation at Serine 235/236 in PHT cells.
(A) Effect of SLC7A5 overexpression on S6Serine−235/236 and total S6 protein expression. Representative western blots of S6Serine−235/236 and total S6 expression in cell lysates of PHT cells transfected with vector control (Control, C) or SLC7A5 cDNA (OX). Equal loading was performed. Summary of the Western blot data. After normalization to β--actin, the mean density of vector control (Control) samples was assigned an arbitrary value of 1. Subsequently, individual control and SLC7A5 group density values were expressed relative to this mean. Values are given as means + SEM. *P < 0.05 versus control, Paired Student’s t test.
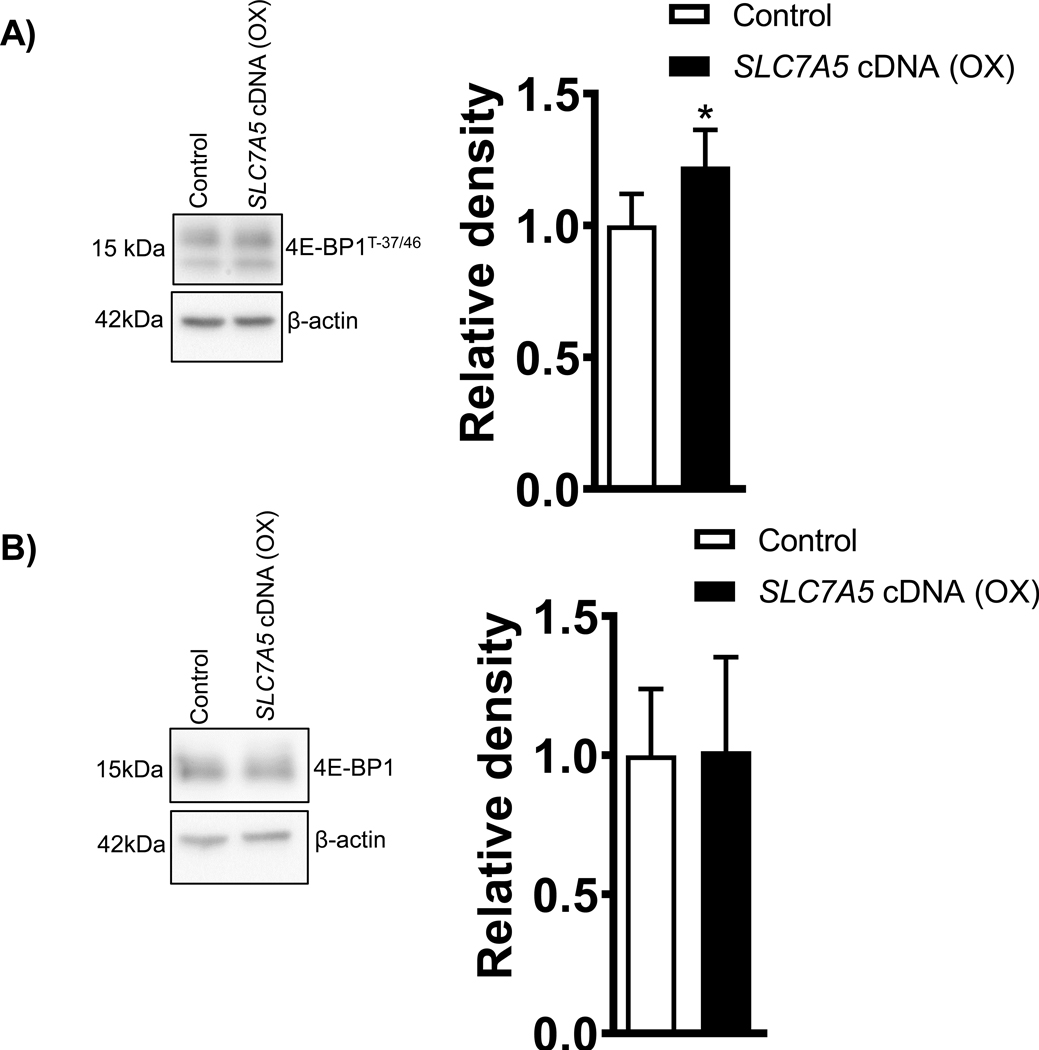
The 4E-BP1 undergoes phosphorylation at multiple sites, and we measured the 4E-BP1 phosphorylation at Thr-37/46. Phosphorylation of 4E-BP1 is hierarchical, in that phosphorylation of Thr-37/46 is required for further phosphorylation at Thr-70. Densitometry analysis showed SLC7A5 overexpression in PHT cells increased (+23%, p=0.01, Figure 4A) the phosphorylation of 4E-BP1 at Thr-37/46 as compared to control. There was no significant difference in total 4E-BP1 expression between groups (Figure 4B).
Figure 4: Overexpression of SLC7A5 activates 4EBP-1 phosphorylation at Threonine 37/46 in PHT cells.
