Abstract
Background
Oropharyngeal dysphagia encompasses problems with the oral preparatory phase of swallowing (chewing and preparing the food), oral phase (moving the food or fluid posteriorly through the oral cavity with the tongue into the back of the throat) and pharyngeal phase (swallowing the food or fluid and moving it through the pharynx to the oesophagus). Populations of children with neurological impairment who commonly experience dysphagia include, but are not limited to, those with acquired brain impairment (for example, cerebral palsy, traumatic brain injury, stroke), genetic syndromes (for example, Down syndrome, Rett syndrome) and degenerative conditions (for example, myotonic dystrophy).
Objectives
To examine the effectiveness of interventions for oropharyngeal dysphagia in children with neurological impairment.
Search methods
We searched the following electronic databases in October 2011: CENTRAL 2011(3), MEDLINE (1948 to September Week 4 2011), EMBASE (1980 to 2011 Week 40) , CINAHL (1937 to current) , ERIC (1966 to current), PsycINFO (1806 to October Week 1 2011), Science Citation Index (1970 to 7 October 2011), Social Science Citation Index (1970 to 7 October 2011), Cochrane Database of Systematic Reviews, 2011(3), DARE 2011(3), Current Controlled Trials (ISRCTN Register) (15 October 2011), ClinicalTrials.gov (15 October 2011) and WHO ICTRP (15 October 2011). We searched for dissertations and theses using Networked Digital Library of Theses and Dissertations, Australasian Digital Theses Program and DART‐Europe E‐theses Portal (11 October 2011). Finally, additional references were also obtained from reference lists from articles.
Selection criteria
The review included randomised controlled trials and quasi‐randomised controlled trials for children with oropharyngeal dysphagia and neurological impairment.
Data collection and analysis
All three review authors (AM, PD and EW) independently screened titles and abstracts for inclusion and discussed results. In cases of uncertainty over whether an abstract met inclusion criterion, review authors obtained the full‐text article and independently evaluated each paper for inclusion. The data were categorised for comparisons depending on the nature of the control group (for example, oral sensorimotor treatment versus no treatment). Effectiveness of the oropharyngeal dysphagia intervention was assessed by considering primary outcomes of physiological functions of the oropharyngeal mechanism for swallowing (for example, lip seal maintenance), the presence of chest infection and pneumonia, and diet consistency a child is able to consume. Secondary outcomes were changes in growth, child’s level of participation in the mealtime routine and the level of parent or carer stress associated with feeding.
Main results
Three studies met the inclusion criteria for the review. Two studies were based on oral sensorimotor interventions for participants with cerebral palsy compared to standard care and a third study trialled lip strengthening exercises for children with myotonic dystrophy type 1 compared to no treatment (Sjogreen 2010). A meta‐analysis combining results across the three studies was not possible because one of the studies had participants with a different condition, and the remaining two, although using oral sensorimotor treatments, used vastly different approaches with different intensities and durations. The decision not to combine these was in line with our protocol. In this review, we present the results from individual studies for four outcomes: physiological functions of the oropharyngeal mechanism for swallowing, the presence of chest infection and pneumonia, diet consistency, and changes in growth. However, it is not possible to reach definitive conclusions on the effectiveness of particular interventions for oropharyngeal dysphagia based on these studies. One study had a high risk of attrition bias owing to missing data, had statistically significant differences (in weight) across experimental and control groups at baseline, and did not describe other aspects of the trial sufficiently to enable assessment of other potential risks of bias. Another study was at high risk of detection bias as some outcomes were assessed by parents who knew whether their child was in the intervention or control group. The third study overall seemed to be at low risk of bias, but like the other two studies, suffered from a small sample size.
Authors' conclusions
The review demonstrates that there is currently insufficient high‐quality evidence from randomised controlled trials or quasi‐randomised controlled trials to provide conclusive results about the effectiveness of any particular type of oral‐motor therapy for children with neurological impairment. There is an urgent need for larger‐scale (appropriately statistically powered), randomised trials to evaluate the efficacy of interventions for oropharyngeal dysphagia.
Keywords: Child, Humans, Cerebral Palsy, Cerebral Palsy/complications, Deglutition, Deglutition Disorders, Deglutition Disorders/etiology, Deglutition Disorders/therapy, Exercise Therapy, Exercise Therapy/methods, Myotonic Dystrophy, Myotonic Dystrophy/complications
Plain language summary
Interventions for swallowing difficulty in children with neurological impairment
Oropharyngeal dysphagia, or swallowing difficulty, can be defined as problems with chewing and preparing food, difficulty moving food through the mouth to the back of the tongue, and difficulty with swallowing and movement of food through the 'throat' or pharynx. Many children with neurological impairment experience swallowing difficulties, including those with acquired brain impairment (for example, cerebral palsy, traumatic brain injury, stroke), genetic syndromes (for example, Down syndrome, Rett syndrome) and degenerative conditions (for example, myotonic dystrophy).
This review examined the effectiveness of interventions for oropharyngeal dysphagia in children with neurological impairment. The three studies included in the review examined oral sensorimotor treatments and lip strengthening interventions. We were interested in three primary outcomes, which were physiological functions of the oropharyngeal mechanism for swallowing (for example, lip seal maintenance), the presence of chest infection and pneumonia, and diet consistency, and three secondary outcomes, which were changes in growth, child's level of participation in the mealtime routine, and the level of parent or carer stress associated with feeding. We concluded that there is currently not enough high‐quality evidence from randomised controlled trials or quasi‐randomised controlled trials for any particular type of oropharyngeal dysphagia intervention in this population of children. There is a need for larger‐scale randomised controlled trials to evaluate the effects of interventions for oropharyngeal dysphagia in children with neurological impairment.
Background
Description of the condition
Dysphagia is a broad term that encompasses many subtypes of swallowing disorder. This review is focused on the efficacy of interventions for oropharyngeal swallowing impairments only. Specifically, difficulty with the oral preparatory phase of swallowing (chewing and preparing the food), the oral phase (moving the food or fluid posteriorly through the oral cavity with the tongue, into the back of the throat) and the pharyngeal phase (swallowing the food or fluid and moving it through the pharynx to the oesophagus). Dysphagia caused by disorders of the oesophageal phase of the swallow (for example, problems such as lower oesophageal sphincter function or gastro‐oesophageal reflux) is excluded from examination in the present review. Oropharyngeal dysphagia is most commonly diagnosed and managed by speech pathologists (also known as speech and language therapists or speech language pathologists) working in a multidisciplinary team of health professionals including occupational therapists, physiotherapists, nurses, radiologists, gastroenterologists and ear, nose and throat specialists.
Dysphagia is common in children who acquire their brain impairment early (for example, cerebral palsy) (Reilly 1996; Calis 2008) or later in life (for example, traumatic brain injury, stroke, encephalitis, brain tumour) (Cornwell 2003; Morgan 2010a). Another group of children experience dysphagia associated with genetic syndromes such as Down syndrome (Faulks 2007) or Rett syndrome (Morton 1997) or neurological degeneration (for example, muscular dystrophy) (Philpot 1999). Few rigorous epidemiological reports of dysphagia prevalence are available for populations of children with neurological impairment, with the exception of cerebral palsy and traumatic brain injury. Available studies report that up to 99% of children with severe generalised cerebral palsy are reported to have dysphagia (Calis 2008), and between 68% and 72% of children with severe traumatic brain injury present with dysphagia during the acute phase of care (Morgan 2003; Morgan 2010a). An association is reported between neurological severity and dysphagia prevalence, with more severely affected children increasingly presenting with dysphagia (Morgan 2010b).
Diagnosis of oropharyngeal dysphagia
Dysphagia in childhood associated with neurological impairment is complex, with many inter‐related factors contributing to its severity and nature of presentation.
A thorough diagnosis of oropharyngeal dysphagia in children with neurological disorder typically involves a clinical swallowing evaluation (CSE), followed by the most appropriate instrumental assessment (for review, see Arvedson 2008). The CSE and instrumental examination are complementary procedures.
In brief, the CSE involves taking a detailed case history, observing the general presentation and cognitive‐behavioural state of the patient; examining oromotor, laryngeal and respiratory status; and determining aspiration risk during trials of foods and fluids. In childhood, a CSE must evaluate a child’s feeding and swallowing function in the context of the skills expected during their particular transitional stage of feeding or developmental level. For example, children from birth to six months of age are predominantly breast or bottle fed, whereas children from six to 18 months are moving towards independent feeding where they are learning to drink from an open cup, to manipulate a spoon, and are moving towards handling increasingly varied textures. These developmental or transitional stages of feeding have important implications for the type of treatment approach used and its success. It is also important to note that the CSE does not allow objective diagnosis of impairment(s) in the pharyngeal phase of swallowing. Objective measurement of the pharyngeal phase requires instrumental diagnostic techniques that provide information on the anatomy and physiology of the swallowing process, including being able to determine the presence of prandial aspiration (aspiration of food or fluid into the trachea and lungs). These are predominantly the videofluoroscopic swallow examination (VFSE) and the endoscopic swallowing examination (ESE) (Arvedson 2008).
Consequences of dysphagia
The direct impacts of oropharyngeal dysphagia include physiological limitations to the oral phase of swallowing (for example, poor lip closure, and poor oral transit owing to reduced mobility of the tongue for propelling food posteriorly into the oropharynx to trigger a swallow) and the pharyngeal phase (for example, inadequate laryngeal elevation and airway closure, resulting in aspiration of food and fluid into the trachea, and inadequate pharyngeal peristalsis, resulting in excessive pooling of food or fluid in the valleculae or pyriform sinuses). In turn, oropharyngeal impairments may disrupt the ingestion of food or fluid and result in further significant adverse consequences, such as nutritional deficiencies or respiratory compromise, both of which are potentially life threatening.
