Abstract
Diabetes Mellitus (DM) is a severe and chronic metabolic disorder characterized by hyperglycaemia and various complications, including cardiovascular disease. It is diagnosed when fasting plasma glucose (FPG) level is126 mg/dL (7.0 mmol/L) or higher [1]. Notable differences in DM prevalence are evident among populations in the United States. While DM affects 13% of the general adult population, specific groups, including American Indians/Alaska Natives (14.7%), Hispanics (12.5%), and non-Hispanic Blacks (11.7%), experience disproportionately higher rates. Conversely, lower prevalence rates are observed among non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%). Black Americans are twice as likely to succumb to diabetes-related mortality compared to Whites [2]. The overall objective of this review article is to comprehensively address racial disparities in DM within the United States, emphasizing prevalence rates, management strategies, and health outcomes across diverse ethnic groups. To achieve this objective, we conducted a systematic review and meta-analysis utilizing data from nationally representative surveys, healthcare databases, and published literature spanning from 2014 to 2023. Our findings highlight significant racial disparities in DM prevalence, with minority populations, including African Americans, Hispanics, and Native Americans, consistently exhibiting higher rates than their Caucasian counterparts. Beyond prevalence, disparities extend to access to healthcare resources, diabetes education, and preventive measures. Additionally, challenges in DM management, including access to optimal treatment modalities, medication adherence, and diabetes self-management education, are identified among minority populations. Socioeconomic factors, particularly income and education, significantly contribute to these disparities. This review article contributes to the growing body of evidence guiding policymakers, healthcare professionals, and researchers in developing targeted strategies to achieve health equity in diabetes management and prevention. Addressing these disparities is crucial for fostering an inclusive and practical approach to DM care within diverse ethnic populations.
Keywords: Diabetes mellitus, Racial disparity, Prevalence, Management, Policies
Introduction
Diabetes Mellitus (DM) is a severe and chronic metabolic disorder characterized by hyperglycaemia and various complications, including cardiovascular disease. Globally, the prevalence of diabetes in the general population is projected to escalate to 643 million by 2030 and 783 million by 2045 [3]. DM results from glucose dysregulation, with type 2 DM comprising 90% to 95% of cases among American populations, while type 1 DM accounts for 5% to 10% [4]. In type 1 diabetes, individuals produce insufficient or no insulin due to the destruction of insulin-producing cells by the body’s immune system. This condition is characterized as an autoimmune disease [5]. On the other hand, in type 2 diabetes, the body initially may produce adequate insulin, but it becomes less responsive over time. As the disease progresses, the pancreas gradually loses its capacity to generate sufficient insulin. Type 2 diabetes is linked to both genetic predisposition and lifestyle factors such as excessive weight or obesity, unhealthy dietary habits, and insufficient physical activity [6,7].
Scientific evidence underscores that diabetes significantly impacts quality of life, increasing the risk of adverse complications such as stroke, amputation, kidney failure, myocardial infarctions, and blindness, leading to substantial morbidity and premature mortality [8–10]. Interestingly, the overall rate of diabetes-related complications has decreased since 1990 due to advancements in early detection, screening, diagnosis, and treatment. However, minority patients with diabetes continue to experience these complications at a disproportionately higher rate compared to non-Hispanic White patients. Moreover, they are less likely to receive recommended diabetes preventive care, including haemoglobin A1c (HbA1c) testing, annual cholesterol screening, and retinal examinations [11]. We focus mainly on type 2 DM in this review paper.
The overarching objective of this review article is to comprehensively address racial disparities in DM within the United States, focusing on prevalence rates, management strategies, policies, and health outcomes across diverse ethnic groups.
Approaches
Data Sources and Data Extraction
We undertook a comprehensive systematic review and meta-analysis, drawing on data sourced from nationally representative surveys, healthcare databases, clinical trials, and published literature covering the period from 2014 to 2023. This review was performed using seven databases: Science Direct, PubMed Central, MEDLINE, Google Scholar, World Health Organization (WHO), Food and Drug Administration (FDA), and Centers for Disease Control and Prevention (CDC). Our search incorporated key terms such as “Diabetes Mellitus (DM)”, “racial disparity”, “prevalence”, “management”, “policies”, and “outcomes”. Selection criteria were limited to peer-reviewed articles with titles and abstracts explicitly addressing these key terms.
The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency and reproducibility. Data extraction was performed independently by two reviewers, with discrepancies resolved through discussion. The quality of included studies was assessed using the Cochrane risk-of-bias tool for randomized trials and the Newcastle-Ottawa Scale for observational studies.
Results and Discussions
We found several peer-reviewed articles that addressed the racial disparities in DM prevalence, management, policies, and outcomes. Our findings highlight significant racial disparities in diabetes prevalence, with minority populations, including African Americans, Hispanics, and Native Americans, consistently exhibiting higher rates than their Caucasian counterparts. Beyond prevalence, disparities extend to access to healthcare resources, diabetes education, and preventive measures. Additionally, challenges in diabetes management, including accessing optimal treatment modalities, medication adherence, and diabetes self-management education, are identified among minority populations. Socioeconomic factors, particularly income and education, emerge as significant contributors to these disparities. In our results and discussion sections, we did not summarize all the racial disparities in DM prevalence, management, and outcomes examined in each study. However, we highlighted their main findings in the present article. These are discussed in more detail in subsequent sections below.
Racial and Ethnic Disparities in Diabetes Mellitus
Diabetes mellitus (DM) has a disproportionately higher impact on minority communities in the United States (US). Presently, 13% of the general adult population in the US has diabetes. Figure 1 illustrates the notable variation in diabetes prevalence among racial and ethnic groups in the US. The data informing the graph, as depicted in Figure 1, were sourced from the Centers for Disease Control and Prevention’s National Diabetes Statistics Report 2020 [12].
Figure 1:
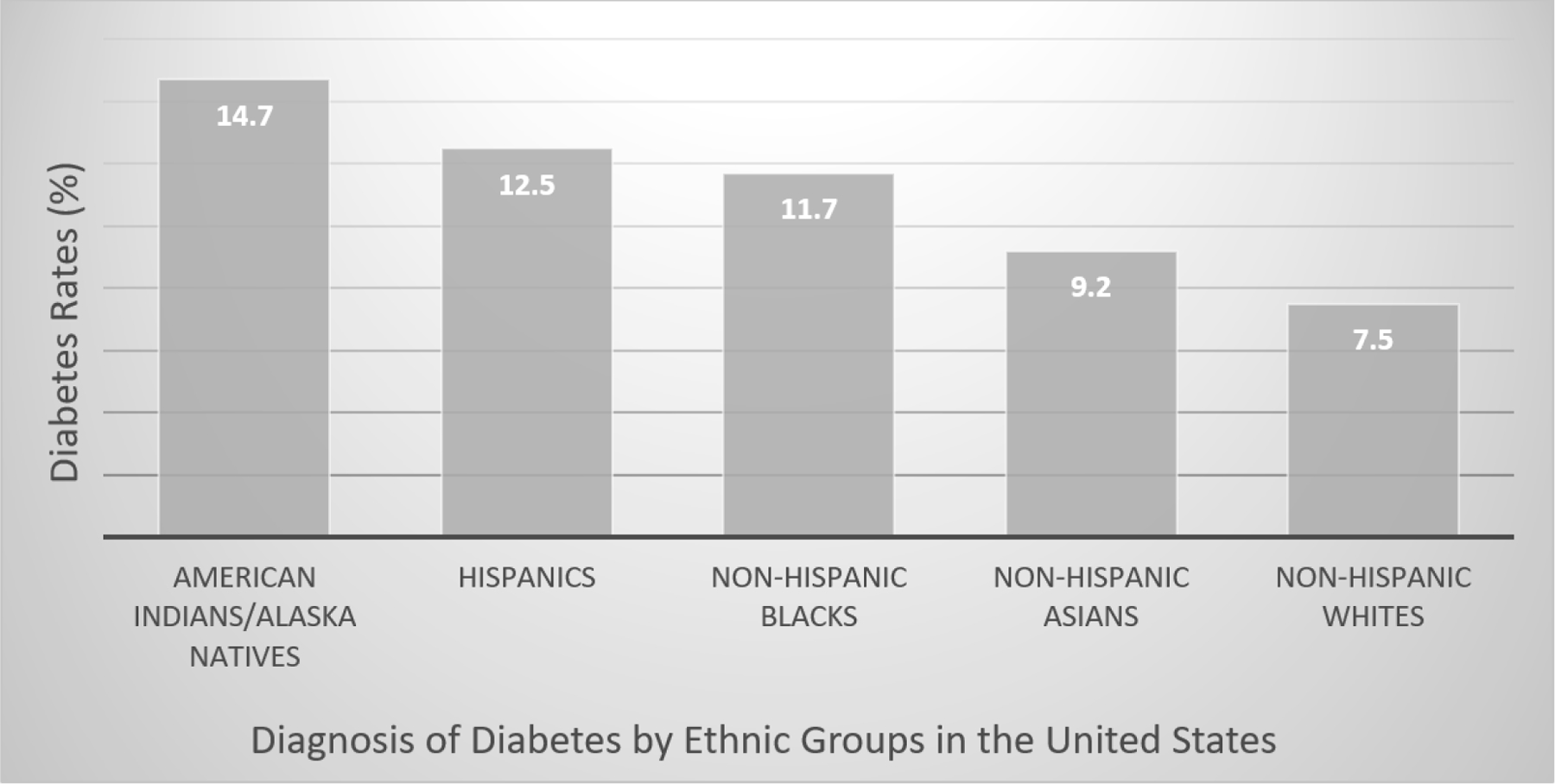
Diabetes diagnosis rates by racial and ethnic groups in the United States.