(A) Effect of SLC7A5 overexpression on 4EBP-1Threonine−37/46 and total 4EBP-1 protein expression in cell lysates of PHT cells transfected with vector control (Control, C) or SLC7A5 cDNA (OX). Representative western blots . Equal loading was performed. After normalization to β-actin, the mean density of vector control (Control) samples was assigned an arbitrary value of 1. Summary of the western blot data. Values are given as means + SEM. *P < 0.05 versus control, Paired Student’s t test.
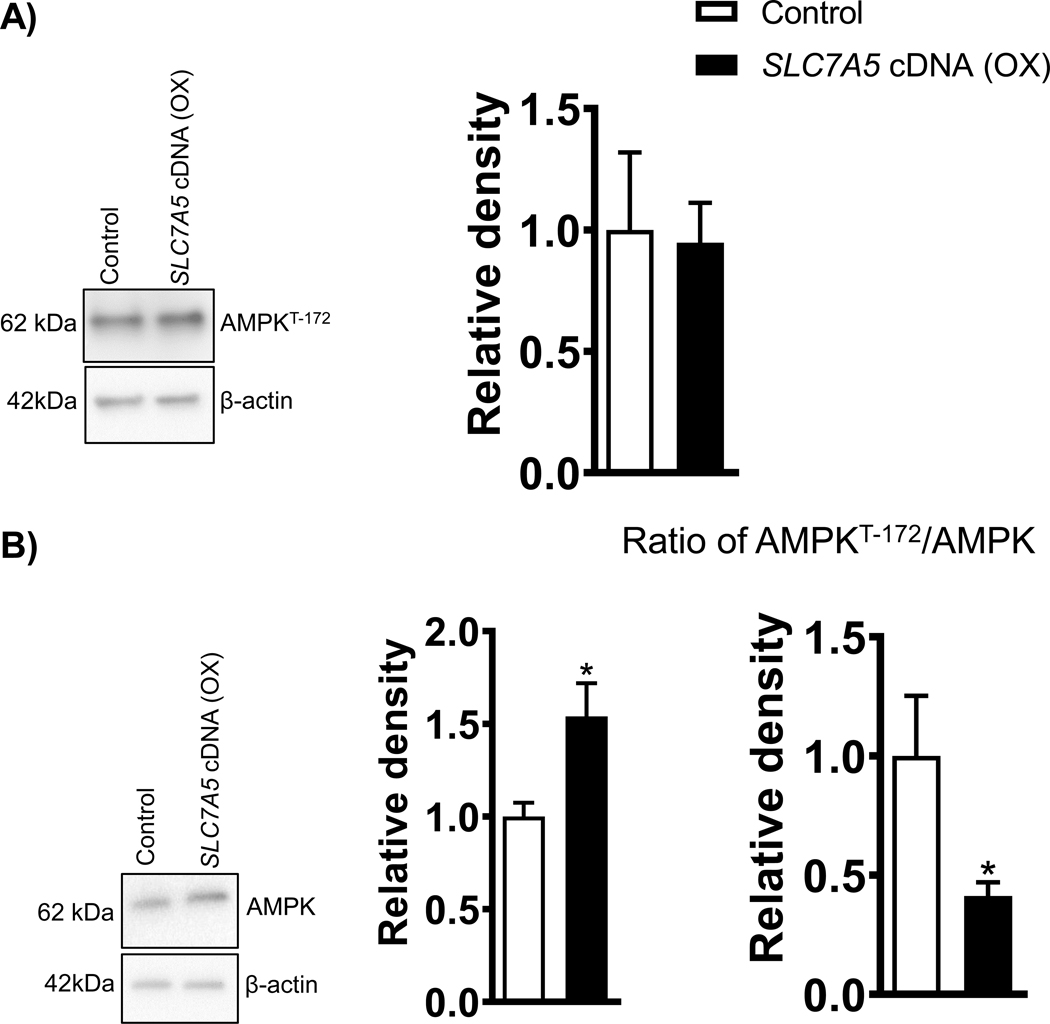
Overexpression of SLC7A5 inhibits Phosphorylation of AMPK at Threonine-172 signaling in PHT cells
As shown in Figure 5A, trophoblast AMPK activity, as determined by Thr-172 phosphorylation, was comparable between PHT cells overexpressing SLC7A5 and PHT cells transfected with a control vector. In contrast, there was a significant increase (+54%, p=0.01; Figure 5B) in total AMPK expression in SLC7A5 overexpressing PHT cells as compared to the vector control (control). The ratio of AMPK phosphorylation at Thr-172 to total AMPK was decreased in the SLC7A5 overexpressing PHT cells (Figure 5B).
Figure 5: Overexpression of SLC7A5 decreases the ratio of AMPKThreonine−172/Total AMPK in PHT cells.
(A) Effect of SLC7A5 overexpression on AMPK Threonine−172 and total AMPK protein expression in cell lysates of PHT cells transfected with vector control (Control, C) or SLC7A5 cDNA (OX). Representative western blots. Equal loading was performed. After normalization to β-actin, the mean density of vector control (Control) samples was assigned an arbitrary value of 1. Summary of the western blot data. Values are given as means + SEM. *P < 0.05 versus control, Paired Student’s t test.