Nutritional deficiencies
Marked oropharyngeal dysphagia places children at risk of reduced energy and nutrient intake, and poor growth (Thommessan 1991; Stallings 1993; Arrowsmith 2006), potentially leading to failure to thrive, or, if left untreated, malnutrition. Prolonged inadequate energy and nutrient intake may have wide‐ranging effects beyond physical growth, with potential impacts on psychomotor development and even neurological development. There are also recognised effects of reduced nutrient intake owing to dysphagia on the immune, skeletal and cardiovascular systems (Rosenbloom 1996). Micronutrient deficiencies have been reported in children with cerebral palsy (Patrick 1990), and specific deficits such as iron deficiency have been reported in children with neurodisability when their diets are limited to specific food sources that may be easier to ingest but reduce the variety of nutrients (Rosenbloom 1996). Children with oropharyngeal dysphagia are often unable to consume sufficient energy and nutrients from an oral diet and require supplemental non‐oral feeding options, such as nasogastric tube feeding or, in severe cases, a gastrostomy.
Respiratory compromise
Oropharyngeal dysphagia puts a child at risk of prandial aspiration (where food or fluid is misdirected from the typical path from pharynx to oesophagus and enters the trachea and lungs instead), as well as choking and increased work of breathing during feeding. Respiratory complications, such as chest infection or pneumonia, may subsequently arise from oropharyngeal dysphagia owing to the presence of aspiration (Loughlin 1989; Arvedson 1994). Children may be required to have a modified diet in an effort to compensate for their feeding difficulties and avoid aspiration. In severe cases of aspiration (that is, where children develop respiratory compromise such as chest infections or pneumonia associated with prandial aspiration), children may be required to use non‐oral methods of feeding such as nasogastric tube feeding or gastrostomy.
Social impacts
Beyond the direct medical impacts of oropharyngeal dysphagia, there are other significant life impacts of the disorder. Dysphagia has impacts on a child’s ability to participate in daily food‐related activities. For example, in the case of a 15‐year‐old girl who returned to school with persistent dysphagia and risk of aspiration one year after a traumatic brain injury; the adolescent remained socially isolated from peers during her lunch break because she needed to receive non‐oral feeds (via gastrostomy) from the school nurse (Morgan 2004). Further social impacts can be seen in relation to mealtime interactions for children with dysphagia and their families. One study reported on the characteristics of mealtime communication between 20 mothers and their children with cerebral palsy (Veness 2008). In contrast to the positive mealtime communication behaviours typically observed in children without feeding impairments, mothers of children with dysphagia and cerebral palsy were found to dominate the mealtime interactions and used more directive communicative functions than their children (Veness 2008). While mealtimes are typically an enjoyable time for socialisation within the family unit, they are often a stressful occasion for the child and family affected by dysphagia.
Description of the intervention
The focus of this review was to examine the effectiveness of interventions for oropharyngeal dysphagia in children with neurological impairment. We examined oropharyngeal dysphagia treatment in any setting at any frequency or duration. The comparison was standard management, defined in this context as the 'normal' care given to children with oropharyngeal dysphagia (for example, support during mealtimes by a carer or therapy aide). The nature of available interventions can be conceptualised as direct, indirect and compensatory. This categorisation of feeding interventions is based on adult dysphagia literature, in the absence of any well‐defined current paediatric dysphagia intervention models. These intervention approaches are largely based on improving the oropharyngeal impairment. By targeting treatments at the impairment level, it is anticipated that participants will also experience associated improvements in levels of activity and participation (WHO 2001) associated with swallowing or oropharyngeal feeding success.
Direct interventions
Direct interventions use food or fluid during swallowing tasks to target the physiological limitations or impairments (WHO 2001) associated with oropharyngeal dysphagia across the oral phase of swallowing (for example, poor lip closure or reduced mobility of the tongue for propelling food posteriorly into the oropharynx to trigger a swallow) and the pharyngeal phase (for example, inadequate laryngeal elevation and airway protection, resulting in aspiration of food and fluid into the trachea, or inadequate pharyngeal peristalsis resulting in excessive pooling of food or fluid in the valleculae or pyriform sinuses). A range of impairment‐level direct intervention methods are available, and we have provided examples of each below.
Motor with swallow: for example, specific movement‐based techniques such as the supraglottic swallow (for example, Logemann 1991; Logemann 1993; Ohmae 1996) or Mendelsohn manoeuvre (for example, Cook 1989; Huckabee 1999).
Sensory with swallow: for example, altering bolus taste or flavour to make it sour or sweet to increase sensory input, increasing or decreasing the temperature of a food or fluid to increase sensory input and improve swallow physiology (Lazarus 1993; Logemann 1995).
Indirect interventions
In contrast to the direct interventions described above, indirect interventions are not based around the use of food or fluid. Similarly to direct interventions, however, indirect interventions also work on targeting the underlying physiological limitation or impairment associated with oropharyngeal dysphagia.
Motor without swallow: for example, exercises to increase oral motor function such as using the Iowa Oral Pressure Instrument to increase tongue strength and function with eventual improvements seen in swallow physiology (for example, Robbins 2007).
Sensory without swallow: for example, techniques of applying thermal tactile stimulation such as icing the faucial arches in an attempt to increase sensation to this region with eventual improvements seen in swallow physiology (Lazzara 1986).
Pharmacological/surgical: for example, interventions such as intrathecal baclofen or botox to increase or decrease tone to enable more functional oropharyngeal swallowing physiology.
Compensatory techniques
Compensatory techniques are based around improving activity limitations and participation restrictions, or removing environmental barriers to enhance oropharyngeal feeding success (WHO 2001). Examples of compensatory techniques include the following.
Postural modifications (for example, altering the child’s seating position to facilitate optimal trunk and body stability to effect improvement of function and control of the oropharyngeal musculature and hence improve swallow physiology) (Larnert 1995).
Products and technology: altering feeding utensils or seating systems to facilitate swallowing (Chadwick 2003; Redstone 2004).
Natural environment: experimentally altering the level of temperature, light or noise in the feeding environment to facilitate swallowing (WHO 2001).
Support networks: altering the level of external support required by children to facilitate swallowing (Ball 2012; Jones 2012).
How the intervention might work
See above.
Why it is important to do this review
As outlined earlier, oropharyngeal dysphagia may have deleterious impacts on health and quality of life for children with neurological impairment. Despite the high rate of dysphagia prevalence in children with marked neurological impairment, there has been a lack of systematic examination of the literature in this field. We intend, therefore, to summarise whatever high‐quality evidence is available about the effectiveness of various interventions for oropharyngeal dysphagia. Speech pathologists and occupational therapists most commonly manage children with dysphagia; however, the lack of evidence in this field has broader implications across a range of health professionals, including medical officers, physiotherapists and dieticians. Further, the prevalence of dysphagia and lack of consensus on optimal interventions results in negative health economic impacts and places pressure on resources. It is, therefore, timely to undertake the present Cochrane review to examine current literature systematically and to encourage funding bodies and clinical researchers to address this striking evidence gap.
Objectives
To examine the effectiveness of interventions for oropharyngeal dysphagia in children with neurological impairment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐randomised trials (for example, where participants are allocated by date of birth, day of the week or alternate allocation) were considered for the review.
Types of participants
Participants for inclusion were children aged under 18 years, presenting with dysphagia of acquired (for example, following traumatic brain injury or stroke), developmental (for example, cerebral palsy), degenerative (for example, Friedrich's ataxia, myotonic dystrophy), or genetic origin (for example, Down syndrome, CHARGE syndrome, Rett syndrome), as diagnosed by a medical officer. We included children with early structural deficits, including oesophageal atresia or tracheo‐oesophageal fistulas, provided they had a coinciding oropharyngeal dysphagia of neurological origin. An age limit of 18 years was chosen because it denotes the upper age limit of paediatric care for the majority of healthcare providers internationally and, as such, it was anticipated that most paediatric‐focused studies would include children of up to 18 years. In fact, some of the studies defined 'child' as up to 21 years of age and so studies about children that included a few participants up to 21 years of age were not excluded. No lower age limit was applied because children can experience difficulties with feeding and swallowing skills from birth.
Types of interventions
Direct and indirect interventions for oropharyngeal dysphagia (see Description of the intervention) targeting impairment, activity or participation levels (WHO 2001) and environmental factors were considered. Intervention studies with drooling as the only dependent variable without measuring oropharyngeal dysphagia as a primary outcome were excluded from the review.
Types of outcome measures
Primary outcomes
The following primary outcomes were considered:
the physiological function of the oropharyngeal mechanism for swallowing as determined through clinical swallowing evaluation (CSE, for example, lip seal, tongue movement, jaw rhythmicity), endoscopic swallowing examination (ESE, for example reduced vocal fold closure) or videofluoroscopic swallowing examination (VFSE, for example reduced pharyngeal peristalsis);
presence of a history of confirmed aspiration pneumonia or recurrent chest infection with or without pneumonia with suspected prandial aspiration aetiology;
diet consistency a child is able to consume, as a possible indicator of oral and pharyngeal skills (that is, whether the child can manage a developmentally appropriate oral diet; whether the texture/consistency of foods and fluids must be modified; whether supplementary feeding is required, such as nasogastric or gastrostomy tube feeding).
Secondary outcomes
The following secondary outcomes were considered:
changes in growth: weight and height percentiles; growth velocity;
child's level of participation in mealtime routine with family, peers or strangers;
level of parent or carer stress associated with feeding.
The following were deemed appropriate for recording primary and secondary outcomes: medical chart review, questionnaires, rating scales, checklists or interviews with a relevant carer or health professional, including parent, carer, speech pathologist, medical officer or teacher. Owing to variance in quality of reporting, we considered all measures but discussed evidence of their reliability and validity. Where studies retrospectively used medical records to determine outcomes, we considered these studies individually.
We intended to group outcome time points for primary and secondary outcome measures as follows: immediately post‐intervention, up to six months post‐intervention and more than six months post‐intervention. It is difficult to anticipate the length of time to follow‐up post‐intervention across studies and hence we altered time points accordingly to best represent follow‐up periods across studies.
Search methods for identification of studies
This review is based on studies identified by searches run in October 2011. We used a sensitive search strategy with a filter to identify randomised trials, but did not limit by publication date or language.
