Abstract
Mucosal vaccination of capsular polysaccharide (PS) of Streptococcus pneumoniae and subsequent creation of the first line of immunological defense in mucosa were examined. Mucosal as well as systemic antibody responses to PS were evoked by peroral or intranasal immunization of BALB/c mice with PS-cholera toxin B subunit (CTB) conjugates entrapped in the alginate microspheres (AM). The bacterial colonization at the lung mucosa was most profoundly inhibited (<95%) by intranasal immunization with the naked conjugate (PS-CTB). The mice vaccinated orally with encapsulated conjugate [AM(PS-CTB)] showed significant reduction on the level of pneumococcal bacteremia (<99%). Eighty percent of the mice perorally immunized with AM (PS-CTB) were protected from lethal intranasal challenge with S. pneumoniae, whereas more than 60% of the mice in the other control groups died of infection. Our novel approach may prove to be important in the development of a mucosal vaccine that will provide protection of mucosal surfaces of host.
Streptococcus pneumoniae is the most important bacterial cause of pneumonia, meningitis, and otitis media in young children, in the elderly, and in patients with chronic medical conditions or immunosuppressive illnesses, particularly AIDS (31). Acute respiratory infection by S. pneumoniae results in more than one million deaths per year worldwide despite the wide use of antibiotics (31). The emergence of antibiotic-resistant S. pneumoniae has had a great impact on the practice of outpatient medicine (24). With the increase in antibiotic resistance (now accounting for ca. 25% of the cases) and with the increased incidence of pneumococcal infection, the need for an effective therapy (8) and vaccine takes on greater importance.
In addition, pneumococcal infections have recently emerged as a major threat to patients with human immunodeficiency virus (HIV) infection or with chronic debilitating diseases (13, 31). S. pneumoniae accounts for over one-quarter of the bacteremic episodes in HIV-infected children (13). Overall, recurrent diseases have been reported in 8 to 25% of cases of the invasive pneumococcal diseases among patients with HIV (13).
Continued progress in prophylaxis for opportunistic infections will undoubtedly prolong survival and improve the quality of life of these patients. The Center for Disease Control in the United States recommends that all persons infected with HIV receive pneumococcal vaccine. Although a pneumococcal vaccine (such as Pneumovax 23) has been available for more than 10 years, its efficacy is only prevalent among healthy adults and it has little effect on the very young (<2 years), the elderly, and those with immunodeficiencies, such as patients with AIDS (1, 14, 18, 28, 31, 32).
HIV-infected subjects can develop normal intestinal antibody responses in striking contrast with their reported inability to respond to systemically administered immunogens (10, 17, 23, 26). This suggests that mucosal immunization is beneficial for HIV patients (30). Since the mucosal immune system develops earlier in infants and lasts longer in the elderly than the systemic immune system (12, 29, 33), the mucosal immunization should be more advantageous to young children and the elderly among whom otitis media and meningitis are most prevalent.
A major entry site of the pneumococci into the human body is through mucosal surfaces (8). Immunization at the mucosal surface against the pneumococci is most effective because the mucosal immune system is capable of responding to the invading pathogens in the respiratory tracts by producing pathogen-specific secretory immunoglobulin A (sIgA) antibodies (30). The local sIgA has been known to prevent both the colonization at the mucosal tissues and the spread into the systemic circulation more efficiently than the systemic antibodies (30). The present study was conducted to examine whether the mucosal administration of pneumococcal capsular polysaccharide induces mucosal as well as systemic immune responses and whether mucosal immunization protects mice from lethal respiratory infections of S. pneumoniae.
MATERIALS AND METHODS
In vivo microsphere uptake study.
Microspheres were fabricated as described previously (7). Female BALB/c mice, 6 to 8 weeks old, were obtained through DAEHAN Laboratory Animal Research Center Co. (Seoul, Korea). Microspheres were suspended in 0.5 ml of bicarbonate buffer and were delivered into the stomach by using a blunt-tipped feeding needle. Mice were administered a single dose of 20 mg of microspheres entrapping dextran-fluorescein isothiocyanate (FITC). The mice were sacrificed 3 or 5 h after the ingestion. The representative Peyer’s patch (PP) from the small intestine was excised, mounted in OCT freezing compound (Miles, Tarrytown, N.Y.), and snap frozen in −70°C deep freezer. The tissues were cut into 7-μm serial sections, and the sections were viewed with a confocal laser scanning microscope (MRC-1024; Bio-Rad Laboratories, Hercules, Calif.).
Conjugation, purification, and encapsulation of PS.