Discussion
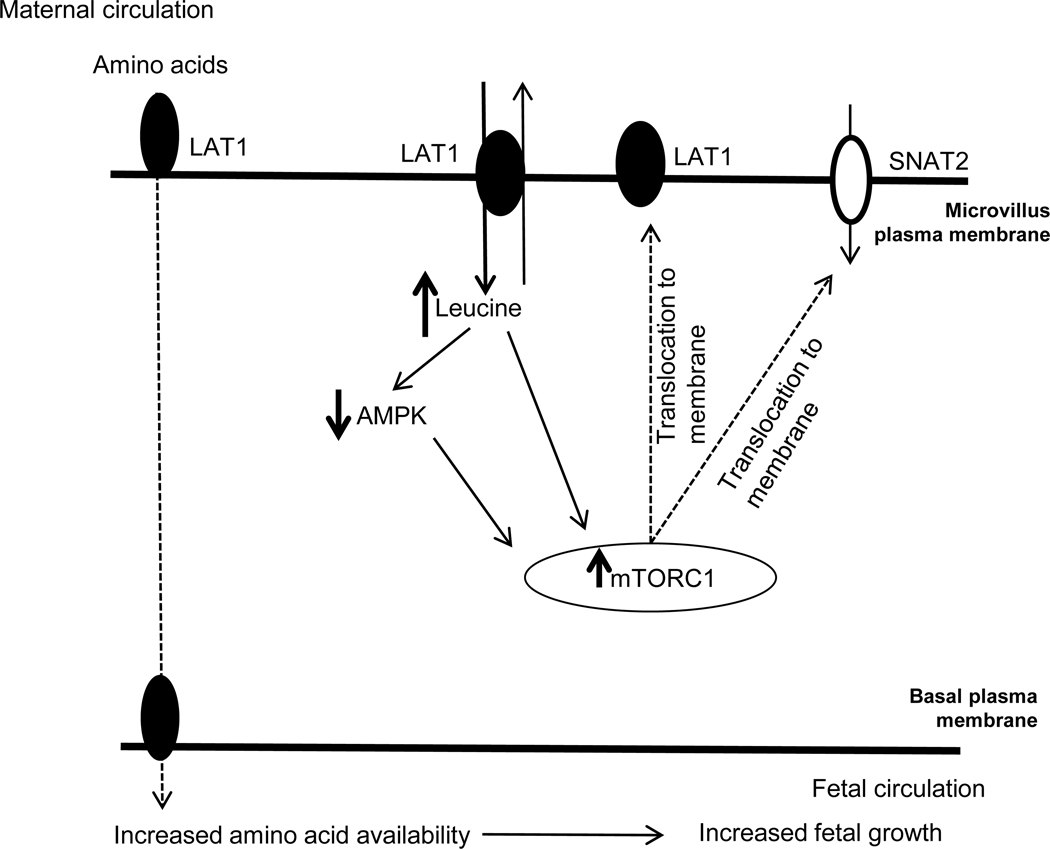
We tested the hypothesis that SLC7A5 (LAT1) overexpression increases the uptake of essential amino acids and activates mTOR signaling in PHT cells and found that overexpression of LAT1 was sufficient to markedly increase system L transport activity in these cells. This is the first time the effect of LAT1 overexpression on the uptake of essential amino acids has been directly studied in primary human trophoblast cells. Moreover, report that SLC7A5 overexpression inhibited AMPK signaling and activated mTOR signaling. Given our previous reports that activation of mTOR promotes the trafficking of specific System L (LAT1) and A (SNAT2) to the trophoblast microvillous plasma membrane we propose that the activation of mTOR signaling in response to SLC7A5 overexpression not only contributes to further LAT1 abundance in the pasma cell microvillous plasma memebrnae but also can explain the increase in System A mediate uptake in PHT cells. These main findings of our study are summarized in Figure 6. We speculate that the decreased placental System L activity reported in human IUGR and the increased placental activity of this transporter system in some cases of fetal overgrowth may directly contribute to changes in fetal amino acid availability and altered fetal growth in these pregnancy complications.
Figure 6: A proposed model linking SLC7A5 overexpression to increase placental System L and System A amino acid transport and activate mTORC1 signaling.
The present study’s findings are consistent with the model that overexpression of SLC7A5 in PHT cells resulted in an increase of the LAT1 protein in the syncytiotrophoblast MVM plasma membrane, in line with the increase in System L activity. This marked increase in trophoblast leucine uptake coincident with significant activation of the mTORC1 signaling, a positive regulator of placental amino acid transport. It is also possible that SLC7A5 overexpression in PHT cells stimulates mTORC1 signaling in part through AMPK inhibition. Activation of placental mTORC1 signaling increased the transporter trafficking of System L amino acid transport isoform LAT1 and System A amino acid transport isoform SNAT2, which contributes to an increased fetal amino acid supply and increased fetal growth.
