Abstract
Burkholderia pseudomallei, the causative agent of melioidosis, is a gram-negative bacterium capable of causing either acute lethal sepsis or chronic but eventually fatal disease in infected individuals. However, despite the clinical importance of this infection in areas where it is endemic, there is essentially no information on the mechanisms of protective immunity to the bacterium. We describe here a murine model of either acute or chronic infection with B. pseudomallei in Taylor Outbred (TO) mice which mimics many features of the human pathology. Intraperitoneal infection of TO mice at doses of >106 CFU resulted in acute septic shock and death within 2 days. In contrast, at lower doses mice were able to clear the inoculum from the liver and spleen over a 3- to 4-week period, but persistence of the organism at other sites resulted in a chronic infection of between 2 and 16 months duration which was eventually lethal in all of the animals tested. Resistance to acute infection with B. pseudomallei was absolutely dependent upon the production of gamma interferon (IFN-γ) in vivo. Administration of neutralizing monoclonal antibody against IFN-γ lowered the 50% lethal dose from >5 × 105 to ca. 2 CFU and was associated with 8,500- and 4,400-fold increases in the bacterial burdens in the liver and spleen, respectively, together with extensive destruction of lymphoid architecture in the latter organ within 48 h. Neutralization of either tumor necrosis factor alpha or interleukin-12 but not granulocyte-macrophage colony-stimulating factor, also increased susceptibility to infection in vivo. Together, these results provide the first evidence of a host protective mechanism against B. pseudomallei. The rapid production of IFN-γ within the first day of infection determines whether the infection proceeds to an acute lethal outcome or becomes chronic.
Melioidosis is a life-threatening infectious disease caused by the gram-negative bacterium Burkholderia (formerly Pseudomonas) pseudomallei. The organism is endemic in Southeast Asia and Northern Australia and can be isolated from a variety of environmental sources, especially soil, ponds, and rice paddies (8). The disease may well be underreported in other areas of the tropics. Infection occurs in humans and other animals. Natural infection is thought to occur via either skin trauma or inhalation of contaminated aerosols (19). Melioidosis is a major cause of mortality in the Northeast of Thailand, where it accounts for approximately 40% of lethal, community-acquired septicemia (5). B. pseudomallei can cause infection in any organ, although pathology occurs mainly in the lungs, spleen, and liver (24, 36). A striking feature of melioidosis is the extremely varied spectrum of clinical presentation. In many cases, infection presents as an acute illness characterized by septic shock. In contrast, some individuals become latently infected with no obvious signs of disease. However, after impairment of immune defenses, such as is seen in diabetes mellitus, renal failure, and steroid treatment, the organism can be reactivated and cause acute lethal sepsis. In fact, relapse of asymptomatic infection has been reported over 20 years after initial exposure to the organism, suggesting an ability to evade immune defenses (18, 20). The ability of the bacterium to cause a long-term latent infection suggests that B. pseudomallei is capable of surviving in an intracellular environment. Results obtained with cell culture systems support this view, in that virulent B. pseudomallei is able to infect and grow within various cell lines as well as human polymorphonuclear leukocytes (PMNL) and monocytes (11, 16, 25). However, neither the site of latency, the mechanisms by which the organism avoids the bactericidal effects of the host immune response, nor the pathways of protective immunity against this bacterium have been identified.
In many other intracellular bacterial infections, the rapid production of proinflammatory and phagocyte-activating cytokines is a key determinant of resistance of the host. The production of gamma interferon (IFN-γ) is of particular importance in controlling the rate of bacterial growth, as shown by the extreme susceptibility to intracellular pathogens of mice depleted of IFN-γ by either gene deletion or neutralizing monoclonal antibody (MAb) (7, 23). However, in the context of gram-negative infection, IFN-γ has also been associated with the development of septic shock, suggesting that in the presence of lipopolysaccharide, IFN-γ can be either beneficial or detrimental depending on the bacterial burden and time of production (1, 4, 15). Other cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-12 (IL-12), and IL-6 may also confer protection or cause pathology, depending on the experimental system. The role of these cytokines in protective immunity against infection with B. pseudomallei has until now not been examined in an experimental model, although serum levels of TNF-α, IFN-γ, and soluble IL-2 receptors, as well as IL-6 and IL-8, are elevated and correlated with disease severity in patients with severe melioidosis, providing predictive markers of outcome (3, 13, 29).
The purpose of this study was to establish a murine model of melioidosis in order to examine the relative importance of IFN-γ and other proinflammatory cytokines in host resistance. Previous attempts to develop animal models of this disease have used a range of species, including ferrets, guinea pigs, hamsters and, to a limited extent, inbred mice (9, 21, 34, 35). In many cases, infection with relatively low numbers of B. pseudomallei has invariably led to acute lethal sepsis, making it difficult to dissect the mechanism of protection. Generation of models of chronic disease have been reported in some species, but the immunological basis of latency has not been examined (21). We now present evidence that infection of outbred TO mice provides a model of both acute and chronic melioidosis, depending upon the initial inoculum. We report here that, in particular, IFN-γ, as well as IL-12 and TNF-α, are essential mediators of protective immunity. These data provide the first evidence for the mechanism of immune control of this clinically important pathogen.
MATERIALS AND METHODS
Bacteria.