S. pneumoniae capsular polysaccharide (PS) was conjugated with cholera toxin B subunit (CTB) or with bovine serum albumin (BSA) by 1-ethyl-1-3-(3-dimethylaminopropyl)carbodiimide coupling procedure as described earlier (6, 27). According to the supplier (List Biologicals Laboratory, Inc., Campbell, Calif.), CTB contained no detectable amount of CT based on the results of an ADP-ribosylation assay. PS type 19F (American Type Culture Collection, Rockville, Md.) was dissolved in deionized water and was activated with cyanogen bromide at pH 10.5 for 10 min. Activated PS was coupled to 6-aminocaproic acid (Sigma, St. Louis, Mo.) at 4°C with gentle stirring for 12 h and then dialyzed against deionized water. The conjugation with the protein by EDC was performed at room temperature for 4 h. After the reaction was stopped, the aggregates were removed by centrifugation. The supernatant was dialyzed against phosphate-buffered saline (PBS). The unreacted proteins were removed by using the Sephacryl S-300 column chromatography in PBS. Before the conjugation, the hexose content was determined by the anthrone reaction (5). Bicinchoninic acid (BCA) protein assay was used to monitor the protein contents as described previously (7). After the conjugation, the GM1–enzyme-linked immunosorbent assay (ELISA) (7) was used to detect PS and CTB by using anti-PS antibody and anti-CTB antibody, respectively, as the primary antibodies. The fractions which contain both the protein and the PS were pooled and concentrated by using Centricon (Amicon, Inc., Beverly, Mass.). The conjugation ratio between the CTB and PS was determined by analyzing the protein and the PS contents. The conjugate was microencapsulated as described earlier (7).
Immunization.
The groups of 10 BALB/c mice (6 to 8 weeks old) were orally immunized three times at an interval of 2 weeks with mock microspheres, naked PS-CTB, microencapsulated PS [AM(PS)], microencapsulated PS-CTB [AM(PS-CTB)], and microencapsulated PS-BSA [AM(PS-BSA)]. Since the serum anti-PS IgG antibody level (11.2 ± 0.6 ng/ml) of the mice 2 weeks after the third vaccination with AM(PS-CTB) was significantly higher than that at 2 weeks after the primary immunization (7.5 ± 2.1 ng/ml), all mice were vaccinated three times at intervals of 2 weeks. Five micrograms of CT were coadministered with antigens in four groups of mice (group 3, 5, 7, and 9 in Table 1). All mice received 20-μg equivalent doses of PS in 500 μl of the bicarbonate buffer at a single time. Two groups of mice were vaccinated with three intranasal administrations of 20 μg of PS (in 20 μl) in either encapsulated [AM(PS-CTB)] or naked (PS-CTB) form. After the mice were sacrificed at 2 weeks after the last immunization, sera and bronchoalveolar lavage fluid were collected as described previously (7, 9). The blood contamination of the bronchoalveolar lavage fluid was assessed by detecting of hemoglobin at an optical density of 575 nm. The murine blood diluted serially in PBS served as a standard. The mean blood contamination was 4.0 ± 1.3%.
TABLE 1.
Serum anti-PS IgM, IgG, and IgA and bronchoalveolar IgA antibody responses of the micea
| Group | Vaccine | RIb | Mean concn (ng/ml) ± SEM of:
|
|||
|---|---|---|---|---|---|---|
| Serum IgM | Serum IgG | Serum IgA | Lung IgA | |||
| 1 | AM(mock) | p.o. | 310.3 ± 17.2 | 4.6 ± 0.4 | 9.8 ± 0.5 | 3.4 ± 0.3 |
| 2 | PS-CTB | p.o. | 536.7 ± 33.5 | 3.9 ± 0.2 | 12.3 ± 0.8 | 3.3 ± 0.2 |
| 3 | PS-CTB+CT | p.o. | 313.5 ± 15.7 | 3.3 ± 0.2 | 7.8 ± 0.4 | 3.0 ± 0.2 |
| 4 | AM(PS) | p.o. | 711.8 ± 59.3 | 4.2 ± 0.3 | 36.8 ± 3.1 | 12.5 ± 1.0 |
| 5 | AM(PS)+CT | p.o. | 616.8 ± 34.3 | 3.0 ± 0.2 | 20.6 ± 1.1 | 6.6 ± 0.4 |
| 6 | AM(PS-CTB) | p.o. | 749.8 ± 37.5 | 11.2 ± 0.6 | 49.9 ± 2.5 | 10.2 ± 1.0 |
| 7 | AM(PS-CTB)+CT | p.o. | 446.6 ± 27.9 | 6.9 ± 0.4 | 18.2 ± 1.1 | 5.0 ± 0.3 |
| 8 | AM(PS-BSA) | p.o. | 442.3 ± 22.1 | 6.5 ± 0.6 | 17.6 ± 1.8 | 12.2 ± 1.2 |
| 9 | AM(PS-BSA)+CT | p.o. | 730.3 ± 52.2 | 9.0 ± 0.6 | 48.2 ± 3.4 | 12.9 ± 0.9 |
| 10 | PS-CTB | i.n. | 637.7 ± 31.9 | 17.9 ± 1.1 | 91.9 ± 4.6 | 23.5 ± 1.5 |
| 11 | AM(PS-CTB) | i.n. | 597.7 ± 37.4 | 9.7 ± 0.6 | 53.6 ± 3.4 | 19.9 ± 1.2 |
Data are presented as geometric mean concentration.