Overexpression of SLC7A5 in PHT cells resulted in an increase of the LAT1 protein in the syncytiotrophoblast MVM plasma membrane, in line with the increase in System L activity. Thus, increased expression of the SLC7A5 gene not only enhanced the translation of the LAT1 protein, it also resulted in increased trafficking of the LAT1 transporter to the plasma membrane. These results are similar to previous studies that overexpression of LAT1 in mouse hepatocytes increased the protein expression of LAT1 in both cytoplasm and plasma membrane, and was sufficient to increase the system L transport activity (38). siRNA mediated silencing of LAT1 decreased the L-leucine uptake in human oral carcinoma (39) and PHT cells (9). Further supporting a mechanistic role of LAT1 for the uptake of essenarial amino acids in cell culture systems, LAT1 overexpression in oral carcinoma cells resulted in elevated transport of neutral amino acids and increased cell growth and proliferation(40). Furthermore, muscle-specific knockout of the Slc7A5 gene in mice results in substantial reductions of both SLC7A5 mRNA expression and large neutral amino acids transport activity in the skeletal muscle (41). In humans, in vitro experiments demonstrate that LAT1 and LAT2 contribute to trophoblast System L-mediated amino acid uptake (9). The 156 % increase in System L activity with SLC7A5 overexpression in PHT cells in the current study was of similar magnitude to that observed in a mouse model of maternal obesity with upregulation of System L amino acid transport activity associated with fetal overgrowth (31, 32). The present study shows that overexpressing placental SLC7A5 is sufficient to increase placental System L amino acid transport activity in PHT cells. During syncytialization in vitro, trophoblast cells become polarized and develop numerous, uniform microvilli on their culture medium-exposed cell surface (42). The measured uptake of labeled amino acid can likely be attributed to transport across the MVM of the syncytiotrophoblast layer, as this is the cell surface that is readily accessible in vitro during transport activity measurements. Leucine is transported across the MVM, mediated almost exclusively by the System L amino acid transport activity (43). Furthermore, in vivo studies using stable isotope techniques indicate that leucine taken up across the MVM from the maternal circulation is rapidly transferred to the fetus (44). Thus, our data strongly support a critical role of LAT1 in mediating the uptake of essential amino acids from the maternal circulation into the synctytiotropoblast, the active step of tranplscental transfer for most amino acids.
There is ample indirect evidence supporting the hypothesis that transplacental transport of essential amino acids regulates fetal growth (45). Trophoblast MVM LAT1 expression is elevated in women with type 2 diabetes and is correlated to birth weight (17) and MVM System L activity is increased in women with gestational diabetes delivering large babies (16). In contrast, MVM System L amino acid transporter activity is decreased in human IUGR (14, 15) and LAT1 and 2 protein expression have been reported to be decreased in placental homogenates from small-for-gestational-age (SGA) fetuses (46) and in pregnancies complicated by preeclampsia (47). Furthermore, maternal infection decreases System L amino acid transport activity and results in fetal growth restriction in rats (48). Moreover, hormones associated with abnormal fetal growth in various animal models have been demonstrated to decrease and/or increase placental LAT1 abundance and System L activity (5, 31). These observations are consistent with the model that placental LAT1 is important in the maternofetal transfer of essential amino acids (49) and that changes in placental LAT1 expression and System L activity contribute to abnormal fetal growth in important pregnancy complications.
Overexpression of SLC7A5 in PHT cells resulted in a marked increase in trophoblast leucine uptake coincident with a significant activation of the mTORC1 signaling, a positive regulator of placental amino acid transporters (18). Leucine has been shown to stimulate the mTOR signaling pathway in porcine trophectoderm cells (50). In addition, leucine supplementation restores placental mTOR signaing and fetal weight in a mouse model of tumor-induced fetal growth restriction (51). Leucine mediated activation of S6K appears to be attenuated in SLC7A5 knockout gastrocnemius muscle (52).The precise molecular mechanism by which leucine activates mTORC1 signaling has not yet been elucidated, but cytosolic and lysosomal leucine-sensors have been identified (53, 54). Our previous studies have shown that mTORC1 is a positive regulator of System L amino acid transport activity (36, 55). Activation of placental mTORC1 signaling and system L transport activity were reported in mouse model of maternal obesity with fetal overgrowth (31). Hence, it is likely that the activation of mTORC1 signaling in SLC7A5 overexpressing PHT cells might have contributed to the activation of System L transport activity.
5’AMP-activated protein kinase (AMPK) is the primary cellular energy sensor and is phosphorylated at Thr-172 in response to increased AMP/ATP ratio associated with energy deprivation. In this study we demonstrated that the activity of PHT cell AMPK, as measured as the ratio of phosphorylated to total AMPK, was decreased in response to SLC7A5 overexpression. These findings are in general agreement with a reported increase in AMPK activity in skeletal muscle of Slc7a5 knock out mice. Because AMPK is a negative regulator of mTORC1, the observed inhibition of AMPK in our study likely contributes to the activation of mTORC1 in PHT cells with SLC7A5 overexpression. We have previously reported that placental AMPK phosphorylation is decreased and mTORC1-S6K signaling pathway is activated in association to increased fetal growth (6). Hung et al (56) reported that women with GDM and LGA babies had lower phosphorylation of AMPKα at Thr-172, and higher phosphorylation of mTOR at Ser2448 levels in the placentas than normal pregnant women. The finding that the SLC7A5 overexpression in PHT cells was able to decrease AMPKα phosphorylation in the current study is consistent with the work from Wilson et al (57)., who demonstrated that leucine supplementation in rats resulted in reduction of phospho-AMPKα activity in the muscle. Leucine incubation in C2C12 myoblasts stimulates mTOR signaling in part through inhibition of AMPK signaling(58). We speculate that elevated energy levels following increased intracellular amino acid concentrations in PHT cells with SLC7A5 overexpression contribute to the observed inhitiion of AMPK signaling. Thus, it is possible that SLC7A5 overexpression in PHT cells stimulates System L activity and mTOR signaling in part through AMPK inhibition.
