Abstract
Background and Objectives
An elevated platelet count may reflect neoplastic and inflammatory states, with cytokine‐driven overproduction of platelets. The objective of this study was to evaluate the prognostic utility of high platelet count among patients undergoing curative‐intent liver surgery for intrahepatic cholangiocarcinoma (ICC).
Methods
An international, multi‐institutional cohort was used to identify patients undergoing curative‐intent liver resection for ICC (2000–2020). A high platelet count was defined as platelets >300 *109/L. The relationship between preoperative platelet count, cancer‐specific survival (CSS), and overall survival (OS) was examined.
Results
Among 825 patients undergoing curative‐intent resection for ICC, 139 had a high platelet count, which correlated with multifocal disease, lymph nodes metastasis, poor to undifferentiated grade, and microvascular invasion. Patients with high platelet counts had worse 5‐year (35.8% vs. 46.7%, p = 0.009) CSS and OS (24.8% vs. 39.8%, p < 0.001), relative to patients with a low platelet count. After controlling for relevant clinicopathologic factors, high platelet count remained an adverse independent predictor of CSS (HR = 1.46, 95% CI 1.02–2.09) and OS (HR = 1.59, 95% CI 1.14–2.22).
Conclusions
High platelet count was associated with worse tumor characteristics and poor long‐term CSS and OS. Platelet count represents a readily‐available laboratory value that may preoperatively improve risk‐stratification of patients undergoing curative‐intent liver resection for ICC.
Keywords: cancer‐specific survival, intrahepatic cholangiocarcinoma, overall survival, platelet count, prognosis, thrombocytosis
Acronyms
- AJCC
American Joint Committee on Cancer
- ASA
American Society of Anesthesiologists
- bFGF
basic Fibroblast Growth Factor
- CA19‐9
Carbohydrate Antigen 19‐9
- CI
Confidence Interval
- CSS
Cancer‐Specific Survival
- EGF
Epidermal Growth Factor
- ICC
Intrahepatic Cholangiocarcinoma
- IG
intraductal growth
- IL
interleukin
- IQR
Interquartile range
- MS
Mass forming
- OS
Overall Survival
- PI
periductal infiltrating
- VGEF
Vascular Endothelial Growth Factor
1. INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer accounting for approximately 15% of all primary liver malignancies. The incidence of ICC has been rising worldwide over the past three decades, with as many as 1.18 cases per 100.000 people diagnosed annually in the United States. 1 , 2 According to the National Comprehensive Cancer Network guidelines, curative‐intent resection is the mainstay of treatment for resectable, non‐metastatic ICC. 3 Even after resection, however, prognosis of patients with ICC remains unsatisfactory, with median overall survival (OS) ranging from 15 to 31 months. 4 , 5 In addition, three out of four patients with ICC will deveZlop recurrence within 2 years following resection. 6 , 7 As such, identification of prognostic factors for accurate risk stratification is important to determine which patients are at risk for adverse outcomes when offered surgery. 8
To date, few prognostic models have been developed for patients with ICC; most rely on pathologic data and are, therefore, not useful in the preoperative setting. 9 , 10 Preoperatively, information that can be clinically useful includes radiologic tumor characteristics (i.e., size, number of tumors, proximity/involvement of vasculature), tumor‐specific biomarkers (i.e., carbohydrate antigen (CA)19‐9), as well as markers of liver function. 10 , 11 Recently, number of platelets has been proposed as a marker of both liver function and inflammation, as well as a possible risk factor for recurrence among patients with certain malignancies. 12 , 13 Indeed, cancer cells release inflammatory cytokines that enhance the production of megakaryocytes in the bone marrow and platelet accumulation in the peripheral circulation. 12 In turn, platelets secrete several angiogenic factors and cytokines, including Vascular Endothelial Growth Factor (VEGF), Epidermal Growth Factor (EGF), basic Fibroblast Growth Factor (bFGF) and interleukin (IL)−8, which promote inflammatory processes and contribute to tumor cell survival, dissemination and metastasis. 8 , 14 To date, little is known regarding the prognostic implications of preoperative platelet count among individuals undergoing resection for ICC. Therefore, the objective of the current study was to assess the association of preoperative platelet count with overall (OS) and cancer‐specific survival (CSS) among patients undergoing curative‐intent resection of ICC.
2. MATERIALS AND METHODS
2.1. Study population and inclusion criteria
A multi‐institutional database incorporating data from 15 tertiary institutions (International Intrahepatic Cholangiocarcinoma Study Group) was utilized to identify patients undergoing curative‐intent liver resection for ICC between 2000 and 2020. 7 , 11 , 15 , 16 Patients were excluded if they (i) did not undergo curative‐intent resection, (ii) had missing preoperative platelet count data, (iii) had residual disease after resection (macroscopically positive [R2]), (iv) had missing follow‐up data or (v) died within 30 days after resection. The Institutional Review Board of all participating institutions approved this study.