As seen in Figure 1, American Indians/Alaska Natives exhibit a disproportionately high rate at 14.7%, followed by Hispanics at 12.5%, and non-Hispanic Blacks at 11.7%. In contrast, lower rates are observed among non-Hispanic Asians at 9.2% and non-Hispanic Whites at 7.5% (DHHS, 2020). Moreover, an estimated 38.0% (96 million) of adults aged 18 and above have prediabetes, as per the National Diabetes Statistics Report 2020. Alarmingly, more than 8 in 10 individuals with prediabetes are unaware of their condition [13]. A recent study indicated a 33% increase in diabetes-related deaths during the COVID-19 pandemic, with two-thirds attributed to SARS-CoV-2 infection. Additionally, this study highlighted a rise in diabetes-related mortality, particularly among young adults, and widened disparities across age, sex, and racial and ethnic subpopulations [14].
In the US, the incidence of type 2 diabetes among children and adolescents aged 10 to 19 years has risen gradually from 2002 to 2015 [15,16]. However, when broken down by race and ethnicity, the incidence remained stable among non-Hispanic white individuals but surged significantly among all other racial and ethnic groups, with particularly marked increases observed in non-Hispanic Black children and adolescents [16].
Epidemiological Data on Diabetes Mellitus
Epidemiological data on DM comprehensively explain its prevalence, trends, and demographic disparities. Major key findings of epidemiological studies into DM are detailed in the sections below.
Prevalence, Trends, and Projection of Diabetes Mellitus:
Over the years, the trends in DM prevalence have been steadily increasing, reflecting a growing public health concern. In 2021, crude estimates revealed that 38.4 million individuals, comprising 11.6% of the population of all ages, were affected by diabetes. Among adults aged 18 years or older, 38.1 million adults, accounting for 14.7% of all US adults were diagnosed with diabetes. Over the period of 2001 to 2020, the age-adjusted prevalence of total diabetes significantly increased among US adults, rising from 10.3% in 2001–2004 to 13.2% in 2017–2020 [17]. Future projections from a recent study indicate a notable uptick in diagnosed diabetes cases, with an estimated 39.7 million adults affected by 2030 and 60.6 million by 2060 [18]. Additionally, the study forecasts a rise in the prevalence rates across all race-sex groups, highlighting that Black women and men are anticipated to have the highest prevalence [18].
Demographic Disparities of Diabetes Mellitus:
Diabetes mellitus (DM) affects different population groups unevenly, with specific demographics experiencing higher rates of the condition. Within the Hispanic population, Mexican American adults exhibited the highest age-adjusted prevalence of diagnosed diabetes at 13.4%, followed by 12.4% in Puerto Rican-American adults. In comparison, Cuban American adults had the lowest prevalence at 6.5% [19]. Recent studies have highlighted the heterogeneity in diabetes prevalence and risk factors among Hispanic subgroups [20,21].
Among Black populations, studies have shown differences between US-born and African-born or Caribbean-born adults, with higher prevalence rates observed among US-born individuals in some instances [22]. However, other studies suggest varying trends, indicating complexities within this population. Acculturation and time spent in the US. also influence diabetes risk among foreign-born Blacks [22].
Diabetes rates among Asian Americans vary, with Filipino and Korean Americans exhibiting higher prevalence rates. The CDC’s data indicate varying prevalence rates among different Asian subgroups, with Filipino and Asian Indian populations showing higher rates compared to other Asian groups [13].
As another minority group in the United States, American Indians also experience a disproportionate prevalence of DM within their communities which is closely tied to economic disparities. Factors such as high neighborhood poverty rates, limited access to healthcare services, and enduring economic challenges contribute to this burden. These circumstances often lead to unhealthy lifestyle practices that exacerbate the development and management of chronic illnesses like diabetes. Furthermore, discrimination, especially within healthcare settings, has been identified as a contributing factor to the heightened prevalence of diabetes [19].
Undiagnosed Cases of Diabetes Mellitus
Asignificant proportion of individuals with DM remain undiagnosed in the US, which has implications for disease management and prevention of complications. Interestingly, epidemiological studies have identified a substantial case of undiagnosed diabetes until clinically apparent, necessitating an understanding of undiagnosed cases. Scientific studies highlight a substantial burden of undiagnosed diabetes, with estimates indicating 8.7 million undiagnosed cases in the US, constituting 22.8% of all adults with diabetes [23,24]. However, a recent study suggests a lower prevalence of undiagnosed DM, employing a two-test criterion for screening [24,25]. In addition, several epidemiological studies have identified higher prevalence rates among older adults, obese individuals, and certain racial and ethnic groups, including Mexican and Asian Americans, and those without healthcare access [26,27]. Specifically, Mexican Americans exhibit significantly higher rates of undiagnosed diabetes compared to non-Hispanic whites, as do Asian Americans [24].
Socioeconomic Disparities in Diabetes Mellitus
Diabetes mellitus (DM) burdens the US population unevenly, with varying rates across socioeconomic statuses (SES), and racial, and ethnic groups. SES, a complex construct including factors like education and income, strongly predicts DM onset and progression [28].
Low Income and Poverty Associated with Diabetes Mellitus Disparity:
Lower income levels are associated with a higher prevalence of DM. Poverty often limits access to healthy food options, safe environments for physical activity, and healthcare services, leading to higher rates of obesity and diabetes within impoverished communities. The low-income population bears a disproportionate burden of DM [29,30]. Numerous studies highlight income as a significant contributor to diabetes disparities [15,31,32]. A previous study reported a surge in diabetes prevalence among middle-income, near-poor, and poor adults between 2011 and 2014 [15]. A recent investigation observed higher diabetes prevalence in low-income communities from 2001 to 2018, with income emerging as the primary driver of diabetes disparities, especially among middle-aged females [32]. This inequality has widened since 2011, potentially due to limited opportunities for preventive screenings, food insecurity, and sedentary lifestyles in low-income areas [33]. Poor minorities are likely to eat unhealthy diets. As a result, unhealthy diet and sedentary habits elevate obesity risk, a known diabetes factor [34,35]. Racial and ethnic minorities face heightened diabetes risk, primarily due to socioeconomic factors. Income-related inequality largely underpins this disparity [31]. Hispanics and non-Hispanic Blacks, often impoverished compared to Whites, face higher diabetes susceptibility, compounded by obesity, food insecurity, and physical inactivity [36]. Addressing healthcare quality and access is crucial for mitigating diabetes disparities in these communities.
Poverty is also a commonly found phenomenon among oppressed or indigenous populations [37]. Studies have confirmed that poverty is associated with the development and complications of type 2 diabetes in adults [33,29]. One study investigated poverty as a risk factor for developing type 2 diabetes in youth and discovered that low family income was associated with a significantly higher hazard of type 2 diabetes in adolescents compared to high family income. The dose-dependent association indicates that the risk of type 2 diabetes in adolescents increases with a decrease in household income 38. Furthermore, research revealed a significant link between children and adolescents from lower-income households and a higher risk of hospitalization for diabetes complications [38].
Scientific studies have been conducted to explore the relationship between neighborhood socioeconomic status and diabetes. For instance, Gaskin et al. studied how individual and neighborhood poverty interact to affect the likelihood of developing diabetes. Their findings revealed that poor adults living in nonpoor neighborhoods are more likely to have diabetes compared to nonpoor adults living in nonpoor neighborhoods [39]. Furthermore, the study found that poor adults living in poor neighborhoods have double the odds of having diabetes. The study also observed a gradient in the relationship between race, poverty, and place. When comparing the likelihood of having diabetes, poor Whites living in poor neighborhoods had the highest odds when compared to nonpoor Whites living in nonpoor neighborhoods (odds ratio [OR] 2.51, 95% CI = 1.31–4.81). Also, poor Blacks residing in poor neighborhoods and nonpoor Blacks living in poor neighborhoods had similar odds of having diabetes (OR 2.45, 95% CI 1.50–4.01 and OR 2.49, 95% CI 1.48–4.19, respectively). Lastly, poor Whites living in nonpoor neighborhoods had the lowest odds of having diabetes (OR 1.73, 95% CI 1.16–2.57) [39].
Moreover, studies have shown variations in diabetes prevalence across census tracks, attributable to the socioeconomic status of each neighborhood. Kolak, et al. discovered that Type 2 DM rates were notably higher in census tracts characterized by lower incomes, reduced high school graduation rates, increased single-parent households, and crowded housing [40]. Additionally, adults with prediabetes face an elevated risk of developing Type 2 DM if they reside in neighborhoods with lower educational attainment, diminished annual income, and a higher percentage of households receiving Supplemental Nutrition Assistance Program benefits [41].
The Centers for Disease Control and Prevention (CDC) indicated that individuals with a family income lower than the federal income poverty level often have the greatest prevalence of diabetes (Table 1) [42]. As seen in table 1, families with less than 100% federal poverty level have a higher incidence of diabetes.