We have previously reported that mTOR signaling is a positive regulator of trophoblast System L and System A transporter activity by promoting the plasma membrane trafficking of LAT1 and SNAT2, respectively (23, 36, 59). Thus, the increased System A activity following trophoblast overexpression of LAT1 in the current study may be due to the activation of mTOR signaling in response to increased trophoblast uptake of essential amino acids. The interaction between System A and L amino acid transporters is bidirectional because System L exchanges intracellular non-essantial amino acids – taken up by System A - with extracellular essenatial amino acids. Thus this is an important example of coordination of trophoblast amino transporter expression and activity to ensure balanced nutrient flux to the fetus.
Alterations in the transfer of nutrients across the placenta may be directly responsible for abnormal fetal growth, which is associated with increased susceptibility to develop chronic diseases such as obesity, T2D and CVD in childhood and adult life (60). Upregulation of LAT1 has been reported to be a key mechanism underlying metabolic re-programming in tumors (61). We recently demonstrated that in MVM LAT1 expression was positively correlated with neonatal fat mass in pregnancy complicated with T2D (17). Increased neonatal adiposity is associated with higher BMI and an increased likelihood of being overweight or obese in adulthood (62). Recent studies suggest that various prenatal factors may specifically influence offspring fat accretion, and neonatal adiposity may be a useful surrogate endpoint for prenatal interventions aimed at reducing future childhood overweight and obesity (63).
There is strong interest in therpautic targeting of LAT1 activity and various selective inhibitors have recently been tested in clinical trials in the cancer field (64). Recent experimental studies provide evidence that activators of LAT1 transporters can be used to prevent or reverse fetal growth restriction. For example, leucine treatment has also been repeatedly shown to improve fetal growth in various species and models of fetal growth restriction (51, 65). Our findings suggest that interventions aiming at decreasing placental System L amino acid transport capacity may prevent fetal overgrowth and long-term metabolic dysfunction in offspring that are exposed to the abnormal metabolic intrauterine environment in maternal obesity.
One limitation of this study is that although gene targeting in PHT cells can provide mechanistic evicence of the critical role of LAT1 in mediating the uptake of essential amino acids across the MVM, representing the the uptake into the placenta from the maternal circulation and the active step in placental amino acid transfer, this experimental system does not allow firm conclusions on the effect of transplacental transfer of esseantial amino acids. To address this issue, future directions include the generation of mouse models of trophoblast-specific targeting of Slc7a5 in mice by, for example, using transduction of blastocysts with lentiviral vectors (26, 66, 67) . This approach will allow the determination of transplacental transfer of amino acids in vivo.
In conclusion, we have demonstrated that LAT1 overexpression results in increased System L amino acid transport activity and activation of mTOR signaling in cultured primary human trophoblast cells. We propose that that trophoblast LAT 1 expression and activity not only regulates placental function mediated by modulating mTOR signaling but also control the transplacental transfer essential amino acids and fetal growth. We speculate that the decreased placental System L activity reported in human IUGR and the increased placental activity of this transporter system in some cases of fetal overgrowth may directly contribute to changes in fetal amino acid availability and altered fetal growth in these pregnancy complications.
Clinical Significance.
The availability of essential amino acids is believed to be one critical factor determining fetal growth. However, the mechanism mediating the placental transport of essential amino acids in women remains to be fully established. We observed that overexpression of the LAT1 isoform (SLC7A5) in cultured primary human trophoblast cells, constituting the placental transporting epithelium, caused increased trafficking of the LAT1 protein to the microvillous plasma membrane and a marked increase in the uptake of essential amino acids. The previously reported decreased placental System L activity in human fetal growth restriction and the increased placental activity of this transporter system in some cases of fetal overgrowth may directly contribute to changes in fetal amino acid availability and altered fetal growth in these pregnancy complications
Funding
NIH-NICHD: R01 HD105701 and R01 HD068370
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Data Availability
All supporting data for this manuscript are included in the Figures and the accompanying Supplementary Files.
