Abstract
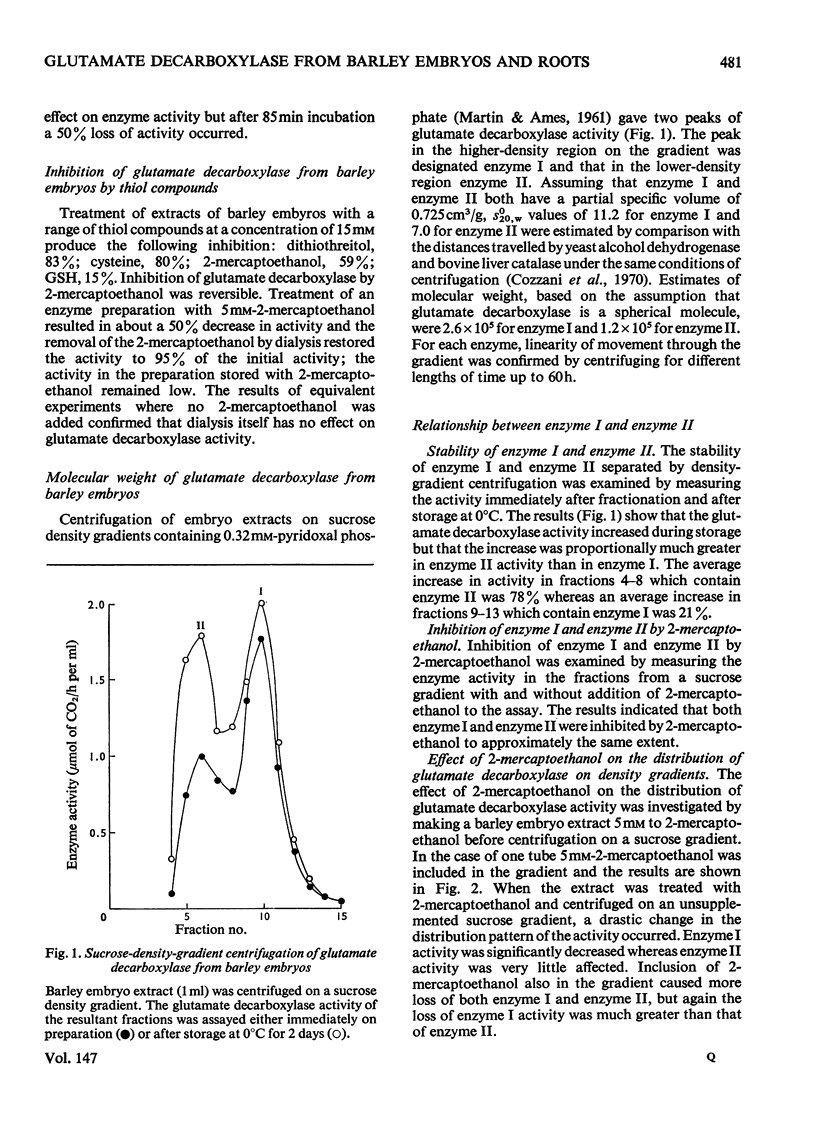
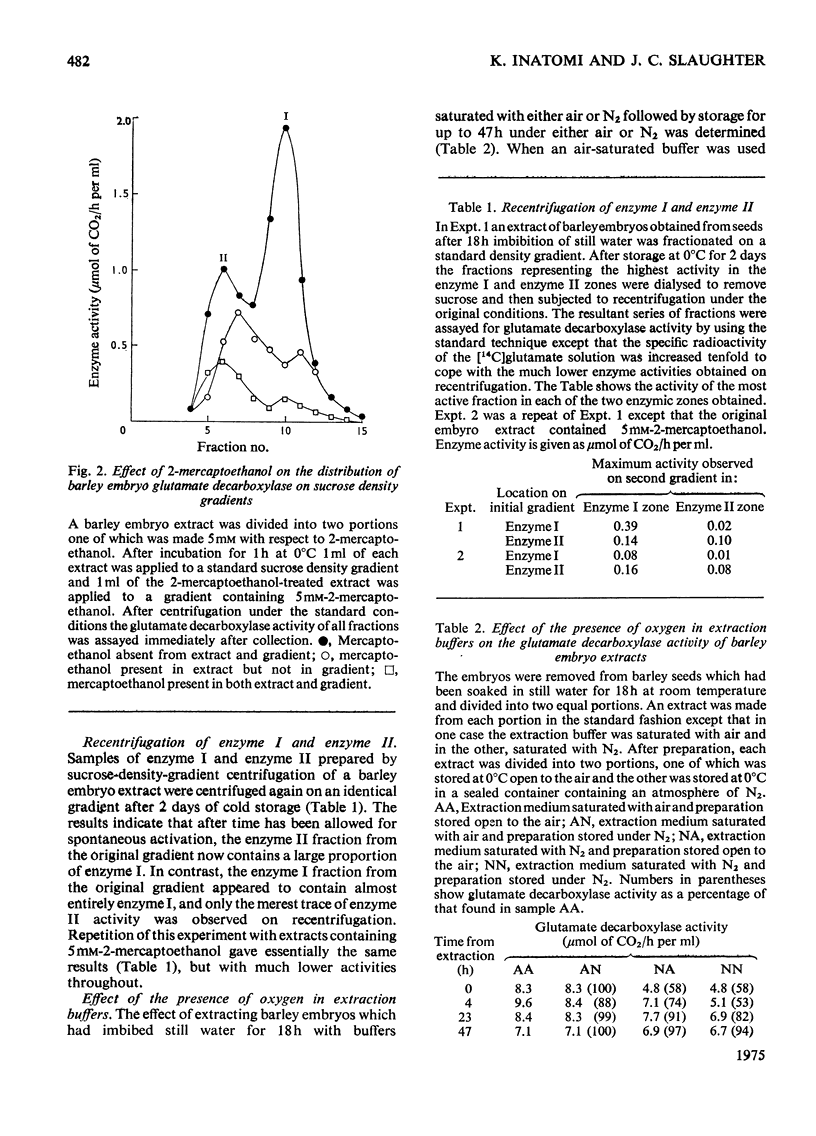
Glutamate decarboxylase in extracts of barley has a Km value for L-glutamate of 22 mM and is activated by the addition of pyridoxal phosphate by up to 3.5 times. Sucrose-density-gradient experiments indicate the presence of two enzyme forms with molecular weights 256000 and 120000. The lower-molecular-weight form appears to be relatively inactive and spontaneously associates to the higher-molecular-weight form on storage. The enzyme is inhibited by thiol reagents and the distribution of activity on density gradients is altered in favour of the lower-molecular-weight form by the presence of 2-mercaptoethanol. After removal of the 2-mercaptoethanol spontaneous association to the higher-molecular-weight form occurs. The presence of oxygen in the extraction buffer and in the water during imbibition leads to a relative increase in the higher-molecular-weight form compared with situations where oxygen is excluded. In contrast, glutamate decarboxylase in extracts of 3-day-old barley roots has a Km value for L-glutamate of 3.1 mM and is activated up to 10% by addition of pyridoxal phosphate. The root enzyme occurs as a single species with molecular weight 310000 and this is unaffected by 2-mercaptoethanol although thiol reagents do act as weak inhibitors. The molecular weight is also unaffected by the presence or absence of oxygen in the extraction buffers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBE L., SOHONIE K. PURIFICATION AND PROPORTIES OF GLUTAMATE DECARBOXYLASE FROM FIELD BEAN (DOLICHOS LABLAB). Enzymologia. 1963 Sep 30;26:98–107. [PubMed] [Google Scholar]
- Cheng Y. Y., Linko P., Milner M. On the Nature of Glutamic Acid Decarboxylase in Wheat Embryos. Plant Physiol. 1960 Jan;35(1):68–71. doi: 10.1104/pp.35.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzani I., Misuri A., Santoni C. Purification and general properties of glutamate decarboxylase from Clostridium perfringens. Biochem J. 1970 Jun;118(1):135–141. doi: 10.1042/bj1180135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]