Table 1:
Diabetes Incidence Rates in US Adults Based on Family Income Level.
| Family Income Level | Percentage |
|---|---|
| Less than 100% Federal Poverty Level | 14.1% |
| 100% to 299% Federal Poverty Level | 10.8% |
| 300% to 499% Federal Poverty Level | 7.8% |
| 500% Federal Poverty Level or More | 5.6% |
Education Level Associated with Diabetes Mellitus Disparity:
Education provides individuals with knowledge about healthy lifestyle choices, access to resources, and the ability to navigate healthcare systems effectively. Higher levels of education are generally associated with better health outcomes, including a lower risk of developing diabetes. Education correlates closely with health, influencing diabetes mellitus prevalence and outcomes. Adults’ diabetes rates in the US vary by educational attainment, with the highest prevalence among those without a high school diploma [17]. Lower educational levels, poor diet, and race intertwine, contributing to diabetes disparities [43,44]. Non-Hispanic Blacks, Hispanics, and individuals with lower education exhibit higher diabetes incidence and complications [43,45]. Systemic and structural racism hampers educational opportunities for Black and Hispanic individuals, affecting health outcomes [46]. Higher education promotes healthy behaviors and better health outcomes, including DM management [47]. It fosters critical thinking, enabling individuals to navigate health information effectively [48]. Moreover, higher education correlates with better employment and healthcare access, ultimately improving health outcomes [49,50].
Based on the CDC’s 2019–2021 National Health Interview Survey, individuals lacking a high school education exhibit a higher propensity for diabetes than those with higher educational attainment [42]. However, limited research has studied the correlation between educational levels and the incidence of diabetes-related complications upon diagnosis. A study by Leahy et al. revealed a significant elevation in the risk of diabetes-related complications among patients with elementary school education compared to those with a university degree or higher [51]. Similarly, Kumar, et al., observed a marked decrease in the risk of diabetes-related complications among individuals with six years of formal education compared to those without any educational background [52]. Moreover, Liao et al., demonstrated that individuals with higher educational achievements are less prone to experiencing complications associated with diabetes at the time of diagnosis [53]. This can be attributed to their enhanced health literacy, facilitating earlier detection of disease-related issues and reducing complications upon diagnosis, potentially delaying the diagnosis.
Limited Access to Health Insurance and Healthcare Associated with Diabetes Mellitus Disparity
Health insurance plays a pivotal role in diabetes management. Coverage facilitates diagnosis, treatment, and preventive care [54]. Under the Affordable Care Act, health insurance coverage increased, benefiting adults with DM [55,56]. Insured individuals with diabetes receive better care and have fewer complications than the uninsured [22]. However, racial and ethnic minorities historically face lower insurance rates, exacerbating diabetes disparities [57]. Insurance coverage significantly influences diabetes care quality [58]. Despite improvements, disparities persist, with minorities and women experiencing treatment gaps [58]. Uninsured individuals face challenges accessing diabetes screenings and care, leading to undiagnosed diabetes and poorer outcomes [13,59]. Tailored diabetes education programs, such as those for Hispanic populations, improve outcomes, underscoring the value of culturally sensitive intervention [60]. Promoting self-care and culturally competent healthcare can enhance diabetes patients’ quality of life.
In a survey conducted between 2015 and 2016, significant disparities in the quality of diabetes care persisted between White individuals and minority groups. Additionally, factors such as lack of health insurance, lower educational attainment, and the presence of obesity and chronic kidney disease emerged as strong predictors of diabetes incidence and mortality among minority populations [61]. Furthermore, Kamat, et al., demonstrated that the prevalence of diabetes increased with lower education levels, and there was an upward trend in the percentage of Hispanics with diabetes over the study period [61]. These shifts could be attributed to inadequate insurance coverage, limited health knowledge, and subsequent suboptimal diabetes preventive care and management, particularly prevalent among Hispanic populations.
DM care remains a great challenge due to its complex interactions with multiple factors such as education, early screening, socioeconomic status, and the healthcare system [33,62]. It poses significant challenges to healthcare systems globally. Therefore, understanding the multifaceted factors associated with the prevalence and management of DM within healthcare systems is paramount for effective prevention and treatment strategies.
The healthcare system is crucial in-patient diabetes self-management and care [63,64]. High-quality care for diabetes decreases the risk of diabetes complications and mortality [65,66]. According to the American Diabetes Association (ADA), it is recommended for diabetic patients with type 2 to frequently visit their healthcare providers to perform haemoglobin A1c tests, foot exams, dilated eyes exams, blood cholesterol tests, and flu vaccines [36]. Regular check-up and testing of HbA1C blood glucose is considered by the ADA as foundational care for patients with type 2 diabetes 1.
In the United States, diabetes care disparities in the healthcare system could be examined in terms of affordability, accessibility, and competency of healthcare workers [67]. In 2018, a scientific study used the category of health insurance coverage, whether private, public, or lack of health insurance, to measure the accessibility of diabetic patients to health [68]. It was noted that uninsured adults were significantly less likely to receive diabetic services compared to those with private while adjusting for possible confounding factors [68]. Furthermore, a population-based study of diabetic patients in the US from 2005–2016 revealed that patients who had health insurance coverage had higher odds of seeking diabetic care and meeting the glycaemic targets set by the ADA compared to those who were uninsured [58]. They also reported a significantly lower odd ratio in NHB achieving the blood pressure, and cholesterol level compared to NHW [58]. Worldwide, patients in rural areas are often challenged with the accessibility to healthcare due to geographic isolation, shortage of healthcare workers, and lower health spending compared to urban areas [69].
An investigation found worse diabetic quality outcomes, low frequent visits for care, and less accessibility to specialist care in rural settings compared to urban areas [67]. In brief, high-quality diabetic management is associated with to social determinants of health and access to healthcare quality [33,70,71]. Therefore, an active interaction involving the patients, healthcare workforce, and health care system would be beneficial to delivering high-quality care for diabetes [72,73]. Nevertheless, the healthcare system worldwide is struggling to improve the health of their population with constrained budgets. The gap between the resources’ availability and the diabetic care adversely impacts not only the treatment but also the health outcome [74]. Consequently, self-management training for diabetes, virtual care, and reducing barriers to access to healthcare have been recommended for diabetic patients [74]. Ensuring of funding on training programs are directed to meet the need for treatment and education on diabetes, to assign pre-deductible insurance coverage for high diabetic treatment, and to enhance patient safety policy in healthcare practice [74]. The system should also implement deploying new value-based approaches to reward financing or penalizing organizations and providers depending on the quality of care they provide.
Management of Diabetes Mellitus
The International Diabetes Federation (IDF) estimated approximately 463 million adults with diabetes in 2019, which is projected to increase to 578 million by 2030 and 700 million by 2045. Current treatment options for DM primarily involve antidiabetic drugs such as metformin, sulfonylurea, thiazolidinedione, or DPP-4 inhibitors [75]. However, these medications often fall short of achieving complete diabetes control, necessitating the exploration of new therapeutic strategies to address the burden of the disease.
One such strategy, accessible and cost-effective for diabetic patients, involves the use of medicinal plants, vitamins, and essential elements. Recent research in our laboratory has highlighted the health benefits of edible medicinal plants such as Allium sativum, Momordica charantia, Hibiscus sabdariffa L., and Zingiber oficinale, as well as vitamins C, D, and E, all of which exhibit anti-hypoglycemic properties and hold promise for the prevention and/ or management of DM [76]. It has been reported that the beneficial health effects of these edible medicinal plants and essential vitamins include their anti-inflammatory, immunomodulatory, cardioprotective, hypolipidemic, hypoglycaemic, antioxidant, antibiotic, antifungal, antimicrobial, antiseptic, anticancer, and antiviral activities [76–80].
Furthermore, incorporating physical activity into daily routines, along with other preventive measures like maintaining a healthy diet, can potentially lead to improved outcomes in managing DM. The World Health Organization (WHO) has pinpointed insufficient physical activity as a significant risk factor driving global mortality rates. Their guidelines stress that even modest levels of physical activity offer benefits over complete inactivity, and higher levels of activity correlate with even better health outcomes [81–83]. While nutritional and physical activity benefits are often studied separately, recent findings underscore the synergistic potential of integrating both to achieve stronger positive health effects and fortify the immune system [84,85]. This integrated approach surpasses the impacts of singular-focused strategies. Research indicates that maintaining a balanced diet and engaging in regular physical activity during childhood can significantly diminish the likelihood of developing chronic diseases in adulthood [86,87]. A balanced diet, characterized by ample intake of fruits, vegetables, whole grains, plant proteins, and dairy products, furnishes the body with vital nutrients crucial for optimal growth and development.
Figure 2 below provides an example of health promotion practices based on a healthy diet and physical activity that individuals are encouraged to adopt in their daily lives.
Figure 2:
Achieve optimal health through consistent engagement in physical exercise and a well-balanced dietary regimen rich in nutrient-packed vegetables, fruits, and essential vitamins.
Addressing Diabetes Mellitus through Healthy Diet Strategies
Maintaining a well-balanced diet is pivotal in fostering a robust immune system, bolstering its ability to prevent diabetes mellitus effectively. Edible medicinal plants, known for their extensive historical use in traditional medicine to address many diseases, have emerged as promising sources of antidiabetic drugs. Edible medicinal plants and/or plant-based medicine have long been employed worldwide as cost-effective remedies for preventing and treating diabetes. Eating plant-based medicine plays an excellent and effective role in addressing diabetes mellitus through healthy diet strategies. Especially in economically disadvantaged regions, reliance on these natural resources is commonplace for managing various health conditions, including diabetes. Notably, many pharmaceuticals in use today are derived from compounds found in traditional medicinal plants. For instance, metformin, a widely prescribed anti-hyperglycaemic drug, traces its origins to the historical use of Galega oficinalis in DM treatment [88,89]. Among the most prominent medicinal plants and vitamins with hypoglycaemic properties, aiding in immune system enhancement and blood sugar regulation in humans, are Allium sativum (garlic), Momordica charantia (bitter melon), Hibiscus sabdariffa L. (roselle plant), Zingiber oficinale Rosc (ginger), as well as vitamins C, D, and E [76]. The accessibility, affordability, and efficacy of these edible medicinal plants render them pivotal in addressing DM management, particularly in resource-constrained settings across the globe.