References
- 1.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–33. doi: 10.1111/j.1471-0528.2006.00989.x [DOI] [PubMed] [Google Scholar]
- 4.Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology. 2011;152(3):1119–29. doi: 10.1210/en.2010-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosario FJ, Schumacher MA, Jiang J, Kanai Y, Powell TL, Jansson T. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J Physiol. 2012;590(6):1495–509. doi: 10.1113/jphysiol.2011.226399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, et al. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98(1):105–13. doi: 10.1210/jc.2012-2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantham P, Rosario FJ, Nijland M, Cheung A, Nathanielsz PW, Powell TL, et al. Reduced placental amino acid transport in response to maternal nutrient restriction in the baboon. Am J Physiol Regul Integr Comp Physiol. 2015;309(7):R740–6. doi: 10.1152/ajpregu.00161.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritchie JW, Taylor PM. Role of the System L permease LAT1 in amino acid and iodothyronine transport in placenta. Biochem J. 2001;356(Pt 3):719–25. doi: 10.1042/0264-6021:3560719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaccioli F, Aye IL, Roos S, Lager S, Ramirez VI, Kanai Y, et al. Expression and functional characterisation of System L amino acid transporters in the human term placenta. Reprod Biol Endocrinol. 2015;13:57. doi: 10.1186/s12958-015-0054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohgaki R, Ohmori T, Hara S, Nakagomi S, Kanai-Azuma M, Kaneda-Nakashima K, et al. Essential Roles of L-Type Amino Acid Transporter 1 in Syncytiotrophoblast Development by Presenting Fusogenic 4F2hc. Mol Cell Biol. 2017;37(11). doi: 10.1128/MCB.00427-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamoto Y, Sakata M, Ogura K, Yamamoto T, Yamaguchi M, Tasaka K, et al. Expression and regulation of 4F2hc and hLAT1 in human trophoblasts. Am J Physiol Cell Physiol. 2002;282(1):C196–204. doi: 10.1152/ajpcell.2002.282.1.C196 [DOI] [PubMed] [Google Scholar]
- 12.James-Allan LB, Teal S, Powell TL, Jansson T. Changes in Placental Nutrient Transporter Protein Expression and Activity Across Gestation in Normal and Obese Women. Reprod Sci. 2020;27(9):1758–69. doi: 10.1007/s43032-020-00173-y [DOI] [PubMed] [Google Scholar]
- 13.Zaugg J, Huang X, Ziegler F, Rubin M, Graff J, Muller J, et al. Small molecule inhibitors provide insights into the relevance of LAT1 and LAT2 in materno-foetal amino acid transport. J Cell Mol Med. 2020;24(21):12681–93. doi: 10.1111/jcmm.15840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44(4):532–7. doi: 10.1203/00006450-199810000-00011 [DOI] [PubMed] [Google Scholar]
- 15.Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, et al. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2001;86(11):5427–32. doi: 10.1210/jcem.86.11.8036 [DOI] [PubMed] [Google Scholar]
- 16.Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51(7):2214–9 [DOI] [PubMed] [Google Scholar]
- 17.Castillo-Castrejon M, Yamaguchi K, Rodel RL, Erickson K, Kramer A, Hirsch NM, et al. Effect of type 2 diabetes mellitus on placental expression and activity of nutrient transporters and their association with birth weight and neonatal adiposity. Mol Cell Endocrinol. 2021;532:111319. doi: 10.1016/j.mce.2021.111319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosario FJ, Kanai Y, Powell TL, Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol. 2013;591:609–25. doi:jphysiol.2012.238014 [pii] 10.1113/jphysiol.2012.238014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosario FJ, Kramer A, Li C, Galan HL, Powell TL, Nathanielsz PW, et al. Reduction of In Vivo Placental Amino Acid Transport Precedes the Development of Intrauterine Growth Restriction in the Non-Human Primate. Nutrients. 2021;13(8). doi: 10.3390/nu13082892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Guan KL. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol. 2019;21(1):63–71. doi: 10.1038/s41556-018-0205-1 [DOI] [PubMed] [Google Scholar]
- 21.Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol. 2007;582(Pt 1):449–59. doi: 10.1113/jphysiol.2007.129676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169(2):361–71. doi: 10.1016/j.cell.2017.03.035 [DOI] [PubMed] [Google Scholar]
- 23.Rosario FJ, Dimasuay KG, Kanai Y, Powell TL, Jansson T. Regulation of Amino Acid Transporter Trafficking by mTORC1 in Primary Human Trophoblast cells is Mediated by the Ubiquitin Ligase Nedd4–2. Clin Sci (Lond). 2015; 10.1042/CS20150554. doi: 10.1042/CS20150554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen HN, Oxender DL, Liang M, Vatz KA. The use of N-methylation to direct route of mediated transport of amino acids. J Biol Chem. 1965;240(9):3609–16 [PubMed] [Google Scholar]