2.2. Variables and outcomes of interest
Demographic and clinicopathologic variables of interest included age, sex, American Society of Anesthesiologists (ASA) classification, history of cirrhosis, tumor location (i.e., uni‐ or multi‐focal), preoperative CA19‐9 levels, platelet count, tumor size, T‐ and N‐stage, margin status, morphology (MF: mass forming, IG: intraductal growth, PI: periductal infiltrating), histological grade, microvascular invasion, major resection and receipt of neoadjuvant and adjuvant chemotherapy.
The primary independent variable was preoperative platelet count. Patients were categorized as having high preoperative count if the number of platelets equaled or exceeded 300*109/L, as previously reported. 17 , 18 , 19 , 20 The American Joint Committee on Cancer (AJCC) 8th edition staging manual was used for T‐stage and N‐category classification. 9 Major liver resection was defined as the resection of three or more Couinaud segments. 21 Microvascular invasion was defined as intra‐parenchymal vascular involvement identified on histological examination. Margin status was defined as R0 for microscopically negative and R1 for microscopically positive resection margin.
The primary outcomes of interest were CSS and OS. CSS was defined as the time interval between resection and death from disease. Patients who died from other causes or were alive at last follow‐up were censored. OS was defined as the time interval between the date of surgery and the date of death from any cause or last follow up.
2.3. Statistical analysis
Continuous variables were presented as median [interquartile range (IQR)] and categorical variables were reported as frequencies (proportion, %). Continuous variables were compared using the Wilcoxon rank sum test. Categorical variables were compared using the Chi‐square test. Differences in OS and CSS were assessed with the Kaplan‐Meier method and the log‐rank test. Cox proportional hazards models were used to assess the association of clinicopathologic variables with CSS and OS. Variables significantly associated with outcomes in univariable analysis (p < 0.05) were included in the multivariable Cox regression models. All tests were two‐sided, and a p‐value < 0.05 was considered statistically significant. All statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Baseline characteristics of patient cohort
A total of 825 patients undergoing curative‐intent resection for ICC met inclusion criteria and were included in the final cohort (Table 1). Median patient age was 59.5 years (IQR: 51.0–68.0). Most patients were male (n = 472, 57.3%) and had an ASA class 2 (n = 542, 70.7%). On final pathology, 568 (77.0%) patients had T2 or more advanced T disease, while approximately one in five patients (n = 181, 21.9%) had lymph node metastasis (N1). The vast majority of patients had negative resection margins (R0 resection) after resection (n = 713, 87.2%) and 244 (30.6%) of patients received adjuvant chemotherapy.
Table 1.
Clinicopathologic characteristics of the study cohort.
| Variables | Overall (N = 825a) | Low/normal platelet count (N = 686a) | High platelet count (N = 139a) | p‐valueb |
|---|---|---|---|---|
| Age (years) | 59.5 (51.0, 68.0) | 59.1 (50.0, 68.0) | 60.0 (52.2, 69.0) | 0.400 |
| Sex | <0.001 | |||
| Female | 352 (42.7%) | 270 (39.4%) | 82 (59.0%) | |
| Male | 472 (57.3%) | 415 (60.6%) | 57 (41.0%) | |
| Race | <0.001 | |||
| Nonwhite | 453 (56.8%) | 396 (59.9%) | 57 (41.9%) | |