Syzygium cumini, commonly known as Black Plum is a medicinal plant that merits attention. This fruit-bearing tree is native to India. In a rat experiment, Black Plum exhibited various beneficial effects, including regulating blood sugar levels and enhancing insulin sensitivity. Oral administration of ethanolic and aqueous extracts derived from the bark of Syzygium cumini (at a dosage of 500 mg/kg for 21 days) demonstrated notable reductions in blood glucose levels in diabetic Wistar rats [90].
The consumption of a healthy diet is a wonderful and strategic way to help address DM, but the patient’s body is very vital as well. That is why the edible medicinal way seems to be effective. To address the healthy diet, patients should be willing to change their eating habits. Minor lifestyle changes, especially healthy diet habits can help with this. Although sugar plays a big role in DM, research shows that fat is the issue when it comes to diabetic patients. Minimizing fat intake and reducing body fat help insulin do its job much better. Newer treatment programs drastically reduce meats, high-fat dairy products, and oils [91,92]. It is even stated that starting a vegan diet can also help by avoiding animal product foods. Animal products contain fat and are usually linked with heart disease as well as insulin resistance. It also affects cholesterol levels as well. Forty-three percent of the vegan group reduced their diabetes medications. Among those participants who didn’t change their lipid-lowering medications, the vegan group also had more substantial decreases in their total and LDL cholesterol levels [93]. Current scientific pieces of evidence indicate that adopting healthy dietary patterns can decrease the likelihood of developing major diet-related chronic diseases, including type 2 diabetes, cardiovascular disease, and certain cancers [94–96].
Diet is a crucial modifiable risk factor for the prevention of non-communicable diseases, including obesity, Cardiovascular Disease (CVD), Type 2 diabetes (T2D), and certain cancers. Several systematic reviews and meta-analyses (SRMA) of Randomized Controlled Trials (RCT)have indicated that interventions aimed at improving diet quality (e.g., low-calorie and low-fat diets) and/or increasing physical activity levels can delay or prevent the onset of T2D [97,98]. However, this evidence primarily focuses on high-risk populations, such as individuals with prediabetes or obesity.
Increasing the consumption of fruits and vegetables among the US population is particularly important given the strong evidence that healthy dietary patterns rich in fruits and vegetables are associated with less long-term weight gain, whereas diets characterized by meat and fried foods are linked to greater weight gain [99]. A recent analysis of US health professionals highlighted that a 4-year weight change was positively and strongly associated with increased daily servings of potato chips, potatoes, Sugar-Sweetened Beverages (SSBs), unprocessed red meats, and processed meats [100]. Conversely, weight change was inversely associated with the intake of vegetables, whole grains, fruits, nuts, and yogurt [101]. In addition to specific foods, poor carbohydrate quality also appears to influence subsequent weight gain and T2D risk.
Addressing Diabetes Mellitus through Physical Activity Strategies
Physical exercise is of the utmost importance in dealing with diabetes. Daily exercise aids in the digestion of food and helps control blood sugar in diabetic patients [91]. Today, exercise is recommended as one of the first management strategies for patients newly diagnosed with diabetes mellitus and, together with diet and behavior modification, is a central component of diabetes mellitus and obesity prevention programs [102,103]. Notably, aerobic exercise is a well-established way to improve HbA1c, and strong evidence exists about the effects of aerobic activity on weight loss and the enhanced regulation of lipid and lipoprotein metabolism [102–104]. There are many more physical activity strategies that researchers can look at. For example, resistance training is necessary. Resistance Training (RT) is when you perform resistance exercises to strengthen and build muscle mass. RT can combat metabolic dysfunction in patients with type 2 diabetes It has the power to combat metabolic dysfunction in patients with type 2 diabetes It seems to effectively improve overall metabolic health and reduce metabolic risk factors in diabetic patients [105]. RT is one way rather than aerobics. It can help improve insulin sensitivity by helping the muscle cells uptake glucose, which can help lower blood sugar levels. This is important because it allows the glycaemic control for a diabetic.
Another physical activity would be Interval Training (IT). It is an exercise type where you alternate between intense and lower activities. For example, when you sprint for 10 minutes, slow down catch your breath, and then continue to sprint. This exercise is used to pace the patient’s body. Research has shown that High-Intensity Interval Training (HIIT) can promote improvements in glucose control and cardiovascular health in individuals with type 2 diabetes [106–108]. HIIT has had a major progression. When it discussed in patients with type 2 DM there have been signs of improvement in insulin sensitivity, blood pressure and cardiovascular, and metabolic health. Lastly, incorporating physical activity into the patient’s daily routine would be beneficial. For example, instead of taking the elevator, taking the stairs, or walking around the neighborhood when given a chance instead of lying on the couch. These little activities can go a long way. The key to addressing diabetes in physical activities is finding something you can integrate into your routine. Regular exercise produces health benefits beyond improvements in cardiovascular fitness. These include enhanced glycaemic control, insulin signaling, and blood lipids, as well as reduced low-grade inflammation, improved vascular function, and weight loss [104,109].
In a comprehensive report published by Health and Human Services, USA, it was reported in 2015 that physical activity significantly improved abnormal glucose tolerance primarily when caused by insulin resistance, rather than by a deficiency of circulating insulin [110]. Additionally, physical activity is likely most beneficial in preventing the progression of Type 2 Diabetes Mellitus (T2DM) during its early stages, before insulin therapy becomes necessary [111]. The protective mechanism of physical activity appears to have a synergistic effect with insulin. During a prolonged session of physical activity, contracting skeletal muscles enhance glucose uptake into the cells, increasing blood flow to the muscles and improving glucose transport into muscle cells [112]. Moreover, physical activity has been found to reduce intra-abdominal fat, a known risk factor for insulin resistance. Other studies have shown that physical activity is inversely associated with intra-abdominal fat distribution and can reduce overall body fat stores [113,114].
Treatment of Diabetes Mellitus
The treatment approach for Diabetes Mellitus (DM) varies depending on the specific type [114] diagnosed in a patient. Two main types, Type 1 Diabetes, and Type 2 Diabetes, have distinct characteristics and underlying causes. Type 1 Diabetes results from an autoimmune attack on the body’s insulin-producing cells and typically manifests from birth. Conversely, Type 2 Diabetes occurs when body cells develop insulin resistance, or the pancreas fails to produce sufficient insulin to meet the body’s needs.
Tailoring treatment for each type and individual involves considering various factors such as symptoms, the type of DM, and the patient’s financial resources or ability to make significant lifestyle changes. While lifestyle modifications have shown benefits, they can present long-term challenges for many patients. Physicians must be knowledgeable about the range of pharmaceutical options available for diabetes treatment and select the most effective, safe, and tolerable medications for their patients [115].
Treatment modalities for DM encompass both oral and injectable medications. For Type 2 DM, numerous oral pharmacological agents exist, including sulfonylureas, meglitinides, metformin, thiazolidinediones (TZDs), alpha-glucosidase inhibitors, dipeptidyl peptidase IV (DPP-4) inhibitors, bile acid sequestrants, dopamine agonists, sodium-glucose transport protein 2 (SGLT2) inhibitors, and oral glucagon-like peptide 1 (GLP-1) receptor agonists. Additionally, injectable GLP-1 agonists are used to lower blood sugar levels in individuals with type 2 DM by stimulating insulin secretion and suppressing glucagon secretion.
In contrast, Type 1 DM requires a different treatment approach due to its autoimmune nature, which destroys insulin-producing β cells. Insulin replacement therapy stands as the primary treatment option for type 1 DM 116. This therapy helps regulate blood sugar levels and enhance the quality of life for patients. In cases of Diabetic Ketoacidosis, a life-threatening condition caused by severe insulin deficiency, intensive insulin therapy becomes crucial.
Management of type 1 DM often involves a combination of intensive dietary control and lifelong exogenous insulin supplementation, administered either through multiple daily injections or insulin pumps [116]. In some cases, transplantation becomes a consideration, with pancreas or islet cell transplants being options for adult patients with type 1 DM [117]. However, these procedures are less common in pediatric or child patients. Dietary modifications and regular physical activity serve as fundamental components of diabetes treatment, aiding in energy balance and overall management of the condition [115].
Policy and Advocacy Addressing Diabetes Mellitus
Diabetes ranks among the top 10 causes of mortality worldwide. Alongside cardiovascular disease, cancer, and respiratory diseases, these ailments contribute to more than 80% of premature deaths from noncommunicable diseases [118]. Those with diabetes face a 2–3 times higher risk of all-cause mortality [119]. Moreover, the presence of diabetes correlates with heightened mortality rates from infections, cardiovascular disease, stroke, chronic kidney disease, chronic liver disease, and cancer [120].
A holistic strategy, integrating public awareness, preventive measures, healthcare access, research, and collaboration, is indispensable in tackling the intricate challenges posed by DM through policy and advocacy endeavors. Figure 3 illustrates a structured framework to address DM epidemic effort comprehensively.