- 25.Matoba S, Nakamuta S, Miura K, Hirose M, Shiura H, Kohda T, et al. Paternal knockout of Slc38a4/SNAT4 causes placental hypoplasia associated with intrauterine growth restriction in mice. Proc Natl Acad Sci U S A. 2019;116(42):21047–53. doi: 10.1073/pnas.1907884116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaughan OR, Maksym K, Silva E, Barentsen K, Anthony RV, Brown TL, et al. Placenta-specific Slc38a2/SNAT2 knockdown causes fetal growth restriction in mice. Clin Sci (Lond). 2021;135(17):2049–66. doi: 10.1042/CS20210575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YY, Rosario FJ, Abu Shehab M, Powell TL, Gupta MB, Jansson T. Increased ubiquitination and reduced plasma membrane trafficking of placental amino acid transporter SNAT-2 in human IUGR. Clin Sci (Lond). 2015; 10.1042/CS20150511. doi: 10.1042/CS20150511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansson T, Ylven K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23(5):392–9. doi: 10.1053/plac.2002.0826 [DOI] [PubMed] [Google Scholar]
- 29.Meier C, Ristic Z, Klauser S, Verrey F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002;21(4):580–9. doi: 10.1093/emboj/21.4.580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kavitha JV, Rosario FJ, Nijland MJ, McDonald TJ, Wu G, Kanai Y, et al. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J. 2014;28(3):1294–305. doi: 10.1096/fj.13-242271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aye IL, Rosario FJ, Powell TL, Jansson T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc Natl Acad Sci U S A. 2015;112(41):12858–63. doi: 10.1073/pnas.1515484112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosario FJ, Kanai Y, Powell TL, Jansson T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity (Silver Spring). 2015;23(8):1663–70. doi: 10.1002/oby.21165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosario FJ, Powell TL, Jansson T. mTOR folate sensing links folate availability to trophoblast cell function. J Physiol. 2017;595(13):4189–206. doi: 10.1113/JP272424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF, 3rd. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118(4):1567–82. doi: 10.1210/endo-118-4-1567 [DOI] [PubMed] [Google Scholar]
- 35.Han ZC, Zhang HN, Wang YZ, Lv CY, Xu ZY. Effect of the human insulin-like growth factor 1 gene transfection to human umbilical cord blood mesenchymal stem cells. Saudi Med J. 2014;35(5):435–41 [PubMed] [Google Scholar]
- 36.Rosario FJ, Kanai Y, Powell TL, Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol. 2013;591(3):609–25. doi: 10.1113/jphysiol.2012.238014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SY, Kim JK, Kim IJ, Choi BK, Jung KY, Lee S, et al. Reabsorption of neutral amino acids mediated by amino acid transporter LAT2 and TAT1 in the basolateral membrane of proximal tubule. Arch Pharm Res. 2005;28(4):421–32 [DOI] [PubMed] [Google Scholar]
- 38.Campbell WA, Thompson NL. Overexpression of LAT1/CD98 light chain is sufficient to increase system L-amino acid transport activity in mouse hepatocytes but not fibroblasts. J Biol Chem. 2001;276(20):16877–84. doi: 10.1074/jbc.M008248200 [DOI] [PubMed] [Google Scholar]
- 39.Kim CH, Park KJ, Park JR, Kanai Y, Endou H, Park JC, et al. The RNA interference of amino acid transporter LAT1 inhibits the growth of KB human oral cancer cells. Anticancer Res. 2006;26(4B):2943–8 [PubMed] [Google Scholar]
- 40.Yoon JH, Kim YB, Kim MS, Park JC, Kook JK, Jung HM, et al. Expression and functional characterization of the system L amino acid transporter in KB human oral epidermoid carcinoma cells. Cancer Lett. 2004;205(2):215–26. doi: 10.1016/j.canlet.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 41.Poncet N, Mitchell FE, Ibrahim AF, McGuire VA, English G, Arthur JS, et al. The catalytic subunit of the system L1 amino acid transporter (slc7a5) facilitates nutrient signalling in mouse skeletal muscle. PLoS One. 2014;9(2):e89547. doi: 10.1371/journal.pone.0089547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farmer DR, Nelson DM. A fibrin matrix modulates the proliferation, hormone secretion and morphologic differentiation of cultured human placental trophoblast. Placenta. 1992;13(2):163–77. doi: 10.1016/0143-4004(92)90031-n [DOI] [PubMed] [Google Scholar]
- 43.Jansson T Amino acid transporters in the human placenta. Pediatr Res. 2001;49(2):141–7. doi: 10.1203/00006450-200102000-00003 [DOI] [PubMed] [Google Scholar]
- 44.Cetin I, Marconi AM, Baggiani AM, Buscaglia M, Pardi G, Fennessey PV, et al. In vivo placental transport of glycine and leucine in human pregnancies. Pediatr Res. 1995;37(5):571–5. doi: 10.1203/00006450-199505000-00002 [DOI] [PubMed] [Google Scholar]