Electronic searches
We searched the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL) 2011 Issue 3, part of The Cochrane Library, searched 7 October 2011
Ovid MEDLINE 1948 to September Week 4 2011, searched 10 October 2011
EMBASE 1980 to 2011 Week 40, searched 10 October 2011
CINAHL 1937 to current, searched 10 October 2011
ERIC 1966 to current, searched 10 October 2011
PsycINFO 1806 to Oct Week 1 2011, searched 10 October 2011
Science Citation Index 1970 to 7 October 2011, searched 10 October 2011
Social Science Citation Index (1970 to 7 October 2011), searched 10 October 2011
Cochrane Database of Systematic Reviews (CDSR) 2011, Issue 3, part of The Cochrane Library, searched 7 October 2011
Database of Abstracts Reviews of Effects (DARE) 2011, Issue 3, part of The Cochrane Library, searched 7 October 2011
Current Controlled Trials (ISRCTN Register), searched 15 October 2011
ClinicalTrials.gov, searched 15 October 2011
WHO International Clinical Trials Registry Portal (ICTRP), searched 15 October 2011
Networked Digital Library of Theses and Dissertations (NDLTD), searched 11 October 2011
Australasian Digital Theses Program (via TROVE), searched 11 October 2011
DART‐Europe E‐theses Portal (DART), searched 11 October 2011
The search strategies for each database are in Appendix 1.
Searching other resources
Grey literature
We sought to identify any unpublished and ongoing trials by searching Current Controlled Trials, ClinicalTrials.gov, and WHO ICTRP. In addition, we searched for dissertations and theses using NDLTD, Australasian Digital Theses Program, and DART.
Handsearching
Handsearches of the journals listed below were conducted by review author AM for relevant trials from 2000 to October 2011.
Developmental Medicine and Child Neurology
Dysphagia
Journal of Speech, Language and Hearing Research
We undertook further handsearches of the reference lists of studies included in this review and relevant papers to identify any additional studies in the published or unpublished literature.
Data collection and analysis
Selection of studies
All three review authors (AM, PD and EW) independently screened titles and abstracts for inclusion and discussed results. In cases of uncertainty over whether an abstract met inclusion criterion, authors obtained the full‐text article and independently evaluated each paper for inclusion. In the event of disagreement over inclusion, authors formed a consensus by re‐evaluating the inclusion criterion together. Additional information was sought from authors of the original three studies that met inclusion criteria (Ottenbacher 1981; Gisel 1996b; Sjogreen 2010) by review author AM, to resolve questions about study methodology that informed the 'Risk of bias' assessment. We recorded the reasons for excluding studies. No review author was blind to the authors, institutions or the journals of publication of the articles.
Data extraction and management
One review author (AM) used a data extraction form to collect information about the population, intervention, randomisation methods, blinding, sample size, outcome measures, follow‐up duration, setting, attrition, and handling of missing data, and methods of analysis. Review authors PM and EW both checked AM's data extraction. Items of disagreement were re‐assessed and a consensus reached.
Assessment of risk of bias in included studies
All three review authors (AM, PD, and EW) independently assessed the risk of bias of included studies using The Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011). We resolved disagreements by discussion until consensus was reached. The tool was used to assess the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias (for example, ceasing the trial early, changing methods during the trial).
The quality of included studies was presented in a 'Risk of bias' table where, for each question‐based entry, a judgement of ‘low risk’, ‘high risk’ or ‘unclear risk’ of bias was made by the review authors, followed by a text box providing details on the available information that led to each judgement. The following sources of bias were assessed. (See 'Risk of bias' tables in Characteristics of included studies.)
Random sequence generation
We judged randomisation as follows.
'Low risk' when participants were allocated to treatment conditions using randomisation, such as computer‐generated random numbers, a random numbers table, or coin‐tossing.
'Unclear risk' when the randomisation method was not clearly stated or unknown.
'High risk' when randomisation did not use any of the above methods.
Allocation concealment
We judged allocation concealment as follows.
'Low risk' when participants and researchers were unaware of participants' future allocation to treatment condition until after decisions about eligibility were made and informed consent was obtained.
'Unclear risk' when allocation concealment was not clearly stated or unknown.
'High risk' when allocation was not concealed from either participants before informed consent or from researchers before decisions about inclusion were made, or allocation concealment was not used.
Blinding of participants, personnel and outcome assessors
We determined quality of participant, personnel and outcome assessor blinding by whether knowledge of the allocated interventions was adequately concealed from these people during the study, by using the following judgements.
'Low risk' when participants, personnel and outcome assessors were blind to the treatment conditions and it was unlikely that the blinding could have been broken; where either participants or some key study personal were not blinded but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
'Unclear risk' when blinding of assessors was not reported and information was not available from researchers.
'High risk' when no blinding or incomplete blinding occurred and the outcome or outcome measurement was likely to be influenced by lack of blinding, or where blinding was attempted but could have been broken.
Incomplete outcome data
Assessment took into account whether incomplete outcome data were adequately addressed by the researchers. The corresponding author of included studies was contacted and asked to provide any data that had not been reported (for example, individual data from participants at time points where outcome data were reportedly as collected, but were not described in the results). Other authors were contacted in instances where a corresponding author failed to respond. Where a study reported outcomes only for participants completing the trial, or only for participants who followed the protocol, we contacted the authors and asked them to provide additional information. We described missing data and drop‐outs/attrition for each included study in the 'Risk of bias' table, and interpreted what effect the missing data may have had on the results and conclusions of the review. We intended to conduct sensitivity assessment of any primary meta‐analyses to missing data using the methodology outlined by Higgins 2011, but no primary meta‐analyses were possible.
We assessed the adequacy of the way trials dealt with missing data using the following judgements.
'Low risk' when there were no missing outcome data, or reasons for missing outcome data were unlikely to be related to true outcome, or where missing outcome data were balanced in numbers across groups with similar reasons for missing data across groups, or where missing data had been imputed using appropriate methods.
'Unclear risk' when information about missing data was not available and could not be acquired by contacting the researchers of the study.
'High risk' when the reason for missing outcome data was likely to be related to the true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
Selective reporting
We assessed the possibility of selective outcome reporting by review authors' judgement on whether reports of the study were free of the suggestion of selective outcome reporting; for example, whether it was clear that other data were collected and not reported.
Other bias
Assessment of the methodology also determined whether any other bias was present in the trial, such as stopping the trial early, changing methods during the trial or other anomalies.
Measures of treatment effect
Given the inability to complete a meta‐analysis here (see Results), Appendix 2 contains methods to be used in any update of this review.
Unit of analysis issues
See Appendix 2 for methods to be used in any update of this review.
Dealing with missing data
We assessed missing data and drop‐outs in the included studies. We investigated and reported reasons, numbers and characteristics of drop‐outs. We attempted to contact the authors when further information or data were necessary. While not applicable in the current version of this systematic review, we intended to conduct any meta‐analyses using data from all original participants when possible and report when that was not the case. For studies in which the missing data were not available, we intended to use a sensitivity analysis to assess potential bias in the analysis and discuss the extent to which the results might be biased by missing data.
Assessment of heterogeneity
See Appendix 2.
Assessment of reporting biases
See Appendix 2.
Data synthesis
See Appendix 2.
Subgroup analysis and investigation of heterogeneity
See Appendix 2.
Sensitivity analysis
See Appendix 2.
Results
Description of studies
Results of the search
The search conducted up until October 2011 identified a total of 2515 citations from the database searches, after duplicates were removed. A further 20 dissertation records were obtained through searching the open‐access databases as outlined earlier in the search methods of the review. On the basis of the titles and abstracts, 10 papers were judged to potentially meet the inclusion criterion and the full‐text versions were obtained. Papers were excluded on the basis of not including the correct participant group (for example, participants older than 18 years of age, participants without neurological impairment, participants with oral structural deficits without comorbid oropharyngeal dysphagia) or if they were not intervention studies (for example, theoretical reviews of diagnostic features for dysphagia). A further eight papers were identified by examining the reference list of other published studies. Thus, in total, 18 full‐text articles were obtained and 15 were excluded following evaluation (see Characteristics of excluded studies); thus, three studies met inclusion criteria. A flow diagram of the study selection process is presented in Figure 1.
1.
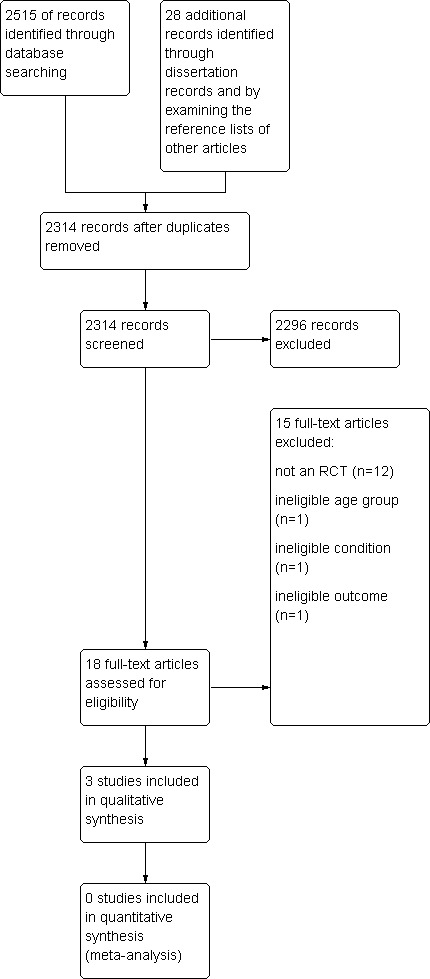
Study flow diagram
Included studies
See Characteristics of included studies.
Study design
All three studies were RCTs, and one of the studies included a counter‐balanced RCT design (Sjogreen 2010).
Setting
Two of the studies were based in Canada (Ottenbacher 1981; Gisel 1996b), with one set in a special school (Gisel 1996b) and one in a state‐based institution for people with intellectual disability (Ottenbacher 1981). The third study was based in Sweden and conducted in the child's school setting or in the child's home (Sjogreen 2010).