Figure 3:
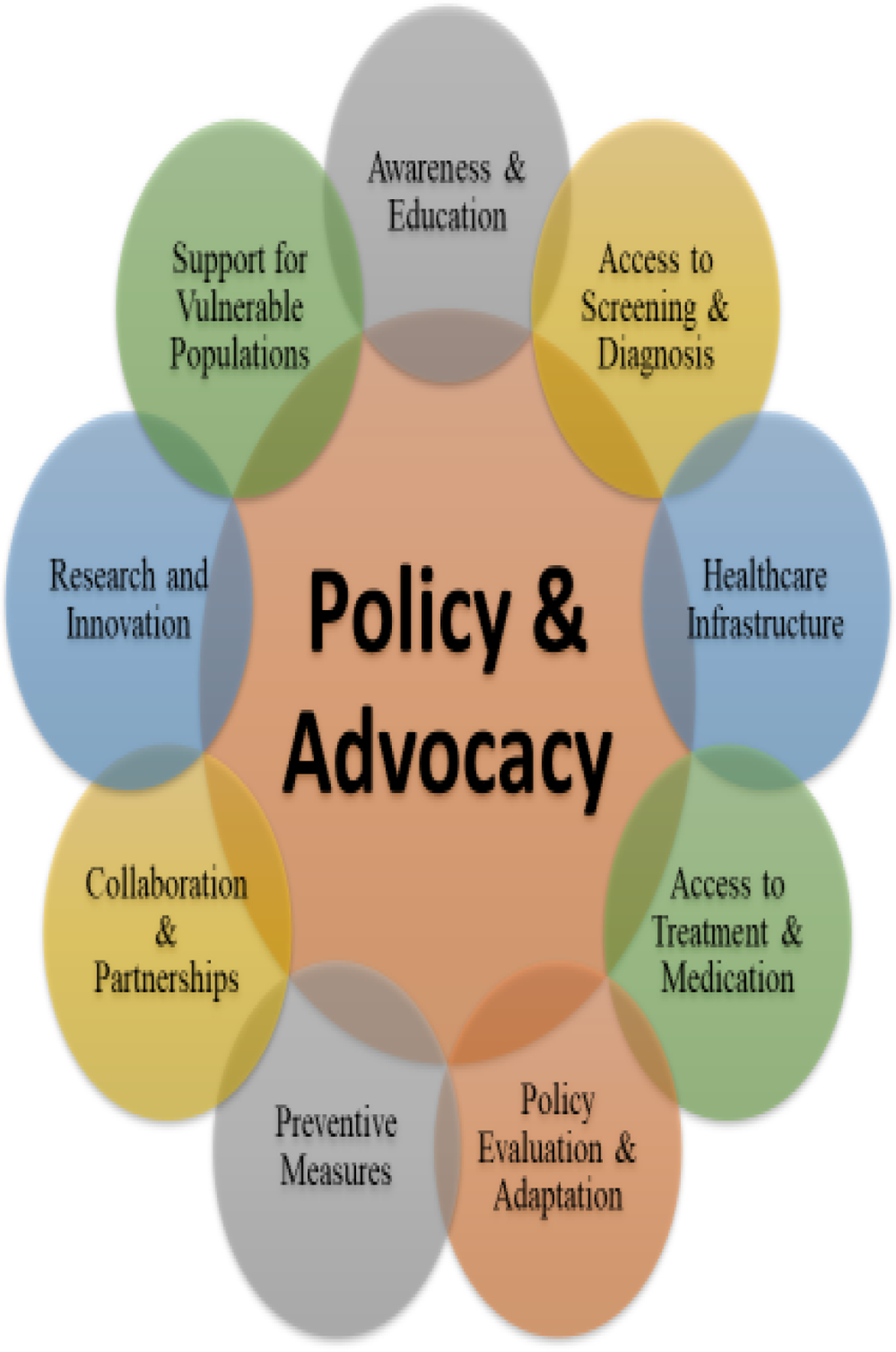
A structured framework for policy and advocacy efforts to comprehensively address the DM epidemic.
Awareness and Education Addressing Diabetes Mellitus
Diabetes Mellitus (DM) imposes significant healthcare burdens, particularly for patients with poor glycaemic control and nephropathy, who face heightened risks of cardiovascular complications and renal failure [121]. Intensive diabetes care, coupled with patient empowerment, has shown promise in sustaining glycaemic control, reducing clinical complications and nephropathy progression, and lowering cardiovascular complication rates [121]. Thus, governments and health organizations must prioritize campaigns aimed at raising awareness about DM, its risk factors, and preventive measures. Additionally, efforts should focus on educating the public about the importance of adopting a healthy lifestyle, maintaining a balanced diet, engaging in regular exercise, and facilitating early detection of diabetes. Previous studies have demonstrated that effective health education significantly improves knowledge, attitude, and practices, leading to better glycemic control. It is widely accepted as an integral part of comprehensive diabetes care for individuals and their families in primary care settings [122,123]. The level of patients’ knowledge about diabetes plays a crucial role in the self-management of the disease. Patients with a thorough understanding of the nature and consequences of diabetes are generally less prone to complications and severe exacerbations [124,125]. The benefits of diabetes self-management education and support include lowering A1C levels, reducing hospital admissions, readmissions, and emergency department visits, reducing diabetes-related distress, and improving self-care behaviors.
Screening and Diagnosis Addressing Diabetes Mellitus
Ensuring proper health education within communities is crucial for promoting regular screening tests and effective management practices to mitigate the risks associated with late diagnosis of DM complications. The IDF has estimated that over half of individuals with diabetes, predominantly Type 2 DM, remain undiagnosed, with cases often only recognized once severe complications arise [3]. The American Diabetes Association (ADA) recommends that screening for diabetes be considered at least every three years, beginning at age 45 for individuals with a BMI greater than 25 (greater than 23 for Asians) and with at least one other risk factor for diabetes [126]. Individuals with multiple risk factors for diabetes may be considered for screening at earlier ages. Additionally, individuals with hypertension (greater than 135/80) should be screened for diabetes. For adults with both hypertension and diabetes, achieving lower blood pressure targets reduces the incidence of cardiovascular events and cardiovascular mortality, justifying the need for regular screening. Individuals diagnosed with prediabetes benefit from diabetes prevention interventions and should be tested annually for progression to diabetes. Therefore, policy initiatives must prioritize access to affordable and convenient screening methods for diabetes mellitus, particularly in underserved communities.
Preventive Measures and/or Treatment of Diabetes Mellitus
Lifestyle modifications, particularly focusing on a healthy diet and physical exercise, are pivotal in slowing the progression of diabetes. Dietary adjustments incorporate nutrient-rich foods like whole grains, vegetables, fruits, legumes, low-fat dairy, lean meats, nuts, and seeds. These dietary choices contribute to maintaining optimal body weight, achieving personalized glycaemic, blood pressure, and lipid targets, and mitigating diabetes-related complications [127]. Policymakers should implement policies that promote healthy behaviors and prevent the development of DM by promoting physical activities and eating a well-balanced diet. Policies should also ensure that individuals with diabetes have access to affordable and effective treatment options, including medications, insulin, and medical devices.
Support for minority Populations with Diabetes Mellitus
Policies should address the specific needs of vulnerable populations, such as low-income individuals, minorities, and those living in rural areas. This may involve targeted interventions to improve access to healthcare services, culturally sensitive educational materials, and community-based programs for diabetes prevention and management.
Collaboration and Partnerships Addressing Diabetes Mellitus
Diabetes stands as one of the foremost global public health challenges, casting a significant burden on both public health systems and socioeconomic progress worldwide. While there are signs of decreasing incidence in certain regions, the prevalence of diabetes has surged over recent decades across the majority of developed and developing nations [128–130].
Policymakers should collaborate with various stakeholders, including healthcare providers, patient advocacy groups, industry partners, and international organizations, to develop and implement comprehensive diabetes policies. These stakeholders can leverage their expertise and resources to achieve common goals and maximize impact by working together.
Limitations
The review paper on racial disparities in diabetes mellitus (DM) within the United States presents valuable insights, but it also has some limitations:
The study focuses exclusively on the United States, potentially limiting the applicability of its findings to other countries or regions with different healthcare systems and socio-economic conditions.
The review relies on data from seven specific databases, which might introduce selection bias. Important studies published in other databases or not indexed in these sources could be omitted. The inclusion criteria are limited to peer-reviewed articles with specific keywords in their titles and abstracts, potentially excluding relevant studies that do not explicitly use these terms.
The data spans from 2014 to 2023, which may not capture more recent trends or changes in diabetes prevalence and management, especially in the context of the COVID-19 pandemic and its impact on healthcare disparities.
While the review highlights disparities among various racial and ethnic groups, it might not fully account for the heterogeneity within these groups. For instance, the Hispanic population includes diverse subgroups with varying health profiles and socio-economic backgrounds.
Although the review addresses socioeconomic factors contributing to disparities, it may not provide a comprehensive analysis of all relevant socio-economic determinants, such as employment status, housing conditions, and social support networks.
The review emphasizes disparities in access to healthcare resources but may lack a detailed exploration of the underlying systemic barriers, such as healthcare policies, insurance coverage disparities, and cultural competence of healthcare providers.
The discussion on diabetes management and prevention strategies among minority populations might benefit from a more in-depth analysis of specific interventions and their effectiveness, including community-based programs and culturally tailored health education.
While the review aims to guide policymakers, it may lack actionable recommendations or a detailed discussion on how to implement effective policies and interventions to reduce racial disparities in diabetes prevalence and management.
The review may not adequately address potential confounding factors, such as comorbidities, lifestyle factors, and genetic predispositions, which could influence diabetes outcomes and disparities among different racial and ethnic groups.