A gentamicin-sensitive strain (708a) of B. pseudomallei, originally obtained from the splenic abscess of a melioidosis patient, was used in all experiments. This strain was isolated from Sappasitprasong Hospital, Ubon Ratchatani, Thailand. Bacteria were subcultured on Columbia agar (Oxoid) and incubated for 24 h at 37°C. Several young colonies were scraped from the agar and suspended in sterile pyrogen-free saline solution (PFS). The suspension was adjusted to an absorbance (optical density) of 0.50 at a 600-nm wavelength. A frozen stock was prepared at a concentration of 108 CFU/ml in PFS with 10% glycerol (vol/vol) and stored at −70°C. When required to inoculate into mice, the bacteria were thawed, washed once with PFS, and diluted to the required concentration in PFS. Identification of the strain both in the initial inoculum and when isolated from acute and chronic infections was confirmed by API 20NE kits (BioMérieux), and a standard disc diffusion method was used to determine susceptibility to gentamicin.
Animal model of infection.
Female, Taylor Outbred (TO) mice (Tuck) or BALB/c mice (Harlan Olac) were used at 10 to 12 weeks of age. In other experiments, mice with a targeted gene deletion of the IFN-γ receptor (G129) were compared with their wild type (129SVEV) as controls. These were originally obtained from Bantin & Kingman (Hull, United Kingdom) and bred at the London School of Hygiene and Tropical Medicine (LSHTM) under aseptic conditions. All animals were maintained with access to food and water ad libitum under Animal Biohazard Containment Level 3 conditions throughout the experiments. Mice were infected intraperitoneally (i.p.) or subcutaneously with various concentrations of viable B. pseudomallei in 0.2 ml of PFS. All infections were performed in a class I Biosafety cabinet, and infected animals were maintained within a negative-pressure, HEPA-filtered flexible film isolator (MDH).
Quantitation of bacterial load and histology.
Small sections of spleen and liver from infected mice were taken immediately after the organs had been harvested and placed in 2% neutral buffered formalin for at least 24 h. This procedure fixed and disinfected the tissues, which were then processed for routine histological examination by using 5-μm-thick paraffin-embedded sections stained with hematoxylin and eosin. The remainder of the organs were placed into 5 ml of PFS containing 0.5% (vol/vol) Triton X-100 (BDH) and disrupted by passing through a 100-μm-pore-size nylon mesh sieve (Falcon). Serial 10-fold dilutions of the resulting cell suspensions were spread in duplicate onto Columbia agar, and colonies were counted after 48 h incubation at 37°C. The remainder of the cell suspensions were added into 10 ml of brain heart infusion broth (Oxoid) and incubated at 37°C for up to 1 week. The positive broths were subcultured onto Columbia agar, and the bacteria were identified. All negative broths were subcultured on day 7 before being discarded.
Neutralizing anti-cytokine antibodies.
To neutralize specific cytokines in vivo, mice were injected i.p. at the time of infection with either 300 μg of hamster anti-mouse IFN-γ MAb (H22) (provided by R. D. Schreiber, Washington University, St. Louis, Mo.), 300 μg of hamster anti-mouse TNF MAb (TN319.12), 1 mg of rat anti-mouse IL-12 MAb (C17.8) (provided by C. Engwerda, Department of Infectious and Tropical Diseases, LSHTM, and originally obtained from G. Trinchieri, Wistar Institute), or 300 μg of rat anti-mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) MAb (MP1-22E9) diluted in PFS. Control mice were injected with appropriate concentrations of either PFS, hamster isotype control antibody (L2), or purified rat immunoglobulin G (IgG; Sigma, Poole, Dorset, United Kingdom).
Statistics.
Statistics analysis (unpaired t test and Kaplan-Meier survival analysis) were performed by using GraphPad Prism software (GraphPad, San Diego, Calif.). A P value of <0.05 was considered significant.
RESULTS
Comparison of susceptibility of BALB/c versus TO mice to B. pseudomallei.
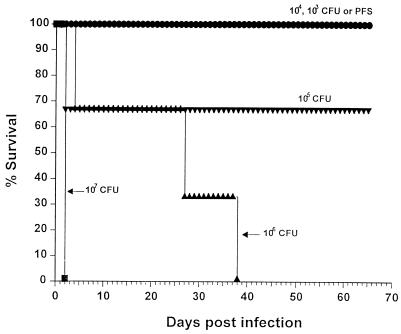
In order to establish a model of melioidosis which would encompass both acute and chronic features of the human disease, we compared the course of infection in inbred BALB/c mice versus the outbred mouse strain TO. Mice were injected i.p. with various doses of B. pseudomallei 708a, and cumulative survival was monitored up to a period of 65 days postinfection. Infection of TO mice resulted in death within 48 h only at a dose of 107 CFU per mouse and delayed mortality between days 25 to 40 at 106 CFU (Fig. 1). Lower doses of bacteria generally resulted in chronic infections, after which death of remaining mice occurred in proportion to the initial inoculating dose of bacteria, i.e., mice receiving the lower dose lived longer. In contrast, acute lethal sepsis occurred within 3 days in all BALB/c mice given 107, 106, or 105 CFU of B. pseudomallei, whereas lower doses were sublethal within the first 2 months of infection (data not shown). In view of their greater resistance, TO mice were subsequently used in all further in vivo experiments.