| White | 344 (43.2%) | 265 (40.1%) | 79 (58.1%) | |
| ASA class | <0.001 | |||
| ≤2 | 542 (70.7%) | 469 (73.1%) | 73 (58.4%) | |
| >2 | 225 (29.3%) | 173 (26.9%) | 52 (41.6%) | |
| History of cirrhosis | 89 (11.6%) | 86 (13.4%) | 3 (2.4%) | <0.001 |
| Location | <0.001 | |||
| Unifocal | 685 (83.6%) | 583 (85.7%) | 102 (73.4%) | |
| Multifocal | 134 (16.4%) | 97 (14.3%) | 37 (26.6%) | |
| CA 19‐9 | 0.200 | |||
| <200 | 499 (75.5%) | 430 (76.4%) | 69 (70.4%) | |
| ≥200 | 162 (24.5%) | 133 (23.6%) | 29 (29.6%) | |
| Tumor size (cm) | 6.0 (4.0, 8.3) | 6.0 (4.0, 8.3) | 6.0 (4.0, 8.4) | >0.900 |
| AJCC 8 th edition T stage | 0.057 | |||
| T1a/T1b | 170 (23.0%) | 151 (24.3%) | 19 (16.2%) | |
| T2/T3/T4 | 568 (77.0%) | 470 (75.7%) | 98 (83.8%) | |
| AJCC 8 th edition N stage | 0.006 | |||
| N0 | 308 (37.3%) | 248 (36.2%) | 60 (43.2%) | |
| N1 | 181 (21.9%) | 142 (20.7%) | 39 (28.1%) | |
| Nx | 336 (40.7%) | 296 (43.1%) | 40 (28.8%) | |
| Margin status | 0.900 | |||
| R0 | 713 (87.2%) | 593 (87.1%) | 120 (87.6%) | |
| R1 | 105 (12.8%) | 88 (12.9%) | 17 (12.4%) | |
| Morphology | <0.001 | |||
| MF, IG | 680 (87.5%) | 583 (89.4%) | 97 (77.6%) | |
| PI, MF + PI | 97 (12.5%) | 69 (10.6%) | 28 (22.4%) | |
| Histological grade | <0.001 | |||
| Well to moderate | 644 (82.2%) | 553 (84.7%) | 91 (70.0%) | |
| Poor to undifferentiated | 139 (17.8%) | 100 (15.3%) | 39 (30.0%) | |
| Major resection | 472 (57.3%) | 368 (53.7%) | 104 (74.8%) | <0.001 |
| Microvascular invasion | 279 (34.2%) | 213 (31.3%) | 66 (48.5%) | <0.001 |
| Neoadjuvant chemotherapy | 73 (8.8%) | 64 (9.3%) | 9 (6.5%) | 0.300 |
| Adjuvant chemotherapy | 244 (30.6%) | 185 (27.9%) | 59 (44.0%) | <0.001 |
Median (IQR); n (%).
Wilcoxon rank sum test; Pearson's Chi‐squared test.
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; CA, Carbohydrate antigen; IG, Intraductal growth; IQR, interquartile range; MF, Mass‐forming; PI, Periductal infiltrating.
3.2. Association of preoperative platelet count with clinicopathologic characteristics
Among the overall cohort, median preoperative platelet count was 213.0 (IQR: 167.0–269.0). A total of 139 patients (16.8%) had high preoperative platelet count, whereas 686 individuals (83.2%) had a normal/low platelet count. Patients with a high platelet count were more likely to have multifocal disease (n = 37, 26.6% vs. n = 97, 14.3%; p < 0.001), N1 lymph node status (n = 39, 28.1% vs. n = 142, 20.7%; p = 0.006) and poor/undifferentiated disease (n = 39, 30.0% vs. n = 100, 15.3%; p < 0.001) (Figure 1A). Additionally, patients with high platelet count more frequently had PI or PI + MF ICC morphologic type (n = 28, 22.4% vs. n = 69, 10.6%; p < 0.001) and microvascular invasion (n = 66, 48.5% vs. n = 213, 31.3%; p < 0.001) (Figure 1B–C), while being less likely to have cirrhosis (n = 86, 13.4% vs. n = 3, 2.4%; p < 0.001) compared with individuals who had a normal/low platelet count (Table 1). Receipt of adjuvant chemotherapy (n = 59, 44.0% vs. n = 185, 27.9%) and major resection (n = 104, 74.8% vs. n = 368, 53.7%) were more frequent in the high platelet count group (both p < 0.05). There was no difference between the two groups regarding CA19‐9 levels, tumor size, T category, incidence of R0 resection and receipt of neoadjuvant chemotherapy (all p > 0.05).
Figure 1.
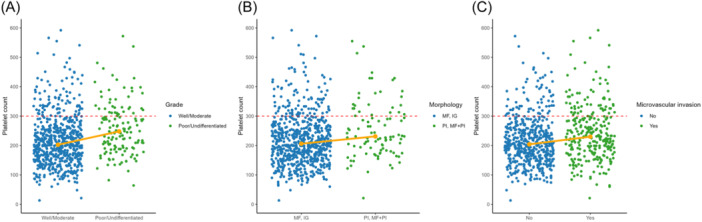
Platelet values according to various clinicopathologic characteristics (MF, Mass‐forming; IG, Intraductal growth; PI, periductal infiltrating).