Addressing these limitations in future research could provide a more nuanced understanding of racial disparities in diabetes and inform more effective strategies to achieve health equity.
Conclusions
This review has underscored the pervasive racial disparities in Diabetes Mellitus (DM) prevalence, management, and health outcomes in the United States. Our systematic analysis of data from 2014 to 2023 has illuminated the stark differences in DM rates among various ethnic groups, highlighting that African Americans, Hispanics, and Native Americans face significantly higher prevalence rates compared to non-Hispanic Whites and Asians. These disparities extend beyond mere prevalence, impacting access to healthcare resources, diabetes education, and preventive measures, which are essential for effective disease management and complication prevention.
Our findings emphasize that socioeconomic factors, particularly income and education, are critical contributors to these disparities. Minority populations often face economic challenges that limit their access to quality healthcare, healthy food options, and safe environments for physical activity. These barriers contribute to higher rates of obesity and diabetes within these communities.
To address these inequities, policymakers, healthcare professionals, and researchers must develop targeted strategies aimed at achieving health equity in diabetes management and prevention. Interventions should focus on improving access to healthcare, enhancing diabetes education, and ensuring that preventive measures are available and affordable for all populations, particularly those at higher risk. Additionally, efforts to address the broader socioeconomic determinants of health, such as poverty and education, are crucial for reducing the burden of diabetes among minority populations
Funding
This work was supported in part by the AIM-AHEAD Coordinating Center, funded by the National Institutes of Health (NIH), Grant # 3OT2OD032581-01S; Grant # U54MD015929-05 at Jackson State University, MS, United States; Grant # U54MD013376-05 at Morgan State University, MD, United States; and by the National Science Foundation (NSF), NSF-IUSE, Grant # 2142465 at Florida Agricultural and Mechanical University, Tallahassee, FL, United States. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH and NSF.
Footnotes
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest.
Data Availability Statement:
The data that support the present review article are included in the article.
References
- 1.Goyal A, Gupta Y, Singla R, Kalra S, Tandon N (2020) American Diabetes Association American Diabetes Association “Standards of Medical Care-2020 for Gestational Diabetes Mellitus”: A Critical Appraisal. Diabetes Ther 11: 1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhaliwal R, Pereira RI, Diaz-Thomas AM, Powe CE, Cardozo LLY, et al. (2022) Eradicating Racism: An Endocrine Society Policy Perspective. J Clin Endocrinol Metab 107: 1205–1215. [DOI] [PubMed] [Google Scholar]
- 3.Shidlovskaya TA, Navalkivska NY (2020) Distortion product otoacoustic emissions among the patients suffering diabetes mellitus type II with hearing impairment. Otorhinolaryngology. [Google Scholar]
- 4.Malla G, Long DL, Judd SE, Irvin MR, Kissela BM, et al. (2019) Does the association of diabetes with stroke risk differ by age, race, and sex? Results from the reasons for geographic and racial differences in stroke (REGARDS) study. Diabetes Care 42: 1966–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redondo MJ, Steck AK, Pugliese A (2018) Genetics of type 1 diabetes. Pediatr Diabetes 19: 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinton W, Nemeth B, de Lusignan S, Field B, Feher MD, et al. (2021) Effect of type 1 diabetes and type 2 diabetes on the risk of venous thromboembolism. Diabet Med 38: e14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, et al. (2020) Epidemiology of Type 2 diabetes - Global burden of disease and forecasted trends. J Epidemiol Glob Health 10: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narváez-Méndez M, Morgan S, Coronado-Malagón M, Arce-Salinas CA, et al. (2018) Biomarkers Through the Development, Progression and Chronic Complications of Diabetes Mellitus: A Mini-Review. J Endocrinol Diab 5: 1–7. [Google Scholar]
- 9.Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E (2016) Chronic Complications of Diabetes Mellitus: A Mini Review. Curr Diabetes Rev 13: 3–10. [DOI] [PubMed] [Google Scholar]
- 10.Ramtahal R, Khan C, Maharaj-Khan K, Nallamothu S, Hinds A, et al. (2015) Prevalence of self-reported sleep duration and sleep habits in type 2 diabetes patients in South Trinidad. J Epidemiol Glob Health 5: S35–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng YY, Diamant A, Jones J, Lin W, Chen X, et al. (2016) Racial and ethnic disparities in diabetes care and impact of vendor-based disease management programs. Diabetes Care 39: 743–749. [DOI] [PubMed] [Google Scholar]
- 12.DHHS (2020) National Diabetes Statistics Report, 2020. [Google Scholar]
- 13.Patel J (2023) Diabetes, A Global Epidemic. SACAD: John Heinrichs Scholarly and Creative Activity Days 2023: 103. [Google Scholar]
- 14.Lv F, Gao X, Huang AH, Zu J, He X, et al. (2022) Excess diabetes mellitus-related deaths during the COVID-19 pandemic in the United States. EClinicalMedicine 54: 101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckles GL, Chou CF (2016) Disparities in the Prevalence of Diagnosed Diabetes - United States, 1999–2002 and 2011–2014. MMWR Morb Mortal Wkly Rep 65: 1265–1269. [DOI] [PubMed] [Google Scholar]
- 16.Hamman RF, Bell RA, Dabelea D, D’Agostino RB Jr, Dolan L, et al. (2014) The SEARCH for diabetes in youth study: Rationale, findings, and future directions. Diabetes Care 37: 3336–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (2020) National Diabetes Statistics Report, 2017. [Google Scholar]
- 18.Lin J, Thompson TJ, Cheng YJ, Zhuo X, Zhang P, et al. (2018) Projection of the future diabetes burden in the United States through 2060. Popul Health Metr 16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan S, Gujral UP, Quarells RC, Rhodes EC, Shah MK, et al. (2023) Disparities in diabetes prevalence and management by race and ethnicity in the USA: defining a path forward. Lancet Diabetes Endocrinol 11: 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamody RC, Grilo CM, Vásquez E, Udo T (2021) Diabetes prevalence among diverse Hispanic populations: considering nativity, ethnic discrimination, acculturation, and BMI. Eat Weight Disord 26: 2673– 2682. [DOI] [PubMed] [Google Scholar]
- 21.Cordero C, Schneiderman N, Llabre MM, Teng Y, Daviglus ML, et al. (2022) Diabetes Incidence Among Hispanic/Latino Adults in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care 45: 1482–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukaz DK, Melby MK, Papas MA, Setiloane K, Nmezi NA, et al. (2022) Diabetes and acculturation in African immigrants to the United States: analysis of the 2010–2017 National Health Interview Survey (NHIS). Ethn Heal 27. [DOI] [PubMed] [Google Scholar]
- 23.Menke A, Casagrande S, Geiss L, Cowie CC (2015) Prevalence of and Trends in Diabetes among Adults in the United States, 1988–2012. JAMA 314: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 24.Fang M, Wang D, Coresh J, Selvin E (2022) Undiagnosed Diabetes in U.S. Adults: Prevalence and Trends. Diabetes Care 45: 1994–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida Y, Wang J, Zu Y, Fonseca VA, Mauvais-Jarvis F (2023) Rising Prediabetes, Undiagnosed Diabetes, and Risk Factors in Young Women. Am J Prev Med 64: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ifedayo J (2023) Assessment of Diabetes Mellitus Perception among Adult Residents in Akure South Local Government Area of Ondo State, South West Nigeria. Am J Phys Educ Heal Sci 1: 1. [Google Scholar]
- 27.Verma H, Chakole S, Laishram G (2021) A Brief Study on Diabetes Mellitus: Pathophysiology and Diagnosis. J Pharm Res Int 33: 1675– 1681. [Google Scholar]
- 28.Piccolo RS, Duncan DT, Pearce N, McKinlay JB (2015) The role of neighborhood characteristics in racial/ethnic disparities in type 2 diabetes: Results from the Boston Area Community Health (BACH) Survey. Soc Sci Med 130: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zolezzi M, Lopez J, Mitchell-Bennet L, Payne LY, McCormick JB, et al. (2022) A Chronic Care Management Framework Bridging Clinic, Home, and Community Care in a Mexican American Population. Health Promot Pract 23: 367–371. [DOI] [PubMed] [Google Scholar]
- 30.Mertens E, Sagastume D, Penalvo JL (2021) Quantification of disparities in the distribution of lifestyle and metabolic risk factors, prevalence of non-communicable diseases and related mortality: The Belgian Health Interview Surveys 1997–2018. BMJ Open 11: e053260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saelee R, Hora IA, Pavkov ME, Imperatore G, Chen Y, et al. (2023) Diabetes Prevalence and Incidence Inequality Trends Among U.S. Adults, 2008–2021. Am J Prev Med 65: 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Zhou X, Bullard KM, Zhang P, Imperatore G, et al. (2021) Income-related inequalities in diagnosed diabetes prevalence among US adults, 2001–2018. PLoS One 18: e0283450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, et al. (2020) Social determinants of health and diabetes: A scientific review. Diabetes Care 44: 258–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malone JI, Hansen BC (2019) Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr Diabetes 20: 5–9. [DOI] [PubMed] [Google Scholar]
- 35.Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE (2022) Why does obesity cause diabetes? Cell Metab 34: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marathe PH, Gao HX, Close KL (2017) American Diabetes Association Standards of Medical Care in Diabetes 2017. J Diabetes 9: 320–324. [DOI] [PubMed] [Google Scholar]
- 37.McGavock J, Wicklow B, Dart AB (2017) Type 2 diabetes in youth is a disease of poverty. Lancet 390: 1829. [DOI] [PubMed] [Google Scholar]