Participants
Children and adolescents with a diagnosis of cerebral palsy were included in two studies (Ottenbacher 1981; Gisel 1996b). Severity of involvement of motor impairment or intellectual ability associated with cerebral palsy was varied across the studies, but children generally presented with deficits on the more severe end of the continuum of impairment (moderate to severe in Gisel 1996b and severe to profound in Ottenbacher 1981). Thirty‐five children (aged 4 years 3 months to 13 years 3 months; 19 males) took part in the study by Gisel 1996b; 27 were wheelchair‐bound, five were ambulatory and three used tricycles for ambulation. Most children were quadriplegic, with variable upper and lower limb spasticity and varying involvement of the left and right sides of the body across the group. All children needed assistance with activities of daily living, including eating, and had a range of hypotonicity and hypertonicity in their trunk and extremities. Similarly, all 20 participants (aged 5 to 21.6 years; 12 males) in the study by Ottenbacher 1981 were dependent on most areas of self‐care, including feeding. The majority of children in the Ottenbacher 1981 study (18 children) had a diagnosis of cerebral palsy (11 spastic quadriplegic, two athetoid, five mixed), but two participants had a non‐specified neuromotor and intellectual impairment. A different population of children were included in the Sjogreen 2010 study, specifically eight participants aged seven to 19 years with a diagnosis of myotonic dystrophy type 1 with onset congenitally or in childhood.
Interventions
An oral sensorimotor programme was used in two studies (Ottenbacher 1981; Gisel 1996b). However, different approaches to oral sensorimotor treatment were taken, with Gisel 1996b targeting oral motor outcomes of improved tongue lateralisation, lip control and vigour of chewing (group A) or chewing only (group B) and Ottenbacher 1981 focusing on (i) inhibition of abnormal oral and postural reflexes, (ii) facilitation of normal muscle tone and (iii) desensitisation of the oral region. The third study (Sjogreen 2010) focused on lip strengthening using a pre‐fabricated 'oral screen'. This screen was placed inside the lips, in front of the teeth, and required the participant either to use it actively (try to keep the oral screen inside the lips while someone pulled at the screen as though trying to remove it) or passively (where the oral screen remained inside closed lips so that the participant experienced the sensation of having a closed mouth and relying on nose rather than mouth breathing). Food stimuli were used in the Gisel 1996b study, yet a lack of detail was provided for the Ottenbacher 1981 study making it difficult to ascertain the exact nature of treatment.
Treatment intensity was similar, with all three studies employing treatment five days per week. However, treatment delivery differed in relation to the duration of daily therapy and in the time course of the entire treatment (that is, five to seven minutes per day, five days a week for 20 weeks for both groups A and B (Gisel 1996b); 30 to 40 minutes per day, five days per week for nine weeks (Ottenbacher 1981); 16 minutes per day, five days per week for 16 weeks (Sjogreen 2010)). The counter‐balanced design of the Sjogreen 2010 study had an intensity of five days per week for 16‐minute sessions with each group receiving 16 weeks of treatment and 16 weeks of their normal routine.
Both oral sensorimotor studies included control comparison groups who received their regular programme of school feeding; however, Gisel 1996b included 10 weeks of this control condition and then 10 weeks of the sensorimotor treatment as per the main experimental group.
Outcome measures
Outcomes were measured at baseline and immediately post‐treatment across all studies, with no further post‐treatment follow‐up. Two studies also conducted additional outcome assessments at the mid‐point of treatment (that is, at 10 weeks of a 20‐week programme; Gisel 1996b) or quarterly (every four weeks of a 16‐week programme; Sjogreen 2010).
Oral sensorimotor treatment
Primary outcome measures as part of a clinical swallowing examination for Gisel 1996b included: (i) eating time (seconds) for three standard textures of food (puree, viscous, solid), (ii) clearing time (time period of 'after swallows' in seconds) for the three standard textures of food, (iii) duration of mealtime for lunch at school (minutes) and (iv) texture changes in lunch meals (for example, notation of the textures able to be consumed at lunch). Primary outcome measures for Ottenbacher 1981 included (i) rating six items of oral‐motor functions and four oral‐motor reflexes, as well as the position of the tongue at rest and the degree of drooling, and (ii) 21 items from the 47‐item Vulpe Assessment Battery, selected because they covered the level of feeding behaviour appropriate to the participants under investigation. All primary measures in the Ottenbacher 1981 study were rated on a four‐point ordinal scale. No further details of these items were provided by the authors but they did provide references to the original source of the assessment battery. The oral sensorimotor treatment studies shared secondary outcome measures of weight (Ottenbacher 1981; Gisel 1996b).
Lip strengthening treatment
Primary outcome measures relevant to oropharyngeal dysphagia in the Sjogreen 2010 lip strengthening intervention study of children with myotonic dystrophy type 1 included: (i) lip closure force and endurance measured with a calibrated lip force meter; (ii) lip mobility measured using three‐dimensional (3D) motion analysis and (iii) eating and saliva control questionnaire. See Characteristics of included studies for further detail on specific measures taken at particular time points in Sjogreen 2010.
Further considerations
As outlined further in the Characteristics of included studies table, the control group (group C) in Gisel 1996b received no therapy for 10 weeks and oral sensorimotor therapy for 10 weeks. Given the lack of a control group for the full 20‐week duration of the treatment programme, it is not possible to make a comparison between control and experimental groups beyond the 10‐week mid‐point of the study.
Replication of the Ottenbacher 1981 study would be challenging because treatments were reported to vary according to the individual oral‐motor assessments and profile of the child, and no treatment protocol detailing the exact procedures followed was provided. A description of the 'three major components' of the treatment programme was provided, but only general treatment principles were included. Further, given that participant's treatment programmes were tailored to their initial oral‐motor assessments, there is a potential risk of variability in treatment protocol within the experimental group.
All three studies included in the review are at risk of selection bias given the very small sample size of participants per intervention group.
Excluded studies
Fifteen studies were excluded following evaluation (see Characteristics of excluded studies). Twelve studies were excluded on the basis of design as they were not RCTs (Ottenbacher 1983; Sobsey 1984; Ganz 1987; Iammatteo 1990; Gisel 1996a; Pinnington 1999; Pinnington 2000; Gisel 2001; Haberfellner 2001; DeMatteo 2002; Alacam 2007; Christiaanse 2011). Detailed descriptions of these 12 studies are provided in Characteristics of excluded studies. One study was excluded for not including original experimental treatment data (that is, it was a review of intervention approaches) (Arvedson 2010). One paper was excluded as it focused on adults (Kiger 2006). The remaining paper was excluded as participants did not have oropharyngeal dysphagia of neurological origin (Korbmacher 2004).
Risk of bias in included studies
See Figure 2, Characteristics of included studies and 'Risk of bias' tables.
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Two of the three included studies had low risk of selection bias (Gisel 1996b; Sjogreen 2010). Authors of these studies used randomisation processes involving participants either having to select a piece of paper from a hat to reveal whether they were taking part in treatment A or B (Sjogreen 2010) or used random number generator tables (Gisel 1996b) to randomise participants to treatment groups. While neither author had stated these methods in their original manuscripts, both provided this detail when contacted via email. Further, both studies concealed the allocation of treatment group from participants and their carers until after consent had been obtained (Gisel 1996b; Sjogreen 2010).
There is unclear risk of selection bias in the study by Ottenbacher 1981, as no details are provided about the presence or absence of random sequence generation or allocation concealment in their paper, and the authors did not respond when contacted.
Blinding
The risk of bias owing to participants and personnel being aware of who was receiving intervention was inevitably high in all studies as it was not possible for these individuals to be blinded to the treatment once intervention occurred given the very direct implementation nature of feeding interventions (for example, administering personnel cannot be blinded to the type of food texture a child is receiving, neither can a child be blinded to the consistency of food they are consuming).
Otherwise, the study by Gisel 1996b had a low risk of performance or detection bias, as the outcome assessor was blinded to treatment group allocation throughout the study. Risk was unclear in this respect for the study by Ottenbacher 1981, because there was no explicit detail regarding the blinding process for outcome assessors. No response was obtained when this author was contacted via email for clarification.
The use of a parent questionnaire as an outcome measure pre‐ and post‐therapy in the Sjogreen 2010 study resulted in high performance and detection bias (that is, high risk rating for blinding of outcome assessors). However, this study also included one speech language pathologist outcome assessor who was blinded for the duration of the study, and two further outcome assessors who were not blinded, but who were collecting objective measures (for example, lip force, grip force).
Incomplete outcome data
Issues with missing data were detailed in all three studies. There was a low risk of attrition bias in the Gisel 1996b study, with one child (no. 12 in group A) dying owing to causes unrelated to the study and, as such, her data were removed from the study. No other evidence of attrition bias was present in Gisel 1996b. There was no evidence of missing data for the eating‐ or feeding‐related scales in the Sjogreen 2010 study.
There was high risk of incomplete outcome data in the Ottenbacher 1981 study. Specifically, the authors reported that owing to 'staffing changes in the institution' not all participants received post‐therapy oral‐motor evaluation (Ottenbacher 1981). Nine of 10 children in the experimental group and only two of 10 children in the control group had post‐therapy evaluations of oral‐motor function.
Selective reporting
There was no evidence of selective reporting bias in any of the three included studies.
Other potential sources of bias
Similarities at baseline
There were significant differences in weight between the treatment and control groups pre‐therapy in the study by Ottenbacher 1981 (participants in the control group had a higher average weight). Further, aside from age and weight, relatively limited details were provided on participant characteristics in Ottenbacher 1981 and the few descriptors included, such as the sub‐types of cerebral palsy, were provided for the control and experimental groups as a whole, making it difficult to make comparisons and interpret the baseline characteristics of experimental versus control groups. There was no discussion of the differences or similarities of the two counter‐balanced groups in Sjogreen 2010, yet the age ranges varied between the two groups (7 to 19 years in Group A; 11 to 17 years in Group B). There were no apparent differences between experimental or control groups in Gisel 1996b.
Effects of interventions
A meta‐analysis was not possible across the three studies owing to significant differences in study methodology (that is, different types of populations, different treatment approaches and highly varied outcome measures across studies). As a result, a narrative summary of the effects of the three intervention studies is provided below.
Oral sensorimotor treatment
Two studies assessed this treatment (Ottenbacher 1981; Gisel 1996b) versus standard care.