3.3. Impact of high versus normal/low platelet count on CSS and OS
After a median follow‐up of 20.9 months (IQR 11.1–39.6), 5‐year OS and CSS in the entire cohort was 36.9% and 44.7%, respectively. Of note, patients with high platelet count had a worse 1‐, 3‐ and 5‐year CSS than patients with normal/low platelet levels (1‐year CSS: 78.5% vs. 87.4%; 3‐year CSS: 51.9% vs. 60.3%, 5‐year CSS: 35.8% vs. 46.7%) (p = 0.009; Figure 2A). Similarly, patients with high platelet count had a worse 1‐, 3‐ and 5‐year OS than patients with normal/low platelet levels (1‐year OS: 71.8% vs. 84.4%; 3‐year OS: 42.5% vs. 54.8%, 5‐year OS: 24.8% vs. 39.8%) (p ≤ 0.001, Figure 2B). On multivariable analysis, after adjusting for relevant clinicopathologic factors, high platelet count remained independently associated with 46% higher hazards of cancer‐specific death (HR = 1.46, 95% CI 1.02–2.09) and 59% higher hazards of all‐cause mortality (HR = 1.59, 95% CI 1.14–2.22) following ICC resection (Tables 2, 3 ).
Figure 2.
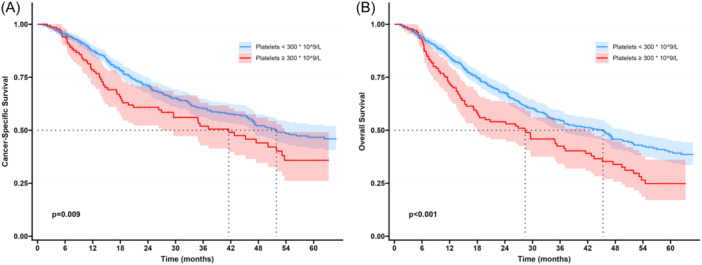
Kaplan Meier curves demonstrating differences in cancer‐specific survival (CSS) and overall survival (OS) among patients with platelet count 300*109/L versus those with <300*109/L.
Table 2.
Cox regression analysis of factors associated with cancer‐specific survival.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | HRa | 95% CIa | p‐value | HRa | 95% CIa | p‐value |
| Age > 60 y (ref: ≤ 60) | 0.79 | 0.63, 1.00 | 0.050 | |||
| Male (ref: Female) | 1.24 | 0.98, 1.56 | 0.071 | |||
| ASA class >2 (ref: ≤ 2) | 1.11 | 0.86, 1.43 | 0.400 | |||
| History of Cirrhosis | 1.05 | 0.74, 1.49 | 0.800 | |||
| CA19‐9 > 200 (ref: ≤ 200) | 2.30 | 1.77, 2.99 | <0.001 | 2.01 | 1.52, 2.66 | <0.001 |
| AJCC 8th edition T stage | ||||||
| T1a/T1b | ref | ref | ref | ref | ||
| T2/T3/T4 | 2.88 | 2.01, 4.13 | <0.001 | 2.79 | 1.84, 4.23 | <0.001 |
| AJCC 8th edition N stage | ||||||
| N0 | ref | ref | ref | ref | ||
| N1 | 1.81 | 1.40, 2.34 | <0.001 | 1.38 | 1.01, 1.88 | 0.046 |
| Nx | 0.91 | 0.63, 1.30 | 0.600 | 0.91 | 0.56, 1.50 | 0.700 |
| Margin status R1 (ref: R0) | 1.23 | 0.86, 1.75 | 0.300 | |||
| Morphologic type | ||||||
| MF, IG | ref | ref | ||||
| PI, MF + PI | 1.06 | 0.74, 1.51 | 0.800 | |||
| Histological grade | ||||||
| Well/moderate | ref | ref | ref | ref | ||
| Poor/undifferentiated | 1.47 | 1.11, 1.95 | 0.008 | 1.33 | 0.96, 1.85 | 0.091 |
| Major resection (ref: Minor) | 1.14 | 0.91, 1.43 | 0.200 | |||
| Microvascular invasion | 1.18 | 0.93, 1.43 | 0.200 | |||
| Neoadjuvant chemotherapy | 1.00 | 0.66, 1.54 | >0.900 | |||
| Adjuvant chemotherapy | 1.17 | 0.92, 1.49 | 0.200 | |||
| Platelets (*10^9/L) ≥ 300 (ref: < 300) | 1.45 | 1.10, 1.91 | 0.009 | 1.46 | 1.02, 2.09 | 0.041 |
Note: Bold values represent statistical significance.
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; CA, Carbohydrate antigen; IG, Intraductal growth; IQR, interquartile range; MF, Mass‐forming; PI, Periductal infiltrating.
HR = Hazard Ratio, CI = Confidence Interval.
Table 3.