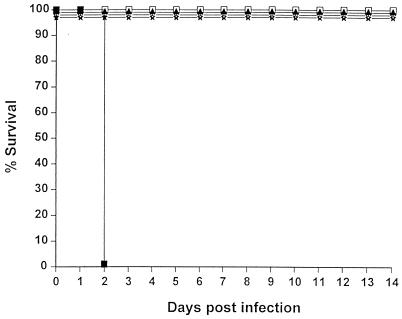
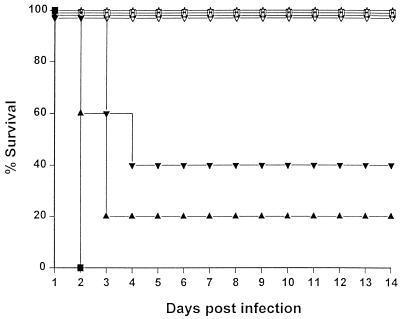
FIG. 1.
Effects of B. pseudomallei on the mortality of TO mice. Mice were infected i.p. with doses of B. pseudomallei from 107 (■), 106 (▴), 105 (▾), 104 (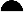 ), or 103 (
), or 103 ( ) CFU per mouse versus use of PFS alone (●). Animals were observed daily, and the percent survival values were plotted against time. Infection with either 107 or 106 CFU of the bacteria (but not lower doses) resulted in a significantly earlier death compared to uninfected animals (P <0.02).
) CFU per mouse versus use of PFS alone (●). Animals were observed daily, and the percent survival values were plotted against time. Infection with either 107 or 106 CFU of the bacteria (but not lower doses) resulted in a significantly earlier death compared to uninfected animals (P <0.02).
Time course of bacterial clearance after infection with B. pseudomallei.
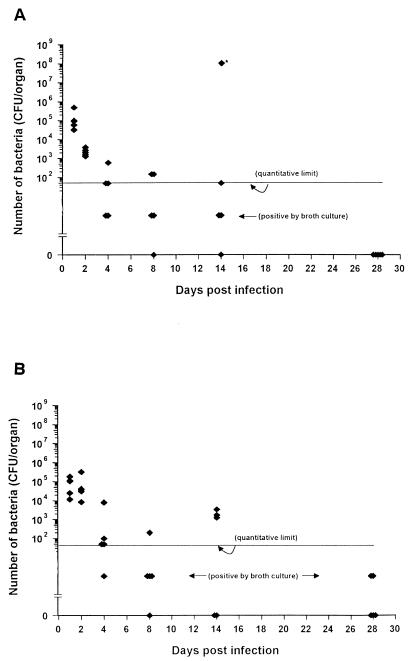
To investigate the kinetics of bacterial replication in the tissues, TO mice were infected with a sublethal dose (2 × 105 CFU) of B. pseudomallei, and the quantity of bacteria in the spleen and liver was determined at various time intervals. At the earliest time point examined (24 h postinfection), the numbers of bacteria in each organ approximated that in the original infecting dose (Fig. 2). However, the splenic load of bacteria progressively decreased over the following 4 days such that by day 8, two of five mice contained only low numbers of bacteria as demonstrated by viable counts, two of five mice were negative on the basis of direct plating of tissue homogenates but showed positive growth when the homogenates were cultured in enrichment broth, and one of the five animals was found to be sterile by both culture criteria. From one experiment, a single animal which had shown no clinical sign of illness was examined, and multiple abscesses were found in the spleen yielding more than 108 CFU per organ, suggesting that in some cases focal growth of the organism can occur without overt signs of infection. Splenomegaly was a prominent feature of the infection and occurred by day 4 and increased progressively with time in contrast to the bacterial burden, which decreased over this period (data not shown). Histopathological examination of the spleens of sublethally infected mice showed an initial infiltration of neutrophils followed by macrophages and lymphocytes (Fig. 3). A similar pattern of progressive reduction of bacterial loads was observed in the liver; however, the clearance of bacteria from this organ was slightly slower than from the spleen (Fig. 2B). Histology sections of the lung showed minimal involvement of this organ by this route of infection.
FIG. 2.
Kinetics of B. pseudomallei clearance from the livers and spleens of infected mice. Mice were infected i.p. with 105 B. pseudomallei, and at various times bacterial loads were determined in the spleen (A) or liver (B) as described in Materials and Methods. Data are presented as the CFU per organ for five individual mice per group. An asterisk represents a single animal with splenic abscesses.
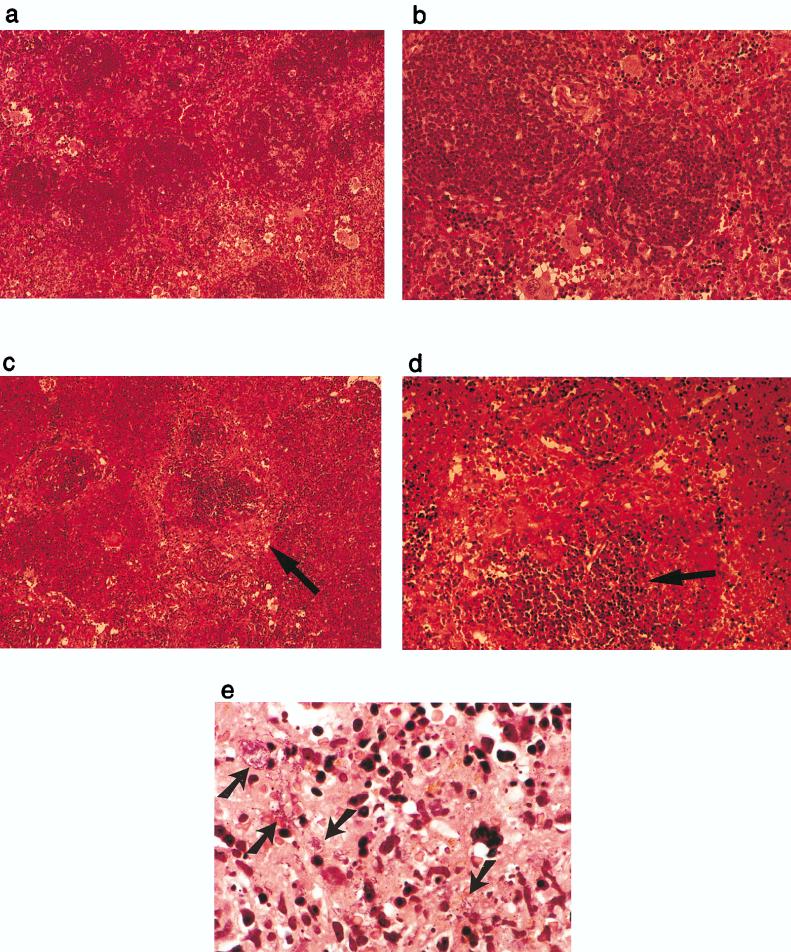
FIG. 3.