- 38.Yen FS, Wei JCC, Liu JS, Hwu CM, Hsu CC (2023) Parental Income Level and Risk of Developing Type 2 Diabetes in Youth. JAMA Netw Open 6: e2345812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaskin DJ, Thorpe RJ Jr, McGinty EE, Bower K, Rohde C, et al. (2014) Disparities in diabetes: The nexus of race, poverty, and place. Am J Public Health 104: 2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolak M, Abraham G, Talen MR (2019) Mapping census tract clusters of type 2 diabetes in a primary care population. Prev Chronic Dis 16: E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmittdiel JA, Dyer WT, Marshall CJ, Bivins R (2018) Using Neighborhood-Level Census Data to Predict Diabetes Progression in Patients with Laboratory-Defined Prediabetes. Perm J 22: 18–096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention (2023) National Diabetes Statistics Report. [Google Scholar]
- 43.Dupre ME, Silberberg M, Willis JM, Feinglos MN (2015) Education, glucose control, and mortality risks among U.S. older adults with diabetes. Diabetes Res Clin Pract 107: 392–399. [DOI] [PubMed] [Google Scholar]
- 44.Mehta N, Stenholm S, Männistö S, Jousilahti P, Elo I (2020) Excess body weight, cigarette smoking, and type II diabetes incidence in the national FINRISK studies. Ann Epidemiol 42: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malayala SV, Raza A (2016) Health behavior and perceptions among African American women with metabolic syndrome. J Community Hosp Intern Med Perspect 6: 30559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braveman PA, Arkin E, Proctor D, Kauh T, Holm N (2022) Systemic and Structural Racism: Definitions, Examples, Health Damages, And Approaches to Dismantling. Health Aff (Millwood) 41: 171–178. [DOI] [PubMed] [Google Scholar]
- 47.Zang E, Lynch SM, Liu C, Lu N, Banas J (2022) Racial/Ethnic and Educational Disparities in the Impact of Diabetes on Population Health Among the U.S.-Born Population. J Gerontol B Psychol Sci Soc Sci 77: 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adu MD, Malabu UH, Malau-Aduli AEO, Malau-Aduli BS (2019) Enablers and barriers to effective diabetes self-management: A multi-national investigation. PLoS One 14: e0217771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zajacova A, Lawrence EM (2018) The Relationship between Education and Health: Reducing Disparities Through a Contextual Approach. Annu Rev Public Health 39: 273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu C, He L, Li Y, Yang A, Zhang K, et al. (2023) Diabetes risk among US adults with different socioeconomic status and behavioral lifestyles: evidence from the National Health and Nutrition Examination Survey. Front Public Health 11: 1197947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leahy S O’ Halloran AM, O’ Leary N, Healy M, McCormack M, et al. (2015) Prevalence and correlates of diagnosed and undiagnosed type 2 diabetes mellitus and pre-diabetes in older adults: Findings from the Irish Longitudinal Study on Ageing (TILDA). Diabetes Res Clin Pract 110: 241–249. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, et al. (2020) Association of Treatment with Hydroxychloroquine or Azithromycin with In-Hospital Mortality in Patients with COVID-19 in New York State. JAMA 323: 2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao YS, Tsai WC, Chiu LT, Kung PT (2023) Educational attainment affects the diagnostic time in type 2 diabetes mellitus and the mortality risk of those enrolled in the diabetes pay-for-performance program. Health Policy 138: 104917. [DOI] [PubMed] [Google Scholar]
- 54.Myerson R, Romley J, Chiou T, Peters AL, Goldman D (2019) The Affordable Care Act and Health Insurance Coverage Among People with Diagnosed and Undiagnosed Diabetes: Data from the National Health and Nutrition Examination Survey. Diabetes Care 42: e179–e180. [DOI] [PubMed] [Google Scholar]
- 55.NCT04980144 (2021) Diquafosol Ophthalmic Solution for Dry Eye Symptoms. [Google Scholar]
- 56.Min HK, Kim SH, Choi JH, Choi K, Kim HR, et al. (2021) Impacts of statin and metformin on neuropathy in patients with type 2 diabetes mellitus: Korean Health Insurance data. World J Clin Cases 9: 10198– 10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angier H, Ezekiel-Herrera D, Marino M, Hoopes M, Jacobs EA, et al. (2019) Racial/Ethnic Disparities in Health Insurance and Differences in Visit Type for a Population of Patients with Diabetes after Medicaid Expansion. J Health Care Poor Underserved 30: 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ (2019) Evaluation of the Cascade of Diabetes Care in the United States, 2005–2016. JAMA Intern Med 179: 1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dall TM, Yang W, Gillespie K, Mocarski M, Byrne E, et al. (2019) The economic burden of elevated blood glucose levels in 2017: Diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 42: 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hildebrand JA, Billimek J, Lee JA, Sorkin DH, Olshansky EF, et al. (2020) Effect of diabetes self-management education on glycemic control in Latino adults with type 2 diabetes: A systematic review and meta-analysis. Patient Educ Couns 103: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamat S, Gousse Y, Muzumdar J, Gu A (2019) Trends and Disparities in Quality of Diabetes Care in the US: The National Health and Nutrition Examination Survey, 1999–2016. Innov Pharm 10: 10.24926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis J, Fischl AH, Beck J, Browning L, Carter A, et al. (2022) 2022 National Standards for Diabetes Self-Management Education and Support. Sci Diabetes Self Manag Care 48: 44–59. [DOI] [PubMed] [Google Scholar]
- 63.Thorsen M, McGarvey R, Thorsen A (2020) Diabetes management at community health centers: Examining associations with patient and regional characteristics, efficiency, and staffing patterns. Soc Sci Med 255: 113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terrie YC (2023) Healthcare Disparities in Diabetes Care. U.S. Pharm 48. [Google Scholar]
- 65.Mizokami-Stout K, Choi H, Richardson CR, Piatt G, Heisler M (2021) Diabetes distress and glycemic control in type 2 diabetes: mediator and moderator analysis of a peer support intervention. JMIR Diabetes 6: e21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klonoff DC, Kerr D, Clark L (2023) Barriers and Solutions to Trends and Disparities in Glycemic Control and Severe Hyperglycemia Among US Adults With Diabetes Using Insulin From 1988 to 2020. J Diabetes Sci Technol 17: 866–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foss R, Fischer K, Lampman MA, Laabs S, Halasy M, et al. (2023) Disparities in diabetes care: Differences between rural and urban patients within a large health system. Ann Fam Med 21: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Canedo JR, Miller ST, Schlundt D, Fadden MK, Sanderson M (2018) Racial/Ethnic Disparities in Diabetes Quality of Care: The Role of Healthcare Access and Socioeconomic Status. J Racial Ethn Health Disparities 5: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strasser R, Kam SM, Regalado SM (2016) Rural Health Care Access and Policy in Developing Countries. Ann Rev Public Health 37: 395– 412. [DOI] [PubMed] [Google Scholar]
- 70.Lu JB, Danko KJ, Elfassy MD, Welch V, Grimshaw JM, et al. (2018) Do quality improvement initiatives for diabetes care address social inequities? Secondary analysis of a systematic review. BMJ Open 8: e018826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hill-Briggs F, Ephraim PL, Vrany EA, Davidson KW, Pekmezaris R, et al. (2022) Social Determinants of Health, Race, and Diabetes Population Health Improvement: Black/African Americans as a Population Exemplar. Curr Diab Rep 22: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heisler M, Simmons D, Piatt GA (2021) Update on approaches to improve delivery and quality of care for people with diabetes. Endocrinol Metab Clin North Am 50: e1–e20. [DOI] [PubMed] [Google Scholar]
- 73.Prestes M, Gayarre MA, Elgart JF, Gonzalez L, Rucci E, et al. (2017) Multistrategic approach to improve quality of care of people with diabetes at the primary care level: Study design and baseline data. Prim Care Diabetes 11: 193–200. [DOI] [PubMed] [Google Scholar]
- 74.Greenlee MC, Bolen S, Chong W, Dokun A, Gonzalvo J, et al. (2023) The National Clinical Care Commission Report to Congress: Leveraging Federal Policies and Programs to Improve Diabetes Treatment and Reduce Complications. Diabetes Care 46: e51–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cloete L (2022) Diabetes mellitus: an overview of the types, symptoms, complications and management. Nurs Stand 37: 61–66. [DOI] [PubMed] [Google Scholar]
- 76.Yedjou CG, Grigsby J, Mbemi A, Nelson D, Mildort B, et al. (2023) The Management of Diabetes Mellitus Using Medicinal Plants and Vitamins. Int J Mol Sci 24: 9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shang A, Cao SY, Xu XY, Gan RY, Tang GY, et al. (2019) Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 8: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bortolotti M, Mercatelli D, Polito L (2019) Momordica charantia, a nutraceutical approach for inflammatory related diseases. Front Pharmacol 10: 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amos A, Khiatah B (2022) Mechanisms of Action of Nutritionally Rich Hibiscus sabdariffa’s Therapeutic Uses in Major Common Chronic Diseases: A Literature Review. J Am Nutr Assoc 41: 116–124. [DOI] [PubMed] [Google Scholar]
- 80.Lechner JF, Stoner GD (2019) Gingers and their purified components as cancer chemopreventative agents. Molecules 24: 2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Ploeg HP, Bull FC (2020) Invest in physical activity to protect and promote health: the 2020 WHO guidelines on physical activity and sedentary behaviour. Int J Behav Nutr Phys Act 17: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, et al. (2020) World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 54: 1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reardon CL, Hainline B, Aron CM, Baron D, Baum AL, et al. (2019) Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med 53: 667–699. [DOI] [PubMed] [Google Scholar]