Cox regression analysis of factors associated with overall survival.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | HR b | 95% CI b | p‐value | HR b | 95% CI b | p‐value |
| Age > 60 y (ref: ≤ 60) | 1.00 | 0.82, 1.23 | >0.900 | |||
| Male (ref: Female) | 1.14 | 0.92, 1.40 | 0.200 | |||
| ASA class >2 (ref: ≤ 2) | 1.21 | 0.97, 1.51 | 0.094 | |||
| History of Cirrhosis | 0.92 | 0.66, 1.27 | 0.600 | |||
| CA19‐9 > 200 (ref: ≤ 200) | 2.24 | 1.76, 2.85 | <0.001 | 2.14 | 1.65, 2.78 | <0.001 |
| AJCC 8th edition T stage | ||||||
| T1a/T1b | ref | ref | ref | ref | ||
| T2/T3/T4 | 2.70 | 1.94, 3.75 | <0.001 | 2.58 | 1.75, 3.78 | <0.001 |
| AJCC 8th edition N stage | ||||||
| N0 | ref | ref | ref | ref | ||
| N1 | 2.27 | 1.82, 2.85 | <0.001 | 1.38 | 1.02, 1.85 | 0.035 |
| Nx | 0.99 | 0.71, 1.37 | >0.900 | 1.08 | 0.70, 1.67 | 0.700 |
| Margin status R1 (ref: R0) | 1.72 | 1.29, 2.28 | <0.001 | 1.47 | 1.02, 2.11 | 0.040 |
| Morphologic type | ||||||
| MF, IG | red | ref | ||||
| PI, MF + PI | 1.29 | 0.95, 1.74 | 0.100 | |||
| Histological grade | ||||||
| Well/moderate | ref | ref | ref | ref | ||
| Poor/undifferentiated | 1.57 | 1.22, 2.01 | <0.001 | 1.28 | 0.94, 1.76 | 0.120 |
| Major resection (ref: Minor) a | 1.34 | 1.09, 1.65 | 0.005 | |||
| Microvascular invasiona | 1.46 | 1.19, 1.80 | <0.001 | |||
| Neoadjuvant chemotherapy | 1.07 | 0.74, 1.56 | 0.700 | |||
| Adjuvant chemotherapy | 1.25 | 1.00, 1.55 | 0.050 | |||
| Platelets (*10^9/L) ≥ 300 (ref: < 300) | 1.58 | 1.24, 2.01 | <0.001 | 1.59 | 1.14, 2.22 | 0.006 |
Note: Bold values represent statistical significance.
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; CA, Carbohydrate antigen; IG, Intraductal growth; IQR, interquartile range; MF, Mass‐forming; PI, Periductal infiltrating.
Microvascular invasion and major resection were not included in the multivariable model to avoid multicollinearity with AJCC T stage.
HR = Hazard Ratio, CI = Confidence Interval.
4. DISCUSSION
ICC is a highly aggressive malignancy of the intrahepatic bile ducts associated with extremely poor prognosis. 1 , 22 Recent efforts have focused on identifying mechanisms of disease development and progression to help optimize prognostication and risk‐stratification among patients who are offered resection for ICC. 10 , 11 Platelets have been recognized as important drivers of tumorigenesis and tumor progression in a number of malignancies. Nevertheless, little is known regarding the prognostic utility of preoperative platelet count among patients undergoing resection for ICC. The current study was important because we investigated the prognostic utility of high platelet count, a routine preoperative laboratory marker, relative to OS and CSS among patients who underwent curative‐intent resection for ICC. Notably, a high preoperative platelet count was associated with adverse clinicopathologic characteristics. In turn, patients with a high preoperative platelet count had worse 5‐year OS and CSS compared with individuals with normal/low platelet count. Even after adjusting for all relevant clinicopathologic characteristics, high platelet count was independently associated with both cancer‐specific, as well as all‐cause mortality following ICC resection.