Primary outcomes
Physiological function of the oropharyngeal mechanism for swallowing
Data on aspects of this outcome were from a clinical swallowing evaluation in Gisel 1996b and Ottenbacher 1981. In the Gisel 1996b study, no statistically significant differences were reported between the experimental groups (A and B) and the control group (C) from baseline (week 0) to weeks 10 or 20 on measures of eating time, clearing time (after swallows) or duration of mealtimes. Similarly, no statistically significant change in oral‐motor function (t = 1.09; P > 0.1; degrees of freedom (df) = 16) was reported post‐treatment for the experimental group in the Ottenbacher 1981 study. No pre‐ and post‐therapy comparison was possible in the control group because post‐therapy evaluations were only available for two of 10 control participants.
Diet consistency child able to consume
In Gisel 1996b, there were no significant differences found for any group on the ability to advance to a more solid texture from baseline to weeks 10 or 20. However, it was difficult to interpret these findings because the control group received no treatment up until week 10, but then received the oral sensorimotor treatment for weeks 10 to 20. As such, because there was no control group after week 10, comparisons can only reliably be made between experimental groups A and B versus control group C up to week 10 of the 20‐week intervention programme. No findings about diet consistency were reported in Ottenbacher 1981.
Presence of a history of confirmed aspiration pneumonia or recurrent chest infection with or without pneumonia with suspected prandial aspiration aetiology
Neither Gisel 1996b nor Ottenbacher 1981 reported on the presence of a history of confirmed aspiration pneumonia or recurrent chest infection with or without pneumonia with suspected prandial aspiration aetiology.
Secondary outcomes
Change in growth (including height and weight percentiles or growth trajectory)
Of these, only weight was reported in the two studies. No statistically significant differences were found between participants in the experimental and control groups in Gisel 1996b and individuals in both groups were found to maintain their weight‐for‐age percentile, at the lower end of expected norms. Some individual participants in the experimental group (six children) in the Ottenbacher 1981 study evidenced weight gains over the treatment period, but three evidenced weight loss. Similarly in the control group, five evidenced weight gains and three evidenced weight loss. Overall, no statistically significant change in weight was found post‐treatment for either the experimental or control groups in Ottenbacher 1981 (after adjustments were made for differences in pre‐therapy weights, see Risk of bias in included studies tables).
Child's level of participation in mealtime routine with family, peers or strangers
Neither Gisel 1996b nor Ottenbacher 1981 reported on this outcome.
Level of parent or carer stress associated with feeding
Neither Gisel 1996b nor Ottenbacher 1981 reported on this outcome.
Lip strengthening treatment
One study assessed this treatment (Sjogreen 2010) versus a no treatment control group.
Primary outcomes
Physiological function of the oropharyngeal mechanism for swallowing
Sjogreen 2010 reported highly variable intra‐ and inter‐individual outcomes for eating and drinking skills following the lip strengthening intervention for children with myotonic dystrophy. The authors suggested the variability in outcome could be related to general health and fatigue/alertness. It is difficult to interpret the significance of the findings of this study because of performance (specifically detection) bias (see Risk of bias in included studies) and because only a very small sample of the original participant group could be included for comparison (that is, only four out of eight children, two in the experimental and two in the control group had eating and drinking difficulties at baseline).
Neither of the other two primary outcomes of interest in this review (that is, diet consistency a child is able to consume or presence of a history of confirmed aspiration pneumonia or recurrent chest infection with or without pneumonia with suspected prandial aspiration aetiology) were reported by Sjogreen 2010.
Secondary outcomes
None of our secondary outcomes (changes in growth; child's level of participation in mealtime routine with family, peers or strangers; level of parent or carer stress associated with feeding) were reported by Sjogreen 2010.
Discussion
Summary of main results
Three studies met inclusion criterion for the review. Two studies were based primarily on oral sensorimotor interventions for participants with cerebral palsy (Ottenbacher 1981; Gisel 1996b) and a third study trialled lip strengthening exercises for children with myotonic dystrophy type 1 (Sjogreen 2010). A meta‐analysis combining results across the three studies was not possible because one of the studies had participants with a different condition (Sjogreen 2010), and the remaining two, although using oral sensorimotor treatments, used vastly different approaches with different intensities and durations. This decision was in line with our protocol. Narrative summaries are provided for each of the three studies individually. It is not possible to reach definitive conclusions on the effectiveness of particular interventions for oropharyngeal dysphagia based on these studies. The Ottenbacher 1981 study had a high risk of attrition bias owing to missing data, had statistically significant differences (in weight) across experimental and control groups at baseline and did not describe other aspects of the trial sufficiently to enable assessment of other potential risks of bias. The Sjogreen 2010 study was at high risk of detection bias as some outcomes were assessed by parents who were not blinded to whether their child was in the intervention or control group. The Gisel 1996b study was consistently assessed as at low risk of bias, but like the other two studies, had a small sample size.
Overall completeness and applicability of evidence
Only three RCTs have been conducted in this field, and the quality of this evidence is limited by biases and limitations in study methodology. To date, the available evidence for treatment of oropharyngeal dysphagia in children with neurological impairment is incomplete. A number of reasons may explain the paucity of research in this field. The first is the complex nature of oropharyngeal dysphagia and the child's underlying neurological impairment, which produce heterogeneous and highly individualised patterns of swallowing difficulty. As a result, clinicians working in this field typically apply a highly individualised approach to patient care and may place less value on treatment approaches that are standardised for application across groups of individuals, such as commonly applied in an RCT. Heterogeneity also results in small subgroups of participants and thus makes recruitment of sufficient numbers to produce appropriately powered studies difficult. A further possible discouraging feature for conducting treatment studies in this field is that it is commonplace for all patients to receive speech pathology input for dysphagia management where required. Thus, rather than being viewed as a less active phase, a no treatment condition is viewed as the withdrawal of services from a patient. It is therefore seen as unethical to apply treatment versus no treatment methodological approaches in intervention trials for dysphagia, making RCT studies inherently more difficult to design. Admittedly, this is a problematic issue common across the paediatric field and is not specific to this particular area. It is also important to note that all of the included studies focused on oral preparatory or oral phase impairments without addressing pharyngeal level deficits. The lack of treatment focus on the pharyngeal phase is potentially owing to goals of the individual studies, as much as to the rationale and limitations associated with the use of diagnostic instrumentation needed to delineate pharyngeal function such as ESE or VFSE, see Diagnosis of oropharyngeal dysphagia section under Description of the condition.
In terms of clinical relevance and applicability of the evidence, there were positive signs regarding the evidence building in this field in regard to the types of participants enrolled in trials, the types of interventions employed, and the setting and duration for the intervention. Specifically, two of the existing RCTs were focused on the group most likely to experience oropharyngeal dysphagia associated with neurological impairment, that is, children with cerebral palsy. It was also encouraging that both studies employed an oral sensorimotor intervention, which is arguably the most commonly applied treatment approach in contemporary clinical settings. The counter‐balanced RCT by Sjogreen 2010 was the first of its kind to explore the efficacy of an oral screen device for managing oropharyngeal dysphagia. While use of this particular tool remains rare in clinical settings, the premise behind the tool (that it can improve lip function) is also in line with commonly applied treatment targets. It was also positive to see that all three studies employed therapy in the child's naturalistic setting wherever possible, for example, treatment occurring before or during regular school mealtime routines. The daily length of therapy in Gisel 1996b and Ottenbacher 1981 was in line with standard clinical practice in that sessions are typically between 30 to 45 minutes. In terms of the ongoing duration of treatment, studies varied between nine and 20 weeks. Again, this length of time could be viewed as complementary to typical treatment models in schools, outpatient health services or community health settings, where a set 'block' of therapy is typically provided, with a set number of weeks offered, which may also coincide with a school term, for example.
One particular methodological aspect of the treatments applied in the existing RCTs may be less transferable to clinical settings, namely intensity of practice. There is an increasing trend for RCTs in speech pathology to deliver treatment 'intensively' (for example, see further discussion in Cherney 2012; Packman 2012; Williams 2012), and typically daily, as seen across the three studies discussed here. Unfortunately, the majority of clinical settings would not easily deliver an intensive model of intervention. Most clinical speech pathology or occupational therapy services in countries such as Australia and the UK, employ a once per week or once per two weeks model of intervention. A cyclical problem arises here. Specifically, intensive daily practice is theoretically reported to be an optimal principle of neuroplasticity (Kleim 2008), yet there is a lack of high‐quality empirical evidence demonstrating the need for intensive therapy within this particular field. In turn, a lack of existing clinical services that could easily accommodate a research trial of intensive daily intervention makes it difficult to generate the evidence to support a significant change in service provision models.
Quality of the evidence
There are a growing number of studies examining the efficacy of interventions for oropharyngeal dysphagia in children with neurological impairment (that is, three studies between 1980 and 1989; four between 1990 and 1999, and 10 from 2000 to 2011). Despite the increasing quantity, there is still a lack in quality. The 15 studies excluded from this review largely on the basis of not being randomised intervention trials. Of the three RCTs available, two of the studies were at high risk of bias including biases of missing data, selective outcome reporting and performance bias (lack of blinding of outcome assessors) (Ottenbacher 1981; Sjogreen 2010). All three studies contained very limited sample sizes (n = 12 per group in Gisel 1996b; n = 10 per group in Ottenbacher 1981; n = 4 per group in Sjogreen 2010), making it difficult to generalise any of the findings to a broader population. Moreover, with such small samples, although the participants are allocated randomly, the individuals in each arm could differ in many characteristics. It was also noticeable that none of the studies included a follow‐up evaluation or longer‐term assessment to determine maintenance post‐treatment. The quality of the current evidence for treatment of oropharyngeal dysphagia is poor.
Potential biases in the review process
There are no known potential biases in this review process. This review searched for related published and unpublished data (including ongoing studies) in this field. We also included studies in languages other than English in our search.
Agreements and disagreements with other studies or reviews
To our knowledge there has been no systematic review of the effectiveness of interventions for oropharyngeal dysphagia in children with neurological impairment and, hence, the review authors were unable to compare findings of the present study with other reviews at this time.