Histology of infection with B. pseudomallei in the spleen after neutralization of IFN-γ in vivo. Mice infected i.p. with B. pseudomallei and treated with either an isotype control antibody L2 (a and b) or anti-IFN-γ MAb H22 (c and d) at 40 h postinfection. Evidence of destruction of the red pulp (arrow in panel c) and disruption of normal lymphoid architecture (arrow in panel d). (e) High-power view of infected, H22-treated mice showing large numbers of the bacteria (arrows). Each photograph represents a typical view of the entire section and for all mice studied (three mice per group).
Role of IFN-γ in resistance to acute infection with B. pseudomallei.
IFN-γ has been implicated in both protective immunity and the pathology associated with infection with gram-negative bacteria. To determine the contribution of IFN-γ in resistance to B. pseudomallei, mice were treated with either saline or the neutralizing hamster anti-mouse IFN-γ MAb (H22) immediately prior to infection with a sublethal dose of viable bacteria, and mortality was monitored. Saline-treated, infected mice survived for the duration of the experiment without any clinical signs of infection. In contrast, mice treated with H22 MAb showed visible signs of acute sepsis (i.e., weight loss and lethargy) within 20 h of infection, and all were dead by 40 h after infection (Fig. 4). Similar conversion to an acutely lethal event was also observed if neutralization of IFN-γ was delayed until 7 days postinfection, although the mortality kinetics observed in this group indicated that death was slightly delayed. All animals died within 5 days of antibody administration, whereas all control infected mice survived until the termination of the experiment on day 30 (data not shown). Treatment of infected mice with an isotype-matched control hamster IgG (L2) resulted in an identical outcome to the treatment with saline plus B. pseudomallei, whereas mice treated with H22 alone and not infected showed no illness at any point. In contrast to the dramatic effects of neutralizing IFN-γ, MAb to murine GM-CSF at concentrations known to inhibit resistance to other pathogens, including Cryptococcus neoformans, Legionella pneumophila, and Candida albicans (6, 37), had no effect on the outcome of infection (Fig. 4).
FIG. 4.
Effects of in vivo neutralization of IFN-γ versus GM-CSF on infection with B. pseudomallei. Mice (three mice per group) were injected with either PFS (⋆); anti-IFN-γ MAb H22 (■), anti-GM-CSF MAb (▴), or their relevant control antibodies; L2 (□), or β-galactosidase MAb (▿) at 300 μg/mouse at the time of i.p. infection with B. pseudomallei (2 × 105 CFU). Results are presented as the percent survival against time. Where multiple groups all showed 100% survival, data points have been separated for clarity of presentation. Infected mice treated with H22 resulted in a significantly earlier death compared to B. pseudomallei plus control antibodies (P < 0.02). The results are representative of two independent experiments.
To examine the cause of death after IFN-γ depletion, the livers and spleens of infected mice were harvested to quantitate the number of bacteria per organ. Respectively, 4,400- and 8,500-fold more bacteria were isolated from the spleen and liver of anti-IFN-γ MAb-treated, infected mice than from the control group (Fig. 5). Although the bacterial loads in both liver and spleen from the IFN-γ-depleted mice were similarly high, the histopathology of these two organs was distinctive. In the spleen, lymphoid architecture, particularly in the red pulp, was extensively destroyed, and large numbers of bacteria were spread throughout the organ and easily observed on hematoxylin and eosin staining (Fig. 3). Spleen sections from control infected mice showed infiltration of lymphocytes and phagocytes, but bacteria were not observed. In contrast to the spleen, the inflammatory response in the liver was more localized, and only a slight increase in tissue damage was observed in IFN-γ-depleted mice compared with infected controls, suggesting that the spleen is particularly sensitive to the necrotic effects of high burdens of B. pseudomallei in vivo.
FIG. 5.
Effect of neutralization of IFN-γ on growth of B. pseudomallei in vivo. Mice were treated with 300 μg per mouse of either control L2 or anti-IFN-γ MAb H22 at the time of infection with 2 × 105 CFU of B. pseudomallei. Viable number of B. pseudomallei (CFU) was determined at 48 h postinfection in the spleen and liver as described in Materials and Methods. Data are presented as individual histograms for each mouse per experimental group. ∗, P < 0.02; ∗∗, P < 0.001 (comparing H22 MAb treatment versus control antibody groups in spleen and liver, respectively).