Produced by megakaryocytes, their precursor cells in the bone marrow, platelets mainly contribute to hemostasis and vascular integrity. In fact, platelets have been demonstrated to stabilize vascular growth during tumor development, and a recent study demonstrated that platelets prevent hemorrhaging within a tumor bed. 23 Mice models have further demonstrated that antibody‐induced thrombocytopenia was subsequently followed by erupted hemorrhage within tumors, limiting tumor cell proliferation and promoting apoptosis. 14 Apart from their role in hemostasis, platelets are activated by cytokines and growth factors; namely VEGF and IL‐6. IL‐6 is elevated in several malignancies, including gastrointestinal, renal, prostate, ovarian and lung cancer. 14 , 24 IL‐6 can be secreted by tumor cells, while concurrently acting as an autocrine growth factor. Furthermore, platelets may affect oncological outcomes by promoting metastatic spread of tumor cells, leading to shorter survival times. 25 , 26 One model proposed that malignant cells enter the blood stream, bind and activate platelets, which, in turn, coat and protect tumor cells from shear stress and immune surveillance, assisting tumor cell arrest at the vessel wall while contributing to extravasation. 14 , 25 , 27 , 28 In fact, studies have demonstrated that in the presence of natural killer (NK) cells, which normally target cancer cells in the circulation, platelet activation is vital for tumor cell survival. 29 , 30 Regarding tumor cell extravasation, platelets can either enhance vascular permeability via growth factors present in their α granules, or tumor‐platelet aggregates can directly embolize the local microvasculature. 25 , 27 , 31
Previous studies have examined the association of thrombocytosis and high platelet count with poor survival among patients with various malignancies. 13 , 32 , 33 , 34 , 35 In analyzing patients undergoing radical nephroureterectomy for upper tract urothelial carcinoma, Milojevic et al. 8 reported that preoperative thrombocytosis was associated with worse clinicopathologic characteristics and was an independent predictor of poor CSS (HR 2.48, 95% CI 1.14–4.31). In a different single‐institution study of 199 patients who underwent R0 resection for non‐small cell lung cancer, Kim et al. 36 noted that individuals with preoperative thrombocytosis had shorter OS and disease‐free survival. Multivariable analysis demonstrated that preoperative thrombocytosis was associated with increased risk of death (2.98, 95% CI 1.22–5.01). In yet another study, Sasaki et al. 37 analyzed a cohort of 636 patients with colorectal cancer undergoing surgical resection between 2002 and 2008. Patients with thrombocytosis had a worse 5‐year CSS versus individuals without thrombocytosis (62.9% vs. 89.9%, p < 0.001). Thrombocytosis was an independent adverse prognosticator of CSS on multivariable analysis (HR 2.96, 95% CI 1.72–5.00). Similarly, in the current study, after adjusting for relevant clinicopathologic factors, high platelet count was independently associated with 46% higher hazards of cancer‐specific death (HR = 1.46, 95% CI 1.02–2.09) and 59% higher hazards of all‐cause mortality (HR = 1.59, 95% CI 1.14–2.22) following ICC resection (Tables 2, 3).
In hepatic malignancies, the interpretation of platelet count is more complex as a cirrhotic liver background or compromised liver function may be related to thrombocytopenia. Liver cancer (frequently developed in the setting of cirrhosis) can produce thrombopoietin, stimulating platelet production and contributing to thrombocytosis. 12 Earlier studies examining the role of platelet count and hepatocellular carcinoma had mostly focused on the association of thrombocytosis with worse tumor characteristics and better liver function. 38 In 2020, Liu et al. 39 published a study of 4706 patients with hepatocellular carcinoma based on two cohorts, one in Taiwan and one in the United States. Individuals with thrombocytosis (≥300*109/L) had worse clinicopathologic characteristics compared with individuals with thrombocytopenia. Furthermore, thrombocytosis was associated with worse OS among all subgroups of different liver disease etiologies, even after adjusting for relevant factors (aHR 1.40, 95% CI 1.23–1.60). In the current study cohort of 825 patients, no differences were observed among patients with ICC who did or did not have thrombocytosis relative to age, CA19‐9 levels, tumor size, AJCC T Stage, resection margin and receipt of neoadjuvant chemotherapy. However, patients with a high platelet count did have some worse clinicopathologic characteristics including metastatic lymph node status, poor or undifferentiated histological grade, PI or PI + MF morphology and microvascular invasion. Collectively, the data suggest that high platelet counts may be correlated with adverse tumor biology.
To the best of our knowledge, the current study was the first study to assess the prognostic utility of preoperative high platelet count relative to long‐term outcomes among patients undergoing curative‐intent resection for ICC. Survival analysis demonstrated that a high platelet count was associated with lower 5‐year CSS compared with individuals who had low platelet levels (35.8% vs. 46.7%, p = 0.009). Patients with a high platelet count also had a 5‐year OS of 25.9%, which was inferior to patients with <300*109/L platelets (40.2%, p < 0.001). This association of high platelet count with CSS and OS may, in part, be attributed to the correlation of high platelet count with worse clinicopathologic characteristics. Aggressive tumor growth and endothelial damage activate platelets, which, in turn, release multiple factors and cytokines with inflammatory, proliferative and angiogenic activity. 14 , 24 This pathway can reinforce a vicious cycle of tumor growth increasing platelet counts, with high platelet count augmenting tumor progression. As such, assessment of the platelet count in the preoperative setting may be useful to stratify patients with ICC relative to anticipated CSS and OS following surgical resection.
The results of the current study should be interpreted in light of certain limitations. Due to the retrospective nature of the study, selection bias was possible. The use of a multi‐institutional database was a strength in terms of generalizability of the results; however, patient selection, surgical techniques and reporting of laboratory values may have varied according to local practices. The absence of a comparison group (i.e., patients receiving treatment other than surgery) limited the application of the prognostic utility of high platelet count only to patients with ICC undergoing curative‐intent liver resection.