RI, route of immunization; p.o., peroral; i.n., intranasal.
ELISA.
Anti-PS antibodies were measured by ELISA (7). Before the anti-PS antibodies were assayed, antipneumococcal cell wall polysaccharide (CPS) activity was neutralized by incubating serum with a CPS solution (20 μg/ml in PBS; Statens Seruminstitut, Copenhagen, Denmark) for 2 h at 37°C. The elimination of anti-CPS activity in serum diluted for anti-PS antibody measurement was controlled by ELISA on a separate plate coated with CPS. These absorbed sera were used in all subsequent steps of the ELISA procedures. To calibrate the specific IgA, IgG, or IgM level, a purified respective immunoglobulin with known concentrations of isotype served as a standard. The absorbance values of the standard immunoglobulin were determined by using sandwich ELISA.
Protection study.
The groups of 10 BALB/c mice, 6 to 8 weeks old, were orally immunized three times at an interval of 2 weeks as described above. Protection of mice against the intranasal challenge with live pneumococci was assessed by recovering viable organisms from the lungs and blood of mice immunized with different vaccines. Two weeks after the last immunization, mice were anesthetized and challenged intranasally with 106 CFU of S. pneumoniae type 19 (ATCC 6319) in 20 μl of medium as described previously (34). The CFU of pneumococci in bronchoalveolar lavage solution and in blood were counted 18 h after the challenge. In order to determine the capability of peroral immunization in inducing protective antipneumococcal immunity in old mice, groups of mice that were immunized as described above were boosted once more at the 32nd week and were challenged 6 weeks after the last immunization by intranasal instillation of live S. pneumoniae (108 CFU/20 μl). Since we used 6- to 8-week-old mice for the first immunization, the challenge study to evaluate the percent survival was performed with 44- to 46-week-old mice.
Statistics.
Unpaired, Student’s t tests were used to compare the mean values of antibody levels and the CFU between groups of mice. Kaplan-Meier analysis was performed to compare survival times of mice after the lethal challenge of S. pneumoniae and to obtain independent variables to predict mortality. The values were considered statistically significant when P was <0.05. Some values (0.05 < P < 0.09) were considered of borderline significance.
RESULTS
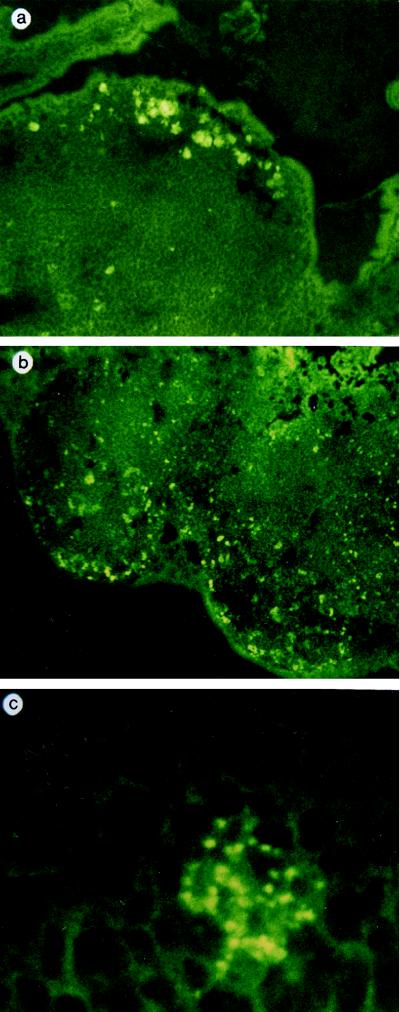
In order to investigate the feasibility of inducing a mucosal immune response by using microspheres, the absorption of the alginate microspheres (AM) into PP through the M cells was studied after gastric administration to the BALB/c mice (Fig. 1). The AM that were <5 μm entrapping FITC-conjugated dextran were prepared as reported earlier (7). The AM started to be absorbed in the dome regions of the PP by 3 h after administration, and those of less than 1 μm were mostly taken up by PP (Fig. 1). None of the AM were observed penetrating into tissues other than the PP.
FIG. 1.
Uptake of AM entrapping FITC-dextran into the PP. Confocal laser scanning microscopic observation of frozen-sectioned tissues of mice revealed that uptaken microspheres were present in the PP at 3 h (A) and 5 h (B and C) after the gastric administration of AM. (C) Part of the PP was magnified (×1,890) to observe the AM remaining in the intercellular space (C).