Susceptibility of IFN-γ-depleted mice to B. pseudomallei.
To identify the minimum infecting dose of B. pseudomallei, mice were challenged with serial 10-fold dilutions of bacteria from 2 × 105 CFU or saline alone and treated with either H22 or control L2 antibody. Mice given control MAb showed no sign of illness and survived throughout the period of the experiment with all bacterial inocula (Fig. 6). In contrast, all IFN-γ-depleted mice infected with 2 × 105 or 2 × 104 CFU died within 48 h. Furthermore, at the lower inocula (2 × 103, 2 × 102, or 2 × 101 CFU), all anti-IFN-γ-treated mice were dead within 5 days of challenge. Even at an inoculum of 2 × 100 CFU, two of three anti-IFN-γ-treated mice died, whereas controls for this inoculum all survived. Finally, all IFN-γ-depleted mice given a further 10-fold dilution (i.e., 2 × 10−1 CFU) survived excluding any nonspecific immunosuppressive effects of IFN-γ neutralization per se. The accuracy of the infecting doses was confirmed by direct plating of the bacterial suspension used in the challenge. Thus, under these conditions, neutralization of IFN-γ lowered the effective lethal dose of B. pseudomallei by 100,000-fold to approximately two bacteria per animal. The importance of IFN-γ was confirmed in experiments examining the sensitivity of IFN-γ receptor knockout mice (G129) to infection with B. pseudomallei. Control wild-type mice (129SVEV) were extremely susceptible to B. pseudomallei infection, and at doses of from 105 to 102 CFU/mouse, both IFN-γR+/+ and IFN-γR−/− mice all succumbed within 2 days. Nevertheless, when given 101 CFU per animal, two of three IFN-γR+/+ mice died at day 12, whereas all IFN-γR−/− mice succumbed within 2 days. Thus, by using either neutralizing MAb in immunocompetent mice or animals lacking the IFN-γ receptor, functional loss of IFN-γ is associated with increased susceptibility to infection with B. pseudomallei.
FIG. 6.
Susceptibility to infection with B. pseudomallei after administration of anti-IFN-γ MAb in vivo. Anti-IFN-γ MAb (H22) or the control antibody (L2) were injected i.p. into mice (three mice per group) at the time of infection with different doses of B. pseudomallei. The doses were 2 × 105 (■), 2 × 104 (●), 2 × 103 (▴), 2 × 102 (▾), 2 × 101 ( ), 2 × 100 (
), 2 × 100 ( ), or 2 × 10−1 (▮) CFU/mouse. Injection of the antibodies into uninfected mice (⧫) was performed as a negative control. Solid symbols represent mice treated with H22 MAb, and open symbols represent L2 treatment. Mice were observed daily for 14 days and the percent survival was plotted against time. Where multiple experimental groups all showed 100% survival, data points have been separated for clarity of presentation. All infected mice treated with 2 × 100 CFU (P < 0.05) and higher doses of B. pseudomallei (P < 0.02) resulted in a significantly earlier time to death when compared to B. pseudomallei plus L2 control antibody. The results are representative of two independent experiments.
), or 2 × 10−1 (▮) CFU/mouse. Injection of the antibodies into uninfected mice (⧫) was performed as a negative control. Solid symbols represent mice treated with H22 MAb, and open symbols represent L2 treatment. Mice were observed daily for 14 days and the percent survival was plotted against time. Where multiple experimental groups all showed 100% survival, data points have been separated for clarity of presentation. All infected mice treated with 2 × 100 CFU (P < 0.05) and higher doses of B. pseudomallei (P < 0.02) resulted in a significantly earlier time to death when compared to B. pseudomallei plus L2 control antibody. The results are representative of two independent experiments.
The role of TNF-α and IL-12 in resistance to acute infection with B. pseudomallei.
In other model systems, the rapid production of IFN-γ is initiated by the production of TNF-α and IL-12 (32). In order to assess the role of these cytokines in melioidosis, TO mice were infected with B. pseudomallei as previously described and given neutralizing MAb specific for either TNF (TN3.19.12), IL-12 (C17.8), or IFN-γ at the time of infection. As expected, neutralization of IFN-γ again resulted in 100% mortality within 48 h (Fig. 7). However, depletion of IL-12 resulted in acute lethal sepsis in four of five animals by 3 days, and three of five animals died by 4 days after neutralization of TNF in vivo. These results suggest that IL-12 and TNF-α, as well as IFN-γ, are also important mediators of resistance to acute infection.
FIG. 7.
Effects of in vivo neutralization of IFN-γ versus TNF and IL-12 on infection with B. pseudomallei. Mice (five mice per group) were injected with either PFS (⋆); anti-IFN-γ MAb H22 (■), anti-TNF MAb (▾), anti-IL-12 MAb (▴), or their relevant control antibodies; L2 (□); rat IgG (▿); or β-galactosidase MAb (▵) at a dose of 300 μg/mouse at the time of i.p. infection with 2 × 105 CFU of B. pseudomallei. All infected mice treated with either H22 (P < 0.002), anti-TNF (P < 0.005), or anti-IL-12 MAb (P < 0.005) resulted in a significantly earlier time to death compared to B. pseudomallei plus control antibodies. Results are presented as the percent survival against time. Where multiple groups all showed 100% survival, data points have been separated for clarity of presentation. The results are representative of two independent experiments.