5. CONCLUSIONS
In conclusion, high preoperative platelet count was associated with adverse clinicopathologic characteristics and worse OS and CSS among patients undergoing resection for ICC. Platelet count—a routine laboratory marker—can have important prognostic implications in the setting of ICC. The current data emphasize the importance of taking advantage of the ease and standardization of platelet count availability in the clinical setting. Platelet count should be considered when stratifying patients with ICC undergoing curative‐intent resection, as it provides a simple, preoperatively available prognostic tool, which simultaneously reflects liver function, tumor inflammatory status, as well as potential for neoplastic progression.
SYNOPSIS
High platelet count represents a routine, preoperatively available laboratory marker that reflects tumor inflammation and progression, and can have important long‐term prognostic implications in the setting of curative‐intent liver resection for intrahepatic cholangiocarcinoma.
Chatzipanagiotou OP, Tsilimigras DI, Catalano G, et al. Preoperative platelet count as an independent predictor of long‐term outcomes among patients undergoing resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2024;130:1042‐1050. 10.1002/jso.27806
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Singal AK, Vauthey J‐N, Grady JJ, Stroehlein JR. Intra‐hepatic cholangiocarcinoma—frequency and demographic patterns: thirty‐year data from the M.D. Anderson Cancer Center. J Cancer Res Clin Oncol. 2011;137(7):1071‐1078. [DOI] [PubMed] [Google Scholar]
- 3. Yang Z, Jiang X. Efficacy and safety comparison of neoadjuvant chemotherapy followed by surgery and upfront surgery for treating intrahepatic cholangiocarcinoma: a systematic review and meta‐analysis. BMC Gastroenterol. 2023;23(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma. JAMA Surg. 2014;149(6):565. [DOI] [PubMed] [Google Scholar]
- 5. De Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi‐institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140‐3145. [DOI] [PubMed] [Google Scholar]
- 6. Zhang XF, Beal EW, Bagante F, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg. 2018;105(7):848‐856. [DOI] [PubMed] [Google Scholar]
- 7. Tsilimigras DI, Mehta R, Aldrighetti L, et al. Development and validation of a laboratory risk score (LabScore) to predict outcomes after resection for intrahepatic cholangiocarcinoma. J Am Coll Surg. 2020;230(4):381‐391.e382. [DOI] [PubMed] [Google Scholar]
- 8. Milojevic B, Janicic A, Grozdic Milojevic I, et al. Prognostic impact of preoperative thrombocytosis on recurrence‐free survival in patients with upper tract urothelial carcinoma. Ann Surg Oncol. 2024;31(4):2538‐2544. [DOI] [PubMed] [Google Scholar]
- 9. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93‐99. [DOI] [PubMed] [Google Scholar]
- 10. Buettner S, Galjart B, Van Vugt JLA, et al. Performance of prognostic scores and staging systems in predicting long‐term survival outcomes after surgery for intrahepatic cholangiocarcinoma. J Surg Oncol. 2017;116(8):1085‐1095. [DOI] [PubMed] [Google Scholar]
- 11. Tsilimigras DI, Hyer JM, Moris D, et al. Prognostic utility of albumin‐bilirubin grade for short‐ and long‐term outcomes following hepatic resection for intrahepatic cholangiocarcinoma: a multi‐institutional analysis of 706 patients. J Surg Oncol. 2019;120(2):206‐213. [DOI] [PubMed] [Google Scholar]
- 12. Voutsadakis IA. Thrombocytosis as a prognostic marker in gastrointestinal cancers. World J Gastrointest Oncol. 2014;6(2):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chakra MA, Azoulai D, Moussa M, et al. The prognostic role of pre‐cystectomy thrombocytosis in invasive bladder cancer. Int Urol Nephrol. 2022;54(12):3153‐3161. [DOI] [PubMed] [Google Scholar]
- 14. Gay LJ, Felding‐Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsilimigras DI, Moris D, Mehta R, et al. The systemic immune‐inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi‐institutional analysis. HPB. 2020;22(12):1667‐1674. [DOI] [PubMed] [Google Scholar]
- 16. Tsilimigras DI, Mehta R, Moris D, et al. A machine‐based approach to preoperatively identify patients with the most and least benefit associated with resection for intrahepatic cholangiocarcinoma: an international multi‐institutional analysis of 1146 patients. Ann Surg Oncol. 2020;27(4):1110‐1119. [DOI] [PubMed] [Google Scholar]