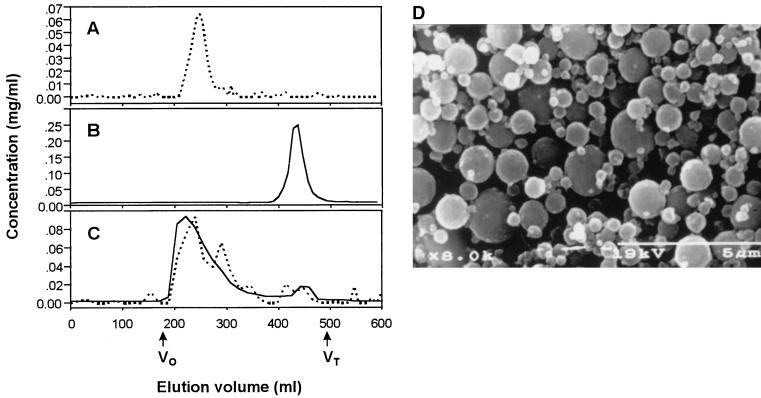
In our previous study (7), we did not observe the induction of serum IgG responses by immunization of mice with PS conjugated with small amount of CTB. Although serum IgM and intestinal IgA responses were significantly higher than those of the nonimmunized group, there was no marked elevation in the serum IgG level, suggesting that the conjugation ratio between PS and CTB (10:1) should be lowered. This was achieved by introducing the spacer molecule (6-aminocaproic acid) between the PS and the protein. The Sephacryl S-300 elution profiles show the crude conjugates containing proteins in the void volume fraction, indicating that the protein has been linked to the PS (Fig. 2C). The completion of the conjugation was also confirmed by ELISA with a GM1-ganglioside (GM1)-coated plate (GM1-ELISA) as described earlier (7). The molar ratio between PS and protein in the conjugate was determined to be 1:1. The microspheres entrapping various vaccines were fabricated by using an interfacial gelation technique as described previously (7). The microspheres were smaller than 5 μm in diameter (Fig. 2D).
FIG. 2.
Purification of PS-CTB conjugate and microspheres entrapping the conjugate. Sephacryl S-300 chromatographs of unconjugated PS (A) and CTB (B) are shown. Before the conjugation, the hexose content was determined by the anthrone reaction (dashed line) (5). BCA protein assay (solid line) was used to monitor the protein content as described earlier (7). After the conjugation, the GM1-ELISA (7) was used to detect PS and CTB by using anti-PS (dashed line) and anti-CTB antibodies (solid line), respectively, as primary antibodies (C). Void volume (V0) and total volume (Vt) of the column are denoted by the arrows (D). The microspheres entrapping PS-CTB conjugate were examined with a scanning electron microscope (S-2460N; Hitachi, Ltd., Tokyo, Japan).
The effects of microencapsulation on the immunogenicity of the orally administered PS were analyzed after the oral immunization of BALB/c mice with the naked conjugates (PS-CTB, group 2) or with the encapsulated conjugates [AM(PS-CTB), group 6] (Table 1). The PS-CTB induced a significant increase in the levels of IgM and serum IgA in serum, as reported previously. No significant serum IgG and bronchoalveolar IgA responses, however, were observed after the oral immunization with the naked conjugate. The AM(PS-CTB) induced bronchoalveolar IgA and serum antibody responses significantly higher than those by PS-CTB (P < 0.05).
We investigated the carrier effect on the immunogenicity of orally administered PS by comparing the immune responses of mice vaccinated with (i) encapsulated PS without CTB [AM(PS)] (group 4), (ii) AM(PS-CTB) (group 6), and (iii) encapsulated PS conjugated to BSA [AM(PS-BSA)] (group 8) (Table 1). When the physical mixture of CTB and PS was microencapsulated and used in immunization, it induced serum and mucosal immune responses not statistically different from those induced by AM(PS) (data not shown). AM(PS-CTB) induced more-prominent serum IgG responses than other groups (P < 0.05). The bronchoalveolar IgA responses were not statistically different in these three groups. Each bronchoalveolar IgA response induced by AM(PS), AM(PS-CTB), and AM(PS-BSA) was significantly higher than that by naked PS-CTB (group 2). The serum IgG levels (15.4 ± 2.3 ng/ml) of the mice immunized with AM(PS-CTB) remained significantly higher than those of mock-immunized mice with empty microspheres (4.0 ± 1.2 ng/ml) at 14 weeks after the first immunization.
To determine whether CT can modulate the systemic and mucosal immune responses against PS, CT was coadministered with vaccines. Interestingly, the PS-specific serum and mucosal antibody responses of mice were significantly reduced when CT was coadministered orally with AM(PS-CTB) (P < 0.05). Coadministration of CT with AM(PS-BSA), however, significantly enhanced the systemic antibody responses of mice (P < 0.05) without significant elevations in the mucosal IgA responses.