DISCUSSION
In this report, we have generated murine models of both acute and chronic infection to investigate the mechanisms of protective immunity against the causative agent of human melioidosis. Three major findings arose from this study. First, immunocompetent mice initially clear viable B. pseudomallei from infected tissues but then enter a chronic phase of infection which is invariably lethal after 2 to 16 months. Second, this early control mechanism is absolutely dependent upon the rapid production of IFN-γ. Finally, TNF and IL-12 also play a role in acute resistance to B. pseudomallei in vivo.
Our initial experiments demonstrated that inbred BALB/c mice are relatively susceptible to acute infection with B. pseudomallei, whereas the outbred TO strain was approximately 100-fold more resistant. Furthermore, 129SVEV mice showed even greater susceptibility, with a 50% lethal dose of ca. 100 CFU. Genetic differences in resistance have also been reported in other gram-negative bacterial infections (14), although the basis of the different susceptibilities of the animals to B. pseudomallei has yet to be clarified. The extreme susceptibility of some species to experimental infection with B. pseudomallei has previously hampered investigation of the protective immune response to this organism. Our ability to challenge TO mice with significant numbers of bacteria and generate either an acutely lethal infection or a chronic infection provided us with a practicable model to dissect the involvement of macrophage-activating cytokines on resistance in vivo. A kinetic study of bacterial clearance from the target organs of sublethally infected TO mice showed little evidence of bacterial growth but did show a progressive decline in viable B. pseudomallei over time. Clearance was marginally slower in the liver compared to the spleen, but in both organs 99% of bacteria were removed within 4 days after infection, and the majority of animals progressed to apparent sterilization of these organs by 28 days after infection. This result was associated with progressive splenomegaly and the rapid development of granulomas containing multinucleated giant cells in infected tissues, a feature characteristic of both human melioidosis and infection in other animal species (9, 30, 36). These observations support our recent description of the ability of Burkholderia species (both B. pseudomallei and B. mallei) to induce cell fusion and giant cell formation in macrophage cell lines in vitro (14a).
Two additional features of infection with B. pseudomallei are worthy of mention. First, splenic abscesses containing a high number of bacteria were observed in some animals which were otherwise asymptomatic and had negligible numbers of bacteria in other organs, indicating that B. pseudomallei can replicate in a focal manner without causing systemic toxicity. Second, at the doses used in this study, all animals that survived the initial infection entered a chronic phase lasting from 2 to 16 months before ultimately progressing to reactivation and death. The cellular and organ location of the few remaining bacteria which go on to cause reactivating disease have not been identified. However, reactivating lesions were observed at multiple tissue sites, including the foot pad and subcutaneous tissue, as well as in the main target organs of liver and spleen. These features mimic the characteristics of clinical melioidosis, further supporting the relevance of the murine model to the human disease (8).
When mice were depleted of IFN-γ at the time of infection, resistance to B. pseudomallei was completely abolished and all animals died within 40 h, whereas the control infected group survived for several months. Neutralization of IFN-γ blocked the generation of multinucleated giant cells (data not shown), increased the bacterial burden in the spleen and liver by 4,400- and 8,500-fold, respectively, and caused massive destruction of splenic lymphoid architecture. Under these conditions, large numbers of bacteria were observed both extracellularly and intracellularly within macrophages but, unlike the case of Listeria monocytogenes (27), there was no evidence that B. pseudomallei invades and replicates in hepatocytes. The fact that as few as two CFU were capable of causing lethal melioidosis in the absence of IFN-γ implies that resident phagocytic cells such as peritoneal macrophages and Küpffer cells are unable to kill B. pseudomallei in vivo. This idea is supported by reports of intracellular survival of B. pseudomallei in both resident macrophages and PMNL in vitro (11, 16, 25). As yet, we have not identified the mechanisms by which IFN-γ mediates its protective effects. IFN-γ is an established activator of macrophage microbicidal activity and mediates resistance against a wide range of other intracellular pathogens, including Listeria spp., Salmonella spp., and Mycobacterium spp., as well as some protozoan parasites (17). Recently, Miyagi and colleagues have demonstrated that IFN-γ activates a murine macrophage cell line to kill B. pseudomallei in vitro by a combination of both oxidative and NO-mediated mechanisms (22). The data presented here are consistent with these in vitro findings and provide direct evidence for the importance of the IFN-γ response in vivo. That IFN-γ depletion increases the susceptibility of mice to B. pseudomallei by more than 100,000-fold (in terms of 50% lethal dose) clearly makes melioidosis one of the most sensitive models of IFN-γ-dependent resistance described to date.