Authors' conclusions
Implications for practice.
The review demonstrates that there is currently not enough high‐quality evidence from RCTs to provide conclusive results about the effectiveness of any particular type of therapy for children with oropharyngeal dysphagia associated with neurological impairment. No clear guidelines or recommendations for clinical practice with this population of children can be made until higher‐quality evidence has been generated.
Implications for research.
There is a critical need for higher‐quality, appropriately statistically powered, RCTs to evaluate the efficacy of interventions for oropharyngeal dysphagia. There are a number of further areas to be carefully addressed in future research studies, as outlined below.
Improvements in methodological design. Few RCTs have been conducted in this field. Future studies should be carefully designed following CONSORT (Consolidated Standards of Reporting Trials) guidelines with particular attention paid to avoiding potential risk of bias.
There is a need for more explicit detail regarding the inclusion and exclusion criteria of study participants and the specific approaches and steps to intervention, that is, detail to a level that would enable exact replication.
Future studies should provide greater detail on the characteristics of study participants to: (i) enable a direct comparison of group characteristics at baseline to interpret similarities or differences between treatment groups at the commencement of the trial, and (ii) enable results to be more easily interpreted in terms of their generalisability back to a broader population of individuals with those same features.
There is a need for longer‐term evaluation of intervention outcomes (for example, three, six, 12 months) to determine whether there is any maintenance of change post‐therapy.
Future studies should include consideration of broader outcome measurements, for example, how did the treatment impact on health‐related quality of life for the individual participant or their family/carer?
-
There are numerous variables regarding the mode, setting, intensity and duration of treatment that are yet to be explored.
Mode of delivery. There is a need to explore new service delivery models (for example, group versus individual treatment) that may enhance access to therapy services for patients and their families. Telehealth is one such option that has not yet been explored in this group, which may help to increase choice and enhance access to therapy services and facilitate a range of socioeconomic benefits (Farmer 2001; Jennett 2003; Wade 2012).
Setting. Future studies should focus on interventions based in real‐world settings such as the school and home environment that are most salient to the participants.
Intensity and duration of treatment. There is a need for further evidence regarding what is the proposed optimal treatment intensity (for example, daily practice?) and duration of therapy. There is also a need for further debate and discussion regarding the current clinical context for service delivery, given that the majority of interventions are still offered on a weekly or two‐weekly basis.
There is a need to consider principles of experience‐dependent neuroplasticity (for example, transference, interference, intensity, specificity, salience, see Kleim 2008) when designing future intervention trials in this field. For example, based on current conceptual theory, arguably the use of food in direct therapy approaches (as opposed to indirect therapy approaches that use no food or fluid) would be viewed as promoting neuroplasticity principles of specificity and salience of the treatment.
Acknowledgements
We are grateful for the guidance and editorial comments from Ms Laura MacDonald, Professor Geraldine Macdonald and other members of the Cochrane Developmental, Psychosocial and Learning Problems Group. We appreciate the help and advice of Margaret Anderson in the development of the search strategy. Thank you sincerely to Erica Gisel and Lotta Sjogreen for providing further information regarding their studies.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL), part of The Cochrane Library searched 7 October 2011 #1 MeSH descriptor Deglutition Disorders, this term only #2 (deglut* NEAR/5 (abnormal* or disorder* or dysfunc* or impair*)):ti,ab,kw #3 (swallow* NEAR/5 (abnormal* or difficult* or disorder* or dysfunc* or function* or impair*)):ti,ab,kw #4 ((oropharynx* or trachea* or lung* or pulmon*) NEAR/5 aspirat*):ti,ab,kw #5 (nasal NEXT regurgit*):ti,ab,kw #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Oropharynx, this term only #8 (oropharyn*):ti,ab,kw #9 (#7 OR #8) #10 (dysphag* or disorder* or dysfunc* or impair*):ti,ab,kw #11 (#9 AND #10) #12 (pharyng* NEAR/5 (dysphag* or dysfunct* or disorder* or impair*)) #13 (#6 OR #11 OR #12) #14 MeSH descriptor Infant explode all trees #15 child NEAR MeSH check #16 MeSH descriptor Adolescent, this term only #17 (baby or babies or newborn* or neonat* or toddler* or child* or preschool* or pre‐school* or schoolchild* or child* or adolescen* or teen* or juvenil* or young people or young person*) #18 (#14 OR #15 OR #16 OR #17) #19 (#13 AND #18) Ovid MEDLINE searched 10 October 2011 1 Deglutition Disorders/ (13226) 2 (deglut$ adj5 (abnormal$ or disorder$ or dysfunc$ or impair$)).tw. (329) 3 (swallow$ adj5 (abnormal$ or difficult$ or disorder$ or dysfunc$ or function$ or impair$)).tw. (4567) 4 ((oropharynx$ or trachea$ or lung$ or pulmon$) adj5 aspirat$).tw. (3958) 5 nasal regurgit$.tw. (87) 6 or/1‐5 (19904) 7 oropharyn$.tw. (11679) 8 Oropharynx/ (2938) 9 7 or 8 (12573) 10 (dysphag$ or disorder$ or dysfunc$ or impair$).tw. (1072281) 11 9 and 10 (1525) 12 (pharyng$ adj5 (dysphag$ or dysfunct$ or disorder$ or impair$)).tw. (706) 13 6 or 11 or 12 (20982) 14 exp Infant/ (865199) 15 exp Child/ (1420327) 16 (baby or babies or infant$ or newborn$ or neonat$ or toddler$ or child$ or preschool$ or pre‐school$ or schoolchild$ or child$ or adolescen$ or teen$ or juvenil$ or young people or young person$).tw. (2872444) 17 Adolescent/ (1456259) 18 or/14‐17 (2946395) 19 randomized controlled trial.pt. (319110) 20 controlled clinical trial.pt. (83678) 21 randomi#ed.ab. (267520) 22 placebo$.ab. (129637) 23 drug therapy.fs. (1505532) 24 randomly.ab. (161540) 25 trial.ab. (232182) 26 groups.ab. (1070965) 27 or/19‐26 (2793973) 28 exp animals/ not humans.sh. (3698786) 29 27 not 28 (2371126) 30 13 and 18 and 29 (953) Lines 19 to 29 form the sensitivity maximising version of the Cochrane highly sensitive search strategy for identifying randomized trials in Ovid MEDLINE (Lefebvre 2008) EMBASE (Ovid) searched 10 October 2011 1 dysphagia/ (28364) 2 (deglut$ adj5 (abnormal$ or disorder$ or dysfunc$ or impair$)).tw. (378) 3 (swallow$ adj5 (abnormal$ or difficult$ or disorder$ or dysfunc$ or function$ or impair$)).tw. (5959) 4 ((oropharynx$ or trachea$ or lung$ or pulmon$) adj5 aspirat$).tw. (4639) 5 nasal regurgit$.tw. (98) 6 or/1‐5 (35965) 7 oropharynx/ (4605) 8 oropharyn$.tw. (13826) 9 7 or 8 (15172) 10 (dysphag$ or disorder$ or dysfunc$ or impair$).tw. (1300510) 11 9 and 10 (1929) 12 (pharyng$ adj5 (dysphag$ or dysfunct$ or disorder$ or impair$)).tw. (859) 13 6 or 11 or 12 (37116) 14 exp infant/ (466419) 15 child/ (1058910) 16 adolescent/ (1110368) 17 (baby or babies or infant$ OR newborn$ or neonat$ or toddler$ or child$ or preschool$ or pre‐school$ or schoolchild$ or child$ or adolescen$ or teen$ or juvenil$ or young people or young person$).tw. (1375171) 18 14 or 15 or 16 or 17 (2515495) 19 exp Clinical trial/ (867367) 20 Randomized controlled trial/ (290224) 21 Randomization/ (54690) 22 Single blind procedure/ (14260) 23 Double blind procedure/ (100996) 24 Crossover procedure/ (30907) 25 Placebo/ (185441) 26 Randomi#ed.tw. (357461) 27 RCT.tw. (7766) 28 (random$ adj3 (allocat$ or assign$)).tw. (85600) 29 randomly.ab. (196338) 30 groups.ab. (1279950) 31 trial.ab. (281742) 32 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (131849) 33 Placebo$.tw. (159940) 34 Prospective study/ (173744) 35 (crossover or cross‐over).tw. (55626) 36 prospective.tw. (336342) 37 or/19‐36 (2553399) 38 13 and 18 and 37 (1217) CINAHL (EBSCOhost) searched 10 October 2011 S16 S11 and S15 S15 S12 or S13 or S14 S14 (baby or babies or infant* or newborn* or neonat* or toddler* or child* or preschool* or pre‐school* or schoolchild* or child* or adolescen* or teen* or juvenil* or young people or young person*) S13 (MH "Adolescence+") S12 (MH "Infant+") OR (MH "Child+") S11 S9 or S10 S10 (pharyng* N5 dysphag*) or (pharyng* N5 dysfunct*) or (pharyng* N5 disorder*) or (pharyng* N5 impair*) S9 S1 or S2 or S3 or S4 or S5 or S8 S8 S6 and S7 S7 (dysphag* or disorder* or dysfunc* or impair*) S6 (MH "Oropharynx") or Oropharyn* S5 nasal regurgit* S4 (oropharynx* N5 aspirat*) or (trachea* N5 aspirat*) or (lung* N5 aspirat*) or (pulmon* N5 aspirat*) S3 (swallow* N5 abnormal*) or (swallow* N5 difficult*) or (swallow* N5 disorder*) or (swallow* N5 dysfunc*) or (swallow* N5 function*) or (swallow* N5 impair*) S2 (deglut* N5 abnormal*) or (deglut* N5 disorder*) or (deglut* N5 dysfunc*) or (deglut* N5 impair*) S1 (MH "Deglutition Disorders") PsycINFO (Ovid) searched 10 October 2011 1 dysphagia/ 2 swallowing/ 3 (deglut$ adj5 (abnormal$ or disorder$ or dysfunc$ or impair$)).tw. 4 (swallow$ adj5 (abnormal$ or difficult$ or disorder$ or dysfunc$ or function$ or impair$)).tw. 5 ((oropharynx$ or trachea$ or lung$ or pulmon$) adj5 aspirat$).tw. 6 nasal regurgit$.tw. 7 (oropharyn$ adj5 (dysphag$ or disorder$ or dysfunc$ or impair$)).tw. 8 (pharyng$ adj5 (dysphag$ or dysfunct$ or disorder$ or impair$)).tw. 9 or/1‐7 (864) 10 ("100" or "120" or `140 or "200").ag. 11 (baby or babies or newborn$ or infant$ or neonat$ or toddler$ or child$ or preschool$ or pre‐school$ or schoolchild$ or child$ or adolescen$ or teen$ or juvenil$ or young people or young person$).tw. 12 10 or 11 13 9 and 12 14 clinical trials/ 15 (randomis* or randomiz*).tw. 16 (random$ adj3 (allocat$ or assign$)).tw. 17 ((clinic$ or control$) adj trial$).tw. 18 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 19 (crossover$ or "cross over$").tw. 20 random sampling/ 21 Experiment Controls/ 22 Placebo/ (2981) 23 placebo$.tw. (26386) 24 exp program evaluation/ 25 treatment effectiveness evaluation/ 26 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. 