Systemic and bronchoalveolar antibody responses of mice were examined after the intranasal immunization. Immunization with PS-CTB (group 10) yielded significantly higher serum IgM responses than immunization with the mock microspheres. The level of serum IgM antibody induced by intranasal administration of PS-CTB, however, was not significantly different from the one by AM(PS-CTB) (group 11). The serum IgA, serum IgG, and bronchoalveolar IgA antibody responses of the mice immunized intranasally with PS-CTB were significantly higher than those immunized with mock microspheres or with AM(PS-CTB).
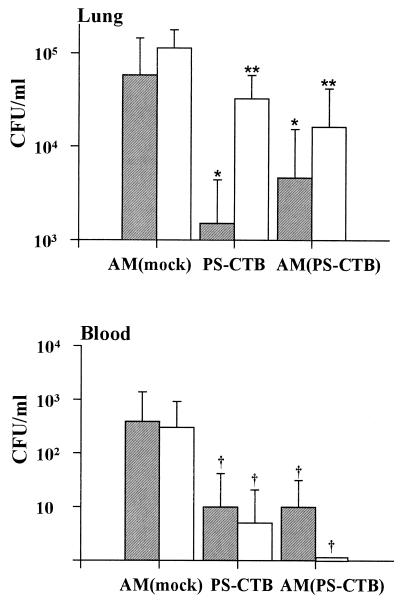
The successful protection against challenge with live S. pneumoniae was demonstrated with the oral or intranasal vaccination (Fig. 3). The control group that received mock AM was highly susceptible to infection with S. pneumoniae, while immunized groups of animals were protected. The number of viable pneumococci recovered from the lungs and the blood of all groups of immunized mice was reduced significantly compared to the control. The extent of inhibition of bacteremia or of bacterial colonization in the bronchoalveoli (i.e., the percent inhibition representing 100 × [1 − CFUtest/CFUcontrol]) was analyzed. When this was compared with the group of mice that were immunized with AM (mock) perorally, more than 90% of bacterial colonization in the lung tissue was inhibited by oral immunization with AM(PS-CTB) (P < 0.01). The bacterial colonization at the lung mucosa was inhibited most profoundly by intranasal immunization with PS-CTB (P < 0.05). Both the mice vaccinated orally with AM(PS-CTB) and the mice vaccinated intranasally with PS-CTB were better protected against pneumococcal bacteremia than those immunized with AM (mock) (P = 0.08).
FIG. 3.
Clearance of pneumococci from bronchoalveoli (top) and from blood (bottom) of mice immunized with vaccines. Three groups of 10 BALB/c mice were immunized three times at an interval of 2 weeks with mock microsphere, naked conjugate (PS-CTB), or encapsulated conjugate [AM(PS-CTB)]. The CFU of pneumococci in bronchoalveolar lavage solution and in blood were counted 18 h after the challenge with S. pneumoniae type 19. Gray and white bars represent the CFU of pneumococci in specimens obtained from the mice after intranasal or peroral vaccination, respectively. Error bars indicate the standard error of the mean. Symbols indicate a statistically significant difference from the mock-immunized group as follows: *, P < 0.05; **, P < 0.01; †, 0.05 < P < 0.09.
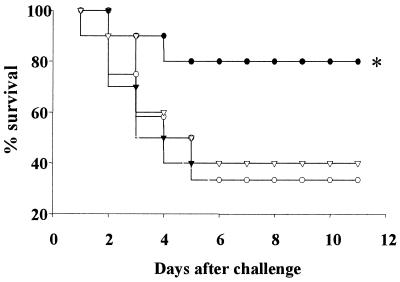
To determine whether PS-specific antibody raised by oral immunization could confer protective immunity on old mice, the mice were boosted at week 32 after the first immunization and were challenged intranasally with live S. pneumoniae. Eighty percent of the mice immunized perorally with AM(PS-CTB) were protected from lethal S. pneumoniae challenge, whereas more than 60% of the mice in the other control groups died of infection (Fig. 4). The mean survival times of mice immunized with AM (PS-CTB) orally, with AM (mock) orally, with AM (PS-CTB) subcutaneously, or with AM (mock) subcutaneously were 10.2 ± 1.2, 6.2 ± 1.3, 6.4 ± 1.5, and 6.7 ± 1.5 days, respectively. The mice immunized with AM (PS-CTB) orally showed significant protection (P < 0.05) against respiratory infection of S. pneumoniae. However, systemic immunization of aged mice with PS did not elicit protective immunity.
FIG. 4.