- 84.Hwang SY, Jang JH, Park JE (2022) Association between Healthy Lifestyle (Diet Quality, Physical Activity, Normal Body Weight) and Periodontal Diseases in Korean Adults. Int J Environ Res Public Health 19: 3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ding D, Lawson KD, Kolbe-Alexander TL, Finkelstein EA, Katzmarzyk PT, et al. (2016) The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet 388: 1311– 1324. [DOI] [PubMed] [Google Scholar]
- 86.Maniaci G, La Cascia C, Giammanco A, Ferraro L, Palummo A, et al. (2023) The impact of healthy lifestyles on academic achievement among Italian adolescents. Curr Psychol 42: 5055–5061. [Google Scholar]
- 87.Neuhouser ML (2019) The importance of healthy dietary patterns in chronic disease prevention. Nutrition Research 70: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nagalievska M, Sabadashka M, Hachkova H, Sybirna N (2018) Galega officinalis extract regulate the diabetes mellitus related violations of proliferation, functions and apoptosis of leukocytes. BMC Complement Altern Med 18: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hachkova H, Nagalievska M, Soliljak Z, Kanyuka O, Kucharska AZ, et al. (2021) Medicinal plants Galega officinalis l. And yacon leaves as potential sources of antidiabetic drugs. Antioxidants (Basel) 10: 1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bindu J, Narendhirakannan RT (2019) Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmad F, Joshi SH (2023) Self-Care Practices and Their Role in the Control of Diabetes: A Narrative Review. Cureus 15: e41409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bode B, King A, Russell-Jones D, Billings LK (2021) Leveraging advances in diabetes technologies in primary care: a narrative review. Ann Med 53: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Asif M (2014) The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, et al. (2020) Diet and healthy lifestyle in the management of gestational diabetes mellitus. Nutrients 12: 3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller V, Mente A, Dehghan M, Rangarajan S, Zhang X, et al. (2017) Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet 390: 2037–2049. [DOI] [PubMed] [Google Scholar]
- 96.Güleryüz C, Eker E, Küçükali GK, Şakar M, Genç FN, et al. (2023) Unfavorable Effects of Low-carbonhydrate Diet in a Pediatric Patient with Type 1 Diabetes Mellitus. J Clin Res Pediatr Endocrinol 15: 444– 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hemmingsen B, Gimenez-Perez G, Mauricio D, Figuls MRI, Metzendorf MI, et al. (2017) Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev 12: CD003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Merlotti C, Morabito A, Ceriani V, Pontiroli AE (2014) Prevention of type 2 diabetes in obese at-risk subjects: a systematic review and meta-analysis. Acta Diabetol 51: 853–863. [DOI] [PubMed] [Google Scholar]
- 99.Schwingshackl L, Hoffmann G, Kalle-Uhlmann T, Arregui M, Buijsse B, et al. (2015) Fruit and vegetable consumption and changes in anthropometric variables in adult populations: A systematic review and meta-analysis of prospective cohort studies. PLoS One 10: e0140846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith JD, Hou T, Ludwig DS, Rimm EB, Willett W, et al. (2015) Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: Results from 3 prospective cohorts. Am J Clin Nutr 101: 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.AlEssa HB, Bhupathiraju SN, Malik VS, Wedick NM, Campos H, et al. (2015) Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am J Clin Nutr 102: 1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, et al. (2016) Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 39: 2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, et al. (2022) Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine Med Sci Sports Exerc 54: 353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kirwan JP, Sacks J, Nieuwoudt S (2017) The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med 84: S15–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Francesconi C, Niebauer J, Haber P, Moser O, Weitgasser R, et al. (2023) Lifestyle: physical activity and training as prevention and therapy of type 2 diabetes mellitus (Update 2023). Wien Klin Wochenschr 135: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Francois ME, Little JP (2015) Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr 28: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hamasaki H (2018) Interval Exercise Therapy for Type 2 Diabetes. Curr Diabetes Rev 14: 129–137. [DOI] [PubMed] [Google Scholar]
- 108.Zugravu C, Petra A, Pietroșel V-A, Mihai B-M, Mihai D-A, et al. (2023) Nutritional Interventions and Lifestyle Changing in Gestational Diabetes Mellitus Prevention: A Narrative Review. Sustainability 15: 1069. [Google Scholar]
- 109.Yun JS, Ko SH (2021) Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism 123: 154838. [DOI] [PubMed] [Google Scholar]
- 110.Charokopou M, Sabater FJ, Townsend R, Roudaut M, McEwan P, et al. (2016) Methods applied in cost-effectiveness models for treatment strategies in type 2 diabetes mellitus and their use in Health Technology Assessments: a systematic review of the literature from 2008 to 2013. Curr Med Res Opin 32: 207–218. [DOI] [PubMed] [Google Scholar]
- 111.Sampath Kumar A, Maiya AG, Shastry BA, Vaishali K, Ravishankar N, et al. (2019) Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann Phys Rehabil Med 62: 98–103. [DOI] [PubMed] [Google Scholar]
- 112.Hejazi K, Mohammad Rahimi GR, Rosenkranz SK (2023) Effects of Exercise Training on Inflammatory and Cardiometabolic Risk Biomarkers in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol Res Nurs 25: 250–266. [DOI] [PubMed] [Google Scholar]
- 113.Doulatyari PK, Ghahramani M, Mozaffari K (2023) Investigating the Effect of Aerobic and Resistance Training on Insulin Resistance and Some Cardiovascular Disease Risk Factors in Type 2 Diabetes Mellitus Patients: A Systematic Review. J Clin Res Paramed Sci 12: e134510. [Google Scholar]
- 114.Dhali B, Chatterjee S, Das SS, Cruz MD (2023) Effect of Yoga and Walking on Glycemic Control for the Management of Type 2 Diabetes: A Systematic Review and Meta-analysis. J ASEAN Fed Endocr Soc 38: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ (2016) Update on the treatment of type 2 diabetes mellitus. World J Diabetes 7: 354–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pathak V, Pathak NM, O’Neill CL, Guduric-Fuchs J, Medina RJ (2019) Therapies for Type 1 Diabetes: Current Scenario and Future Perspectives. Clin Med Insights Endocrinol Diabetes 12: 1179551419844521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kochar IS, Jain R (2021) Pancreas transplant in type 1 diabetes mellitus: The emerging role of islet cell transplant. Ann Pediatr Endocrinol Metab 26: 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.GBD 2015 Risk Factors Collaborators (2016) Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang J, Zheng Y, Gou X, Pu K, Chen Z, et al. (2020) Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int J Infect Dis 94: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Policardo L, Seghieri G, Anichini R, De Bellis A, Franconi F, et al. (2015) Effect of diabetes on hospitalization for ischemic stroke and related in-hospital mortality: A study in Tuscany, Italy, over years 2004– 2011. Diabetes Metab Res Rev 31: 280–286. [DOI] [PubMed] [Google Scholar]
- 121.Tan E, Khoo J, Gani LU, Malakar RD, Tay TL, et al. (2019) Effect of multidisciplinary intensive targeted care in improving diabetes mellitus outcomes: A randomized controlled pilot study - The Integrated Diabetes Education, Awareness and Lifestyle modification in Singapore (IDEALS) Program. Trials 20: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chawla SPS, Kaur S, Bharti A, Garg R, Kauret M, et al. (2019) Impact of health education on knowledge, attitude, practices and glycemic control in type 2 diabetes mellitus. J Family Med Prim Care 8: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jaiswal K, Moghe N, Mehta MC, Khaladkar K, Vaishnao L (2019) Knowledge, attitude & practices of type II diabetes mellitus patients in a tertiary care teaching institute of central India. J Diabetes Metab Disord Control 6: 1–4. [Google Scholar]
- 124.Waheedi M, Awad A, Hatoum HT, Enlund H (2017) The relationship between patients’ knowledge of diabetes therapeutic goals and self-management behaviour, including adherence. Int J Clin Pharm 39: 45–51. [DOI] [PubMed] [Google Scholar]
- 125.Chavan GM, Waghachavare VB, Gore AD, Chavan VM, Dhobale RV, et al. (2015) Knowledge about diabetes and relationship between compliance to the management among the diabetic patients from Rural Area of Sangli District, Maharashtra, India. J Family Med Prim Care 4: 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Turdu G, Gao H, Jiang Y, Kabas M (2018) Plant dipeptidyl peptidase-IV inhibitors as antidiabetic agents: A brief review. Future Med Chem 10: 1229–1239. [DOI] [PubMed] [Google Scholar]
- 127.NCT (2021) Role of Synchronized Lifestyle Modification Program in Insulin Dependent Diabetic Peripheral Neuropathy Patients. [Google Scholar]
- 128.Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, et al. (2019) Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25-year period 1989–2013: a multicentre prospective registration study. Diabetologia 62: 408–417. [DOI] [PubMed] [Google Scholar]
- 129.Wang J, Zhang X, Lan H, Wang W (2017) Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): A meta-analysis of randomized controlled trials. Food Nutr Res 61: 1377571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dwyer-Lindgren L, Mackenbach JP, Van Lenthe FJ, Flaxman AD, Mokdad AH (2016) Diagnosed and undiagnosed diabetes prevalence by County in the U.S., 1999–2012. Diabetes Care 39: 1556–1562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the present review article are included in the article.