The rapidity with which anti-IFN-γ MAb abolishes resistance suggests an innate rather than an acquired source of this cytokine in the initial phase of infection. H22 MAb-treated mice showed signs of illness within 18 to 24 h, suggesting that even in a naive host, B. pseudomallei stimulates IFN-γ production within this period. To date, there is no information on T-cell responses to this organism in either humans or experimental animals. However, such rapid IFN-γ responses are unlikely to be mediated by the clonal expansion of antigen-specific T cells. Natural killer (NK) cells are an alternative source of IFN-γ and represent the first secreting cells after infection with other intracellular pathogens (2, 10, 28). Several pieces of evidence suggest this pathway may contribute to resistance against B. pseudomallei. First, other gram-negative bacteria, such as Salmonella spp. and Francisella spp., can also stimulate T-cell-independent IFN-γ responses (12, 26). Second, as shown here, neutralization of either TNF-α or IL-12, which stimulate secretion of IFN-γ by NK cells, increases susceptibility to B. pseudomallei. Third, SCID mice infected with B. pseudomallei show an intermediate pattern of resistance between that of infected versus IFN-γ-depleted mice, surviving up to 20 days (data not shown). This pattern of partial resistance in SCID mice is typical of other pathogens which are known to activate the NK cell IFN-γ pathway (2). Thus, T cells are not required for initial protection against lethal melioidosis but are absolutely necessary for maintenance of the chronic phase of infection. Finally, irradiated B. pseudomallei activate naive SCID spleen cells (which are enriched in NK cells but devoid of both α/β and γ/δ T cells) for secretion of IFN-γ in vitro (19a). Together, these results point to an initial, T-cell-independent pathway of resistance prior to the generation of antigen-specific immunity. It should be noted that TNF-α and IL-12 have other important proinflammatory actions, including the upregulation of adhesion molecules and the promotion of Th1-type T-cell responses, which may also contribute to resistance in the immunocompetent host (17). Finally, the course of chronic infection may also be influenced by the production of IFN-γ since in preliminary experiments neutralization of IFN-γ promoted reactivation and shortened the duration of the latent state (data not shown). Experiments are in progress to identify the cellular source of IFN-γ in the chronically infected host, which is likely to be derived from antigen-specific T cells rather than NK cells (31).
In conclusion, the results presented here provide the first evidence for the cellular basis of protective immunity against B. pseudomallei. A rapid IFN-γ response protects the naive host from acute sepsis after initial contact with the organisms and allows progression into the chronic phase of the disease. This distinguishes B. pseudomallei from immunity to L. monocytogenes, the classical paradigm of IFN-γ-mediated resistance, where infection leads to complete and protective sterilizing immunity (33). Instead, melioidosis shares characteristics with other pathogens, such as M. tuberculosis and Toxoplasma gondii, where a successful IFN-γ response is still unable to eliminate the organism, placing the host at risk of reactivation if immune competence becomes impaired at a later time. It will be interesting to determine whether sterilizing immunity to B. pseudomallei can be achieved under other circumstances, such as after cytokine therapy with exogenous IFN-γ or IL-12 or after vaccination. To date, no information is yet available on the nature of protective immunity in humans infected with this organism. However, patients presenting with lethal melioidosis express high levels of circulating cytokines, including TNF-α and IFN-γ, which correlate with mortality (3, 29). Animal models of septic shock indicate that the same cytokines which confer resistance when produced in a controlled and localized manner (including IFN-γ, TNF-α, and IL-12) promote pathology when produced systemically to an overwhelming bacterial load (1, 4, 15). From the data presented here, we predict that the generation of IFN-γ-dominant NK cell and T-cell responses will be essential for the control of acute human melioidosis and entry into the chronic phase. Experiments to test this hypothesis and assess the effect of known risk factors for reactivation (such as diabetes) on these responses should provide a better understanding of the immunological processes which control infection with this important pathogen.
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust (project grant 034656/2/91/2). P.S. is supported by grant from the Ministry of Sciences and Technologies of Thailand.
REFERENCES
- 1.Authenrieth I B, Beer M, Bohn E, Kaufmann S H E, Heesemann J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancroft G J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 3.Brown A E, Dance D A B, Suputtamongkol Y, Chaowagul W, Kongchareon S, Webster H K, White N J. Immune cell activation in melioidosis: increased serum levels of interferon-γ and soluble interleukin-2 receptors without change in soluble CD8 protein. J Infect Dis. 1990;163:1145–1148. doi: 10.1093/infdis/163.5.1145. [DOI] [PubMed] [Google Scholar]
- 4.Car B D, Eng V M, Schnyder B, Ozmen L, Huang S, Gallay P, Heumann D, Agnet M, Ryffel B. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaowakul W, White N J, Dance D A B, Wattanagoon Y, Naigowit P, Davis T M E, Looareesuwan S, Pitakwatchara N. Melioidosis: a major cause of community-acquired septicemia in Northeastern Thailand. J Infect Dis. 1989;159:890–899. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 6.Collins H L, Bancroft G J. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-α and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992;22:1447–1454. doi: 10.1002/eji.1830220617. [DOI] [PubMed] [Google Scholar]
- 7.Cooper A M, Dalton D K, Stewart T A. Disseminated tuberculosis in gamma interferon gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dance D A B. Melioidosis. Rev Med Microbiol. 1990;4:143–150. [Google Scholar]
- 9.Dannenberg A M, Jr, Scott E M. Melioidosis: pathogenesis and immunity in mice and hamsters. I. Studies with virulent strains of Malleomyces pseudomallei. J Exp Med. 1958;107:153–187. doi: 10.1084/jem.107.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn P, North R J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan A M, Gordon D L. Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect Immun. 1996;64:4952–4959. doi: 10.1128/iai.64.12.4952-4959.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkins K L, Rhinehart-Jones T R, Culkin S J, Yee D, Winegar R K. Minimal requirements of murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64:3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedland J S, Suputtamongkol Y, Remick D G, Chaowagul W, Strieter R M, Kunkel S L, White N J, Griffin G E. Prolonged elevation of interleukin-8 and interleukin-6 concentrations in plasma and of leukocyte interleukin-8 mRNA levels during septicemic and localized Pseudomonas pseudomallei infection. Infect Immun. 1992;60:2402–2408. doi: 10.1128/iai.60.6.2402-2408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock G E, Schaedler R W, MacDonald T T. Yersinia enterocolitica infection in resistant and susceptible strains of mice. Infect Immun. 1986;53:26–31. doi: 10.1128/iai.53.1.26-31.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Harley V S, Dance D A B, Drasar B S, Tovey G. Effect of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios. 1998;96:71–93. [PubMed] [Google Scholar]
- 15.Heremans H, van Damme J, Dillen C, Dijkmans R, Billiau A. Interferon, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reaction in mice. J Exp Med. 1990;171:1853–1869. doi: 10.1084/jem.171.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones A L, Beveridge T J, Woods D E. Intracellular survival of Burkholderia pseudomallei. Infect Immun. 1996;64:782–790. doi: 10.1128/iai.64.3.782-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann S H E. Immunity to intracellular microbial pathogens. Immunol Today. 1995;16:338–342. doi: 10.1016/0167-5699(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 18.Kingston C W. Chronic and latent melioidosis. Med J Aust. 1971;2:618–621. doi: 10.5694/j.1326-5377.1971.tb92445.x. [DOI] [PubMed] [Google Scholar]
- 19.Leelarasamee A, Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989;11:413–425. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- 19a.Lertmemongkolchai, G., and G. J. Bancroft. Unpublished data.