Immunization of old mice by peroral delivery of AM(PS-CTB) induces PS-specific antibody-mediated protection from lethal challenge with S. pneumoniae. Mice were immunized with AM(PS-CTB) (solid symbols) or with AM(mock) (open symbols) subcutaneously (triangles) or perorally (circles). Groups of mice that were immunized as described in Fig. 3 were boosted once more at week 32 and were challenged 6 weeks after the last immunization by intranasal instillation of live S. pneumoniae (108 CFU/20 μl). Survival is reported as the percentage of surviving mice. All experimental groups include 10 mice except a group of mice immunized perorally with AM(mock), where n = 12. The asterisk indicates a statistically significant difference from the mock-immunized group (P < 0.05). Symbols: ●, AM(PS-CTB) (peroral); ○, AM(mock) (peroral); ▾, AM(PS-CTB) (subcutaneous); ▿, AM(mock) (subcutaneous).
DISCUSSION
It could be considered that mucosal immune defects might contribute to the high rates of pneumococcal pneumonia among infants, the elderly, and patients with HIV infection. However, there are indications that this may not be the case. Similar mucosal levels of IgA and other immune factors were observed between these groups and healthy volunteers (2, 26, 29). Gut IgA antitoxin antibody-forming cells in most HIV-infected subjects after oral immunization with CTB were detected, and the extent of mucosal IgA response was similar to that of healthy individuals (10, 17). Neonates reach adult levels of sIgA by as early as 3 months of age, at which time they can produce IgA-secreting cells in response to an antigen delivered mucosally (29). Elderly humans had significantly higher concentrations of serum and salivary IgM and of salivary IgA antibodies than younger subjects, and the amount of immunoglobulin in whole-gut lavage fluid was similar for these two age groups (2). These observations suggest that overt humoral defects in mucosal immunity are not associated with HIV infection, infantile immunological prematurity, and immunosenescence. These reasons provide a motivation to develop and to use novel mucosal vaccines especially for these immunocompromised groups of people.
Higher induction of sIgA responses could often be achieved through a direct immunization via mucosa-associated lymphoid tissue (MALT), specifically through the PP of the gastrointestinal tract. Effective immunization of the MALT has been limited due to degradation and poor absorption of vaccine at the mucosal surfaces. Numerous approaches to mucosal immunization by using biodegradable microspheres are currently being developed (19). When administered orally, the microsphere protects antigen from acid hydrolysis and enzymatic degradation, is absorbed via M cells, and thus potentiates immune responses in the common mucosal immune systems (19–22, 25). B cells are stimulated by pneumococcal polysaccharide at these organs. Because the uptake of the microspheres into PP is the first step in immunization, we analyzed the uptake of the microspheres by the PP. If the physicochemical properties are considered, the alginate microspheres could be absorbed specifically into the PP through the M cells. The so-called “targeted delivery” could be achieved. These findings suggest that AM could potentially serve as an oral delivery system for the targeted delivery of vaccine antigens into or through the PP.
Despite the poor responses to pneumococcal vaccines in persons with chronic debilitating conditions, the immunization with polysaccharide vaccines conjugated with proteins can enhance prevention of pneumococcal infections (15). The PS-protein conjugates have been shown to be highly immunogenic in both animals and humans (31). S. pneumoniae has been known to colonize at nasopharynx and to invade bloodstreams after the inflammatory activation of lining cells (8). Therefore, the immune exclusion and elimination at the mucosal surfaces are crucial in preventing pneumococcal bacteremia (30). CTB has recently attracted much attention as a promising mucosal carrier for different antigens (11, 16). Therefore, it could be important to determine whether CTB would also provide a mucosal immune response to a conjugated pneumococcal PS antigen. The AM(PS-CTB) induced bronchoalveolar IgA and serum antibody responses that were significantly higher than those induced by naked PS-CTB, indicating that microencapsulation of PS-CTB enhances not only systemic IgG responses responsible for the long-term immunity but also mucosal IgA responses in remote effector sites where the bacteria colonize. AM(PS-CTB) induced prominent serum IgG responses among test groups, suggesting that the conjugation of PS with CTB enhances systemic IgG antibody responses to PS.
Although CT has been known to modulate systemic and mucosal immune responses against protein antigens, little is known about its effect on the immunogenicity of polysaccharide antigen. Interestingly, we observed that the PS-specific serum and mucosal antibody responses of mice significantly changed depending on the carrier protein. While coadministration of CT with AM(PS-BSA) significantly enhanced the systemic antibody responses of mice, those responses were significantly reduced when CT were used with AM(PS-CTB). The serum anti-CTB IgG and IgA levels, as well as the mucosal anti-CTB IgA level, increased when CT was coadministered, as documented previously (11). A recent study shows that the antibody response to the polysaccharide antigen in serum and in lungs after mucosal immunization was suppressed when the mice were preimmunized with the carrier protein (4). It has also been observed that in humans, anti-carrier antibodies in serum can suppress the immune response against polysaccharides after a systemic vaccination with its conjugate (3). Taken together, the suppression in this study is regarded as an immune exclusion by anti-CTB antibody. Since BSA is not important in the uptake efficiency of the conjugate, it can be postulated that anti-BSA antibody response augmented by CT did not decrease the anti-PS antibody responses induced by AM(PS-BSA). In this case, CT presumably has increased anti-PS antibody response by augmenting the anti-carrier T-cell response.