- 17. Domínguez I, Crippa S, Thayer SP, et al. Preoperative platelet count and survival prognosis in resected pancreatic ductal adenocarcinoma. World J Surg. 2008;32(6):1051‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown KM, Domin C, Aranha GV, Yong S, Shoup M. Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas. Am J Surg. 2005;189(3):278‐282. [DOI] [PubMed] [Google Scholar]
- 19. Wang H, Gao J, Bai M, et al. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets. 2014;25(5):382‐387. [DOI] [PubMed] [Google Scholar]
- 20. Schwarz RE, Keny H. Preoperative platelet count predicts survival after resection of periampullary adenocarcinoma. Hepato‐gastroenterol. 2001;48(41):1493‐1498. [PubMed] [Google Scholar]
- 21. Pang YY, Strasberg SM. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333‐39. HPB. 2002;4(2):99‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu L, Tsilimigras DI, Paredes AZ, et al. Trends in the incidence, treatment and outcomes of patients with intrahepatic cholangiocarcinoma in the USA: facility type is associated with margin status, use of lymphadenectomy and overall survival. World J Surg. 2019;43(7):1777‐1787. [DOI] [PubMed] [Google Scholar]
- 23. Ho‐Tin‐Noé B, Carbo C, Demers M, Cifuni SM, Goerge T, Wagner DD. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am J Pathol. 2009;175(4):1699‐1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maass T, Thieringer FR, Mann A, et al. Liver specific overexpression of platelet‐derived growth factor‐B accelerates liver cancer development in chemically induced liver carcinogenesis. Int J Cancer. 2011;128(6):1259‐1268. [DOI] [PubMed] [Google Scholar]
- 25. Buergy D, Wenz F, Groden C, Brockmann MA. Tumor–platelet interaction in solid tumors. Int J Cancer. 2012;130(12):2747‐2760. [DOI] [PubMed] [Google Scholar]
- 26. Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease‐activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104(2):397‐401. [DOI] [PubMed] [Google Scholar]
- 27. Burdick MM, Konstantopoulos K. Platelet‐induced enhancement of LS174T colon carcinoma and THP‐1 monocytoid cell adhesion to vascular endothelium under flow. Am J Physiol Cell Physiol. 2004;287(2):C539‐C547. [DOI] [PubMed] [Google Scholar]
- 28. Jurasz P, Alonso‐Escolano D, Radomski MW. Platelet–cancer interactions: mechanisms and pharmacology of tumour cell‐induced platelet aggregation. Br J Pharmacol. 2004;143(7):819‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palumbo J. Mechanisms linking tumor cell–associated procoagulant function to tumor dissemination. Semin Thromb Hemost. 2008;34(02):154‐160. [DOI] [PubMed] [Google Scholar]
- 30. Kopp H‐G, Placke T, Salih HR. Platelet‐derived transforming growth factor‐β down‐regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69(19):7775‐7783. [DOI] [PubMed] [Google Scholar]
- 31. Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemostasis. 2009;7(11):1759‐1766. [DOI] [PubMed] [Google Scholar]
- 32. Jokisch J‐F, Grimm T, Buchner A, et al. Preoperative thrombocytosis in patients undergoing radical cystectomy for urothelial cancer of the bladder: an independent prognostic parameter for an impaired oncological outcome. Urol Int. 2020;104(1‐2):36‐41. [DOI] [PubMed] [Google Scholar]
- 33. Aoe K, Hiraki A, Ueoka H, et al. Thrombocytosis as a useful prognostic indicator in patients with lung cancer. Respiration. 2004;71(2):170‐173. [DOI] [PubMed] [Google Scholar]
- 34. Barber EL, Boggess JF, Van Le L, et al. Association of preoperative thrombocytosis and leukocytosis with postoperative morbidity and mortality among patients with ovarian cancer. Obstet Gynecol. 2015;126(6):1191‐1197. [DOI] [PubMed] [Google Scholar]
- 35. Shimada H, Oohira G, Okazumi S, et al. Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma1. J Am Coll Surg. 2004;198(5):737‐741. [DOI] [PubMed] [Google Scholar]
- 36. Kim M, Chang H, Yang HC, et al. Preoperative thrombocytosis is a significant unfavorable prognostic factor for patients with resectable non‐small cell lung cancer. World J Surg Oncol. 2014;12(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg. 2012;36(1):192‐200. [DOI] [PubMed] [Google Scholar]
- 38. Carr BI, Guerra V. Thrombocytosis and hepatocellular carcinoma. Dig Dis Sci. 2013;58(6):1790‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu PH, Hsu CY, Su CW, et al. Thrombocytosis is associated with worse survival in patients with hepatocellular carcinoma. Liver Int. 2020;40(10):2522‐2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