27 or/14‐26 28 13 and 27 ERIC (Dialog Datastar) searched 10 October 2011 (( SWALLOW$ NEAR ( ABNORMAL$ OR DIFFICULT$ OR DISORDER$ OR DYSFUNC$ OR FUNCTION$ OR IMPAIR$ ) ) .TI,AB.) OR (( PHARYNG$ NEAR ( DYSPHAG$ OR DYSFUNCT$ OR DISORDER$ OR IMPAIR$ ) ) .TI,AB.) OR (( OROPHARYN$ NEAR ( DYSPHAG$ OR DYSFUNCT$ OR DISORDER$ OR IMPAIR$ ) ) .TI,AB.) OR (( deglut$ NEAR ( abnormal$ OR disorder$ OR dysfunc$ OR impair$ ) ) .TI,AB.) OR (( ( oropharynx$ OR trachea$ OR lung$ OR pulmon$ ) NEAR aspirat$ ) .TI,AB.) OR (( nasal ADJ regurgit$ ) .TI,AB.) Database of Abstracts Reviews of Effects (DARE), part of The Cochrane Library searched 7 October 2011 #1 MeSH descriptor Deglutition Disorders, this term only #2 (deglut* NEAR/5 (abnormal* or disorder* or dysfunc* or impair*)):ti,ab,kw #3 (swallow* NEAR/5 (abnormal* or difficult* or disorder* or dysfunc* or function* or impair*)):ti,ab,kw #4 ((oropharynx* or trachea* or lung* or pulmon*) NEAR/5 aspirat*):ti,ab,kw #5 (nasal NEXT regurgit*):ti,ab,kw #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Oropharynx, this term only #8 (oropharyn*):ti,ab,kw #9 (#7 OR #8) #10 (dysphag* or disorder* or dysfunc* or impair*):ti,ab,kw #11 (#9 AND #10) #12 (pharyng* NEAR/5 (dysphag* or dysfunct* or disorder* or impair*)) #13 (#6 OR #11 OR #12) #14 MeSH descriptor Infant explode all trees #15 child NEAR MeSH check #16 MeSH descriptor Adolescent, this term only #17 (baby or babies or newborn* or neonat* or toddler* or child* or preschool* or pre‐school* or schoolchild* or child* or adolescen* or teen* or juvenil* or young people or young person*) #18 (#14 OR #15 OR #16 OR #17) #19 (#13 AND #18) Current Controlled Trials (ISRCTN Register) searched 15 October 2011
(dysphag* OR swallow* OR deglutition) AND (oropharyn* or pharyn*) ClinicalTrials.gov searched 15 October 2011
(dysphag* OR swallow* OR deglutition ) AND oropharynx* or pharynx* WHO International Clinical Trials Registry Portal (ICTRP) http://apps.who.int/trialsearch/ searched 15 October 2011
(dysphag* OR swallow* OR deglutition) AND (oropharyn* or pharyn*) Networked Digital Library of Theses and Dissertations (NDLTD)http://www.ndltd.org/serviceproviders/scirus‐etd‐search searched 11 October 2011 (dysphag* OR swallow* OR deglutition) AND (oropharyn* or pharyn*) Australasian Digital Theses Program (via TROVE) http://trove.nla.gov.au/ searched 11 October 2011 (dysphag* OR deglutit* OR swallow*) (oropharyn* OR pharyn*) (child* OR infant* OR adolescen* OR teen*) Refine results by limiting to format: Thesis DART‐Europe E‐theses Portal (DART) http://www.dart‐europe.eu/ searched 11 October 2011 (dysphag* OR swallow* OR deglutition) AND (oropharyn* OR pharyn*)
Appendix 2. Methods to be used in any update incorporating meta‐analysis
| Measures of treatment effect |
Dichotomous data Where dichotomous data are present, we will calculate a risk ratio (RR) with a 95% confidence interval (CI) for each outcome in each trial (Higgins 2008). Continuous data We will analyse continuous data when means and standard deviations are presented in the study papers, are made available by the authors of the trials, or are calculable from the available data. Where outcomes are measured using the same scale, we will calculate a mean difference (MD) to determine the differences in mean scores between groups. Where similar outcomes are measured using different scales, we will calculate a standardised mean difference (SMD) using Hedges g Time‐to‐event data We will present the treatment effects of time‐to‐event data or survival data (e.g. child maltreatment incidence data) as a hazard ratio with 95% CIs |
| Unit of analysis issues |
Cluster‐randomised trials It is possible that participants will be randomised to groups in clusters (e.g. when participants are randomised by treatment locality or clinic). For trials that use clustered randomisation, we will present results with proper controls for clustering (robust standard errors or hierarchical linear models). If appropriate controls are not used and it is impossible to obtain the full set of individual participant data, we will control the data for clustering using the procedures outlined in Higgins 2008. That is, when outcome measures are dichotomous, the number of events and number of participants per trial arm will be divided by the design effect (1 + (1 ‐ m) * r), where m is the average cluster size and r is the intra‐cluster correlation coefficient (ICC). When outcome measures are continuous, we will divide the number of participants per trial arm by the design effect, while leaving the mean values unchanged. To determine the ICC, the review authors will use estimates in the primary trials on a study‐by‐study basis. However, where these values are not reported, the review authors will use external estimates of the ICC that are appropriate to each trial context and average cluster size by contacting the trialists and if they are not available, the reviewers will seek statistical assistance from the Cochrane Methods Group (Higgins 2008) Multiple time points When results are measured at multiple time points, we will analyse each outcome at each point in a separate meta‐analysis with other comparable studies taking measures at a similar time point post‐intervention, as described in the outcomes, that is immediately post‐intervention, up to 6 months post‐intervention, more than 6 months post‐intervention. If this is not possible we will define time frames to reflect short‐term (up to 2 months), medium‐term (2 to 6 months) and long‐term follow‐up (more than 6 months). Studies with multiple treatment groups For trials where there are multiple treatment groups, we will not analyse data from the same group twice. We will select the treatment condition for meta‐analysis according to which ones best match the inclusion criteria. The comparison condition will be treatment‐as‐usual or the least active treatment offered |
| Assessment of heterogeneity | We will examine heterogeneity among included studies through the use of the Chi2 test, where a low P value indicates heterogeneity of treatment effects. We will use the I2 statistic (Higgins 2011) to determine the percentage of variability that is because of heterogeneity rather than sampling error or chance. We will discuss possible reasons for heterogeneity and conduct sensitivity analyses accordingly, where data permit. We may also use subgroup analyses to investigate this further, as described below |
| Data synthesis |
Where the interventions are similar in 1) type of intervention, 2) type of participants and 3) intensity, frequency and duration of the intervention, we plan to synthesise results in a meta‐analysis. We will use both a fixed‐effect and a random‐effects model and compare to assess the impact of statistical heterogeneity. Unless the model is contraindicated (e.g. if there is funnel plot asymmetry), we plan to present the results from the random‐effects model. In the presence of severe funnel plot asymmetry, we will present both fixed‐effect and random‐effects analyses, under the assumption that asymmetry suggests that neither model is appropriate. If both indicate a presence (or absence) of effect we will be reassured; if they do not agree we will report this. We will calculate all overall effects using inverse variance methods. If some primary studies report an outcome as a dichotomous measure and others use a continuous measure of the same construct, we will convert results for the former from an odds ratio (OR) to an SMD, provided that we can assume the underlying continuous measure has approximately a normal or logistic distribution (otherwise we will carry out 2 separate analyses) |
| Subgroup analysis and investigation of heterogeneity |
We may conduct further investigation of the causes of heterogeneity using subgroup analyses. We will consider developmental levels for all children. For children born preterm, we will use the corrected age to 2 years. We will stratify children by dependent age at onset of treatment, and according to neurological group status, as follows.
|
| Sensitivity analysis |
If the methodology or analyses in the trials might conceivably have affected the robustness of the results of the review, we will conduct sensitivity analyses by removing studies with particular characteristics and re‐analysing the remaining studies to determine whether the relevant factors affect the results. We will restrict analyses to studies judged to be at low risk of bias. Specifically, we will restrict the analysis to: (a) studies with low risk of selection bias (e.g. associated with sequence generation or allocation concealment); (b) studies with low risk of performance or detection bias (e.g. associated with issues of blinding); (c) studies with low risk of attrition bias (e.g. associated with completeness of data). We will also assess the sensitivity of findings to any imputed data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gisel 1996b.