The serum and mucosal antibody responses of the mice immunized intranasally with naked antigen were significantly higher than those with mock microspheres, as well as in those with microencapsulated ones. The higher immunogenicity of PS-CTB over AM(PS-CTB) given via the intranasal route may be achieved because the unencapsulated vaccine antigen may degrade less in the nasal cavity than in the intestine and because the smaller-sized PS-CTB could be taken up preferentially by the microencapsulated one.
This study provides evidence that protection against respiratory infection by S. pneumoniae can be achieved through mucosal vaccination of pneumococcal capsular polysaccharide. We observed that mucosal immunization conferred protection on mice against an intranasal challenge with live bacteria. In contrast, systemic immunization of the aged mice with PS did not elicit protective immunity against S. pneumoniae respiratory infection. This clearly shows that future antipneumococcal vaccines for hosts who are immunocompromised systemically must be designed to induce mucosal immunity.
ACKNOWLEDGMENT
This work was supported by grant 2V00242 from the KIST 2000 program.
REFERENCES
- 1.Ammann A J, Schiffman G, Abrams D, Volberding P, Ziegler J, Conant M. B-cell immunodeficiency in acquired immune deficiency syndrome. JAMA. 1984;251:1447–1449. [PubMed] [Google Scholar]
- 2.Arranz E, O’Mahoney S, Barton J R, Ferguson A. Immunosenescence and mucosal immunity: significant effects of old age on secretory IgA concentrations and intraepithelial lymphocyte counts. Gut. 1992;33:882–886. doi: 10.1136/gut.33.7.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barington T, Skettrup M, Juul L, Heilmann C. Non-epitope-specific suppression of the antibody response to Haemophilus influenzae type b conjugate vaccines by preimmunization with vaccine components. Infect Immun. 1993;61:432–438. doi: 10.1128/iai.61.2.432-438.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergquist C, Lagergard T, Holmgren J. Anticarrier immunity suppresses the antibody response to polysaccharide antigens after intranasal immunization with the polysaccharide-protein conjugate. Infect Immun. 1997;65:1579–1583. doi: 10.1128/iai.65.5.1579-1583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergquist C, Lagergard T, Lindblad M, Holmgren J. Local and systemic antibody responses to dextran-cholera toxin B subunit conjugates. Infect Immun. 1995;63:2021–2025. doi: 10.1128/iai.63.5.2021-2025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beuvery E C, van Rossum F, Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982;37:15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho N H, Seong S Y, Cheon K H, Kim Y H, Kwon I C, Jeong S Y. A novel mucosal immunization with polysaccharide-protein conjugates entrapped in alginate microsphere. J Controlled Release. 1998;53:215–224. doi: 10.1016/s0168-3659(97)00255-1. [DOI] [PubMed] [Google Scholar]
- 8.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 9.Elson C O, Ealding W, Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984;67:101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson K, Kilander A, Hagberg L, Norkrans G, Holmgren J, Czerkinsky C. Intestinal immune responsiveness in HIV-infected individuals. Adv Exp Med Biol. 1995;371:1011–1014. [PubMed] [Google Scholar]
- 11.Holmgren J, Czerkinsky C, Lycke N, Svennerholm A M. Strategies for the induction of immune responses at mucosal surfaces making use of cholera toxin B subunit as immunogen, carrier, and adjuvant. Am J Trop Med Hyg. 1994;50:42–54. [PubMed] [Google Scholar]
- 12.Horan M A. Immunosenescence and mucosal immunity. Lancet. 1993;341:793–794. doi: 10.1016/0140-6736(93)90566-y. [DOI] [PubMed] [Google Scholar]
- 13.Janoff E N, Breiman R F, Daley C L, Hopewell P C. Pneumococcal disease during HIV infection. Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992;117:314–324. doi: 10.7326/0003-4819-117-4-314. [DOI] [PubMed] [Google Scholar]
- 14.Janoff E N, Douglas J M, Jr, Gabriel M, Blaser M J, Davidson A J, Cohn D L, Judson F N. Class-specific antibody response to pneumococcal capsular polysaccharides in men infected with human immunodeficiency virus type 1. J Infect Dis. 1988;158:983–990. doi: 10.1093/infdis/158.5.983. [DOI] [PubMed] [Google Scholar]
- 15.Laferriere C A, Sood R K, de Muys J M, Michon F, Jennings H J. The synthesis of Streptococcus pneumoniae polysaccharide-tetanus toxoid conjugates and the effect of chain length on immunogenicity. Vaccine. 1997;15:179–186. doi: 10.1016/s0264-410x(96)00148-x. [DOI] [PubMed] [Google Scholar]