- 20.Mays E E, Ricketts E A. Melioidosis: recrudescence associated with bronchogenic carcinoma twenty-six years following initial geographic exposure. Chest. 1975;68:567–577. doi: 10.1378/chest.68.2.261. [DOI] [PubMed] [Google Scholar]
- 21.Miller W R, Panneli L, Cravitz L, Tanner W A, Rosebury T. Studies on certain biological characteristics of Malleomyces mallei and Malleomyces pseudomallei. II. Virulence and infectivity for animals. J Bacteriol. 1948;55:127–135. [PMC free article] [PubMed] [Google Scholar]
- 22.Miyagi K, Kawakami K, Saito A. Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect Immun. 1997;65:4108–4113. doi: 10.1128/iai.65.10.4108-4113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muotiala A, Mäkelä P H. The role of IFN-γ in murine Salmonella typhimurium infection. Microb Pathog. 1990;8:135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- 24.Piggott J A, Hochholzer L. Human melioidosis: a histopathologic study of acute and chronic melioidosis. Arch Pathol. 1970;90:101–111. [PubMed] [Google Scholar]
- 25.Pruksachartvuthi S, Aswapokee N, Thankerngpol K. Survival of Pseudomonas pseudomallei in human phagocytes. J Med Microbiol. 1990;31:109–114. doi: 10.1099/00222615-31-2-109. [DOI] [PubMed] [Google Scholar]
- 26.Ramarathinam L, Niesel D W, Klimpel G R. Salmonella typhimurium induces IFN gamma production in murine splenocytes. Role of natural killer cells and macrophages. J Immunol. 1993;150:3973–3981. [PubMed] [Google Scholar]
- 27.Rogers H W, Callery M P, Deck B, Unanue E R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 28.Scott P, Trinchieri G. The role of natural killer cells in host-parasite relationships. Curr Opin Immunol. 1995;7:34–40. doi: 10.1016/0952-7915(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 29.Suputtamongkol Y, Kwaitkowski D, Dance D A B, Chaowagul W, White N J. Tumor necrosis factor in septicemic melioidosis. J Infect Dis. 1992;165:561–564. doi: 10.1093/infdis/165.3.561. [DOI] [PubMed] [Google Scholar]
- 30.Tarlow M J. Melioidosis and chronic granulomatous disease. Proc R Soc Med. 1971;64:19–20. doi: 10.1177/003591577106400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripp C S, Kanagawa O, Unanue E R. Secondary response to Listeria infection requires IFN-γ but is partially independent of IL-12. J Immunol. 1995;155:3427–3432. [PubMed] [Google Scholar]
- 32.Unanue E R. Interrelationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr Opin Immunol. 1997;9:35–43. doi: 10.1016/s0952-7915(97)80156-2. [DOI] [PubMed] [Google Scholar]
- 33.Unanue E R. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T cell response. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 34.Veljanov D, Vesselinova A, Nikolova S, Najdenski H, Kussovski V, Markova N. Experimental melioidosis in inbred mouse strains. Int J Med Microbiol (Zentbl Bakteriol) 1996;879:351–359. doi: 10.1016/s0934-8840(96)80071-5. [DOI] [PubMed] [Google Scholar]
- 35.Vorachit M, Lam K, Jayanetra P, Costerton J W. Study of the pathogenicity of Burkholderia pseudomallei—a guinea pig model. J Infect Dis Antimicrob Agents. 1995;12:115–121. [Google Scholar]
- 36.Wong K T, Puthucheary S D, Vadivelu J. The histopathology of human melioidosis. Histopathology. 1995;26:51–55. doi: 10.1111/j.1365-2559.1995.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Klein T M, Tomioko M, Friedman H. Differential effects of granulocyte/macrophage colony-stimulating factor (GM-CSF) in enhancing macrophage resistance to Legionella pneumophilia vs. Candida albicans. Cell Immunol. 1997;176:75–81. doi: 10.1006/cimm.1996.1074. [DOI] [PubMed] [Google Scholar]