- 45.Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006;572(Pt 1):5–15. doi: 10.1113/jphysiol.2005.104141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Z, He P, Ding X, Huang Y, Gu H, Ni X. PPARgamma stimulates expression of L-type amino acid and taurine transporters in human placentas: the evidence of PPARgamma regulating fetal growth. Sci Rep. 2015;5:12650. doi: 10.1038/srep12650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia X, Cao Y, Ye L, Liu X, Huang Y, Yuan X, et al. Vitamin D stimulates placental L-type amino acid transporter 1 (LAT1) in preeclampsia. Sci Rep. 2022;12(1):4651. doi: 10.1038/s41598-022-08641-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kowash HM, Potter HG, Woods RM, Ashton N, Hager R, Neill JC, et al. Maternal immune activation in rats induces dysfunction of placental leucine transport and alters fetal brain growth. Clin Sci (Lond). 2022;136(15):1117–37. doi: 10.1042/CS20220245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20(4):419–26. doi: 10.1111/j.1365-2826.2008.01662.x [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Song G, Wu G, Gao H, Johnson GA, Bazer FW. Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol Reprod. 2013;88(5):113. doi: 10.1095/biolreprod.112.105080 [DOI] [PubMed] [Google Scholar]
- 51.Viana LR, Gomes-Marcondes MC. A leucine-rich diet modulates the tumor-induced down-regulation of the MAPK/ERK and PI3K/Akt/mTOR signaling pathways and maintains the expression of the ubiquitin-proteasome pathway in the placental tissue of NMRI mice. Biol Reprod. 2015;92(2):49. doi: 10.1095/biolreprod.114.123307 [DOI] [PubMed] [Google Scholar]
- 52.Schriever SC, Deutsch MJ, Adamski J, Roscher AA, Ensenauer R. Cellular signaling of amino acids towards mTORC1 activation in impaired human leucine catabolism. J Nutr Biochem. 2013;24(5):824–31. doi: 10.1016/j.jnutbio.2012.04.018 [DOI] [PubMed] [Google Scholar]
- 53.Kim SG, Buel GR, Blenis J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol Cells. 2013;35(6):463–73. doi: 10.1007/s10059-013-0138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012;18(9):524–33. doi: 10.1016/j.molmed.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosario FJ, Dimasuay KG, Kanai Y, Powell TL, Jansson T. Regulation of amino acid transporter trafficking by mTORC1 in primary human trophoblast cells is mediated by the ubiquitin ligase Nedd4–2. Clin Sci (Lond). 2016;130(7):499–512. doi: 10.1042/CS20150554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hung TH, Wu CP, Chen SF. Differential Changes in Akt and AMPK Phosphorylation Regulating mTOR Activity in the Placentas of Pregnancies Complicated by Fetal Growth Restriction and Gestational Diabetes Mellitus With Large-For-Gestational Age Infants. Front Med (Lausanne). 2021;8:788969. doi: 10.3389/fmed.2021.788969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson GJ, Layman DK, Moulton CJ, Norton LE, Anthony TG, Proud CG, et al. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab. 2011;301(6):E1236–42. doi: 10.1152/ajpendo.00242.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du M, Shen QW, Zhu MJ, Ford SP. Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci. 2007;85(4):919–27. doi: 10.2527/jas.2006-342 [DOI] [PubMed] [Google Scholar]
- 59.Jansson T, Castillo-Castrejon M, Gupta MB, Powell TL, Rosario FJ. Down-regulation of placental Cdc42 and Rac1 links mTORC2 inhibition to decreased trophoblast amino acid transport in human intrauterine growth restriction. Clin Sci (Lond). 2020;134(1):53–70. doi: 10.1042/CS20190794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jansson T, Powell TL. Role of placental nutrient sensing in developmental programming. Clin Obstet Gynecol. 2013;56(3):591–601. doi: 10.1097/GRF.0b013e3182993a2e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu X The Role of Large Neutral Amino Acid Transporter (LAT1) in Cancer. Curr Cancer Drug Targets. 2019;19(11):863–76. doi: 10.2174/1568009619666190802135714 [DOI] [PubMed] [Google Scholar]
- 62.Moore BF, Harrall KK, Sauder KA, Glueck DH, Dabelea D. Neonatal Adiposity and Childhood Obesity. Pediatrics. 2020;146(3). doi: 10.1542/peds.2020-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrod CS, Chasan-Taber L, Reynolds RM, Fingerlin TE, Glueck DH, Brinton JT, et al. Physical activity in pregnancy and neonatal body composition: the Healthy Start study. Obstet Gynecol. 2014;124(2 Pt 1):257–64. doi: 10.1097/AOG.0000000000000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanai Y Amino acid transporter LAT1 (SLC7A5) as a molecular target for cancer diagnosis and therapeutics. Pharmacol Ther. 2022;230:107964. doi: 10.1016/j.pharmthera.2021.107964 [DOI] [PubMed] [Google Scholar]
- 65.Teodoro GF, Vianna D, Torres-Leal FL, Pantaleao LC, Matos-Neto EM, Donato J Jr., et al. Leucine is essential for attenuating fetal growth restriction caused by a protein-restricted diet in rats. J Nutr. 2012;142(5):924–30. doi: 10.3945/jn.111.146266 [DOI] [PubMed] [Google Scholar]
- 66.Kaufman MR, Albers RE, Keoni C, Kulkarni-Datar K, Natale DR, Brown TL. Important aspects of placental-specific gene transfer. Theriogenology. 2014;82(7):1043–8. doi: 10.1016/j.theriogenology.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okada Y, Ueshin Y, Isotani A, Saito-Fujita T, Nakashima H, Kimura K, et al. Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat Biotechnol. 2007;25(2):233–7. doi: 10.1038/nbt1280 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supporting data for this manuscript are included in the Figures and the accompanying Supplementary Files.