- 16.Lebens M, Shahabi V, Backstrom M, Houze T, Lindblad N, Holmgren J. Synthesis of hybrid molecules between heat-labile enterotoxin and cholera toxin B subunits: potential for use in a broad-spectrum vaccine. Infect Immun. 1996;64:2144–2150. doi: 10.1128/iai.64.6.2144-2150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis D J, Gilks C F, Ojoo S, Castello-Branco L R, Dougan G, Evans M R, McDermott S, Griffin G E. Immune response following oral administration of cholera toxin B subunit to HIV-1-infected UK and Kenyan subjects. AIDS. 1994;8:779–785. doi: 10.1097/00002030-199406000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Leliger A E, Rijkers G T, Aerts P, Been-Tiktak A, Hoepelman A I, van Dijk H, Borleffs J C. Deficient antipneumococcal polysaccharide responses in HIV-seropositive patients. FEMS Immunol Med Microbiol. 1995;12:33–41. doi: 10.1111/j.1574-695X.1995.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 19.Marx P A, Compans R W, Gettie A, Staas J K, Gilley R M, Mulligan M J, Yamschikov G V, Chen D, Eldridge J H. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323–1327. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 20.Mathiowitz E, Jacob J S, Jong Y S, Carino G P, Chickering D E, Chaturvedi P, Santos C A, Vijayaraghavan K, Montgomery S, Bassett M, Morrell C. Biologically erodable microspheres as potential oral drug delivery systems. Nature. 1997;386:410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- 21.Mestecky, J., and J. R. McGhee. Prospects for human mucosal vaccines. 1992. Adv. Exp. Med. Biol. 327:13–23. [DOI] [PubMed]
- 22.Morris W, Steinhoff M C, Russell P K. Potential of polymer microencapsulation technology for vaccine innovation. Vaccine. 1994;12:5–11. doi: 10.1016/0264-410x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 23.Murray H W, Gellene R A, Libby D M, Rothermel C D, Rubin B Y. Activation of tissue macrophages from AIDS patients: in vitro response of AIDS alveolar macrophages to lymphokines and interferon-gamma. J Immunol. 1985;135:2374–2377. [PubMed] [Google Scholar]
- 24.Neu H C. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 25.O’Hagan D T, Jeffery H, Roberts M J, McGee J P, Davis S S. Controlled release microparticles for vaccine development. Vaccine. 1991;9:768–771. doi: 10.1016/0264-410x(91)90295-h. [DOI] [PubMed] [Google Scholar]
- 26.Opstad N L, Daley C L, Thurn J R, Rubins J B, Merrifield C, Hopewell P C, Janoff E N. Impact of Streptococcus pneumoniae bacteremia and human immunodeficiency virus type 1 on oral mucosal immunity. J Infect Dis. 1995;172:566–570. doi: 10.1093/infdis/172.2.566. [DOI] [PubMed] [Google Scholar]
- 27.Peeters C C, Tenbergen-Meekes A M, Evenberg D E, Poolman J T, Zegers B J, Rijkers G T. A comparative study of the immunogenicity of pneumococcal type 4 polysaccharide and oligosaccharide tetanus toxoid conjugates in adult mice. J Immunol. 1991;146:4308–4314. [PubMed] [Google Scholar]
- 28.Peters V B, Diamant E P, Hodes D S, Cimino C O. Impaired immunity to pneumococcal polysaccharide antigens in children with human immunodeficiency virus infection immunized with pneumococcal vaccine. Pediatr Infect Dis J. 1994;13:933–934. doi: 10.1097/00006454-199410000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Rognum T O, Thrane S, Stoltenberg L, Vege A, Brandtzaeg P. Development of intestinal mucosal immunity in fetal life and the first postnatal months. Pediatr Res. 1992;32:145–149. doi: 10.1203/00006450-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Service R F. Triggering the first line of defense. Science. 1994;265:1522–1524. doi: 10.1126/science.8079164. [DOI] [PubMed] [Google Scholar]
- 31.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 32.Simberkoff M S, El Sadr W, Schiffman G, Rahal J J., Jr Streptococcus pneumoniae infections and bacteremia in patients with acquired immune deficiency syndrome, with report of a pneumococcal vaccine failure. Am Rev Respir Dis. 1984;130:1174–1176. doi: 10.1164/arrd.1984.130.6.1174. [DOI] [PubMed] [Google Scholar]
- 33.Szewczuk M R, Campbell R J, Jung L K. Lack of age-associated immune dysfunction in mucosal-associated lymph nodes. J Immunol. 1981;126:2200–2204. [PubMed] [Google Scholar]
- 34.Weiser J N, Austrian R, Sreenivasan P K, Masure H R. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]