| Methods | Randomised controlled trial | |
| Participants | 35 children (19 males) aged 4 years 3 months to 13 years 3 months across 3 groups (A, B, C) A: 11 participants, 5 males; X = 6 years 3 months, SD = 1.4 years B: 12 participants, 8 males; X = 7 years 3 months, SD = 2.1 years C: 12 participants, 6 males; X = 7 years 7 months SD = 2.7 years All children had a diagnosis of cerebral palsy with moderate to severe motor impairment Children's weight was at 5th percentile for age and at or below the 35th percentile for skin‐fold measures (triceps and subscapular) 27 children were wheelchair‐bound, 5 were ambulatory, 3 used tricycles for ambulation All children needed assistance with activities of daily living, including eating, and had a range of hypotonicity and hypertonicity in their trunk and extremities |
|
| Interventions | A: sensorimotor treatment for 20 weeks Involved 5‐7 minutes daily, 5 days per week, prior to lunch. Emphasis placed on tongue lateralisation, lip control and vigour of chewing (exact protocol detailed in the manuscript). All treatment conducted with small food stimuli B: chewing only treatment for 20 weeks Involved 5‐7 minutes daily, 5 days per week, prior to lunch. Children were offered small pieces of fruit gelatin of medium to hard viscosity. Number of pieces varied depending on child's chewing ability. Children given harder textures (i.e. firm cereal bits of pieces of hard biscuits) as they progressed C: control group who followed the school feeding routine for 10 weeks (control) and then received 10 weeks of sensorimotor treatment as for A |
|
| Outcomes | Outcome assessments conducted at 3 time points: the onset of the study (0 weeks), at 10 weeks, and at 20 weeks. Outcome measures included:
No statistically significant differences between groups from baseline (week 0) to weeks 10 or 20 on measures of eating time, clearing time (after swallows) or duration of mealtime. There were no significant differences found for any group on the ability to advance to a more solid texture from baseline to weeks 10 or 20 Participants maintained their weight‐for‐age percentile, at the lower end of expected norms |
|
| Notes | One child in group A died owing to causes unrelated to the study. Authors reported that it was not possible to recruit another child because the death occurred past the mid‐point of the study. Difficult to interpret findings because the control group received no treatment up until week 10 only, and then received the oral sensorimotor treatment as for experimental group A. As such, comparisons can only be made between experimental versus control groups up to week 10 of the intervention. Further, this study is at risk of selection bias given the very small sample size of participants per group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table used |
| Allocation concealment (selection bias) | Low risk | Random assignment occurred after informed consent was obtained |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of the treatment conditions resulted in neither participant or personnel being blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one child was unable to complete the treatment and final outcome assessment (she died for reasons external to the study). Treatment compliance was reported (average of 78.6 ± 2.1%) and was not statistically significantly different between the 3 groups. No mention made of whether any children missed any outcome assessments |
| Selective reporting (reporting bias) | Low risk | Average group data was reported on all outcome measures |
| Other bias | Unclear risk | No obvious further sources of bias |
Ottenbacher 1981.
| Methods | Randomised controlled trial | |
| Participants | 20 participants (aged 5 to 21.6 years, 12 males) with severe or profound ID who were residents in a state‐supported institution for people with ID. All participants had some degree of neuromotor disorder, with 18 participants having a diagnosis of cerebral palsy (11 spastic quadriplegic, 2 athetoid, 5 mixed). All dependent on most areas of self‐care, including needing assistance in feeding, and had some degree of oral‐motor pathology 10 participants randomly selected to take part in oral‐motor therapy and 10 participants served as a control group The control group received their regular programme of therapy and education with no specific treatment of oral‐motor dysfunction or feeding. As for the experimental children, the control children received their regular diet and were fed by their regular aides |
|
| Interventions | Each participant received approximately 30 to 40 minutes of therapy daily, 5 days a week for 9 weeks. Some participants received therapy just prior to or in conjunction with their meals, and others were scheduled for therapy at various times during the day. The treating therapist would determine which children received therapy during or just before mealtimes based on the nature of the oral‐motor/feeding problem exhibited by the participant. There were 3 major components to the treatment:
The exact treatment programme for each participant was developed based on the initial oral‐motor evaluation and an observation of the individual subject's feeding pattern. Specific treatment techniques were drawn from those proposed by Gallander 1979; Gallander 1980. All therapy was administered on an individual basis by 2 occupational therapists experienced in paediatric treatment |
|
| Outcomes | Outcome assessments were conducted pre‐ and post‐therapy. Outcome measures included:
|
|
| Notes | Potential for a high degree of variability in the treatment administered. Difficult to replicate this study owing to lack of detail on treatment protocol. Further, this study is at risk of selection bias given the very small sample size of participants per group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of the treatment resulted in neither participant nor personnel being blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Owing to staffing changes at the institution where the study took place, not all participants were able to be administered post‐therapy oral motor evaluations. Post‐therapy evaluations were conducted with 9 participants in the treatment group and 2 in the control |
| Selective reporting (reporting bias) | Low risk | Data were reported for all outcome measures where available (and missing data were specified as above) |
| Other bias | High risk | Significant difference in weight between the treatment and control groups at baseline (participants in the control group had a higher average weight). Difficult to determine whether experimental and control groups were similar on other characteristics at baseline as little information provided |
Sjogreen 2010.
| Methods | 1 group, single‐treatment, counterbalanced design | |
| Participants | 8 children and adolescents aged 7 to 19 years with myotonic dystrophy type 1 with onset congenitally or in childhood. Participants randomly assigned to 2 groups (A, B). A: treatment group ‐ n = 4; 7 to 19 years; 2 males; 3 congenital onset B: control group without treatment ‐ n = 4; 11 to 17 years; 3 males; 2 congenital onset |
|
| Interventions | The intervention consisted of 16 minutes of training with a pre‐fabricated 'oral screen', 5 days per week. The 16 minutes were divided into 3 sessions:
A sand glass or a timer was used as a reminder of time. A log book was used to record the training. The authors specified that it was preferable for training to be done at school during the day as individuals with myotonic dystrophy generally tired in the morning and in the evening, but that this was not always possible. Intervention was conducted at school (n = 4), home (n = 3), or mixture of 2 sessions at school and 1 at home (n = 1). |
|
| Outcomes | 3 outcome measures were relevant to oropharyngeal dysphagia
Outcome assessments of lip force and endurance measured with a calibrated lip force meter were conducted at school or in the participant's home every fourth week. Lip mobility measured using 3D motion analysis, and parental completion of a questionnaire of eating ability and saliva control was conducted at the clinic at baseline, immediately after the treatment and following the 16‐week non‐treatment period |
|
| Notes | There were inconsistencies between data tables in the Sjogreen study (specifically Tables 1 and 4 in the manuscript) on the outcomes reported on the parent questionnaire at baseline. Further, this study is at risk of selection bias given the very small sample size of participants per group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 6 folded pieces of paper with information about subgroup allocation (A or B) were placed in a box. Participants were asked to pick a piece of paper to find out when they would start treatment. 2 children were enrolled later in the study and had the opportunity to choose whether they wanted to start treatment immediately or wait for 16 weeks, as the intervention interfered with the summer holidays |
| Allocation concealment (selection bias) | Low risk | Random assignment occurred after informed consent was obtained |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of the treatment resulted in neither participant or personnel being blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 1 outcome assessor (speech language pathologist not involved in the study) was blinded for the entire study. 2 personnel outcome assessors (speech language pathologist and dental nurse) were not blinded, but were documenting objectively obtained measures (lip closure force, grip force and 3D video analysis of lip mobility). Parents of children also completed a questionnaire on eating and drinking difficulties. Parents could not be blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were clearly reported in the manuscript. Few instances of missing data |
| Selective reporting (reporting bias) | Low risk | Individual data were reported for all outcome measures |
| Other bias | Low risk | No obvious further sources of bias |
3D: 3 dimensional; ID: intellectual disability.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alacam 2007 | No randomisation, no control group Characteristics of the study Participants: 50 children aged 3 to 7 years with orofacial muscle dysfunction (31 CP; 8 DS; 13 motor‐mental retardation) Intervention type: Dr. Hinz oral‐screen appliance ("Pearl Oral Screen"): applied to 27 children. Castillo‐Morales palatal plate: applied to the 23 children who refused to use a Dr. Hinz oral‐screen. All children also received physiotherapy for orofacial stimulation Intervention duration: appliance recommended to be used for 1 hour per application for 3 to 4 hours a day over 12 months Outcome measures: pre‐treatment: type of swallow, number of chews before swallowing, mouth vs. nose breathing, lip seal at rest, tongue posture and presence of drooling During treatment: chewing and feeding problems, drooling, lip seal and tongue posture 3 months post‐treatment: regression, continuous improvement or stabilisation of orofacial functions Timing of outcome measures: pre‐treatment, end of treatment and 3 months' post‐treatment Results: authors report significant improvements in swallowing function (P < 0.05) after 2 to 3 hours and 3 to 4 hours usage of the Castillo‐Morales plate over 1 month, and improvement in tongue function (only at 1 to 2 hours of daily usage). Lip seal improved with the Dr. Hinz oral‐screen appliance after 3 months (3 to 4 hours of usage) and after 12 months (3 to ‐4 hours of usage) for the Castillo‐Morales palatal plate. Drooling significant decreased at 12 months (P < 0.05) with the Castillo‐Morales palatal plate. No difference between groups in chewing Post‐treatment: oral motor function regressed in 5, continuously improved in 15 and remained stable in 16 (14 lost to follow‐up) Further limitations: Not clear whether outcome assessors were blinded to intervention Absence of a control group Difficult to replicate methodology owing to lack of detail Significant loss to follow‐up |
| Arvedson 2010 | Systematic review |
| Christiaanse 2011 | No randomisation Characteristics of the study Participants: Treatment group: 47 children (17 female) aged 31 ± 19.9 months) who underwent a VFSS pre and post intervention. Primary dysphagia (29/47; 26/29 congenital, 3/29 unknown antenatal onset). Acquired dysphagia (18/47) Control group: 46 children (19 female) aged 9.9 ± 8.1 months with dysphagia who had undergone 2 VFSS within at least 6 months of each other. Primary dysphagia (23/46). Acquired dysphagia (23/46). Children with an initially abnormal VFSS were included Intervention type: NMES sessions conducted using 2 electrodes positioned below the jaw approximating the anterior belly of the digastrics muscles and 2 electrodes positioned above the thyroid notch approximating the infrahyoid muscles. Parents provided with instructions on performing oral motor exercises (without the NMES device) between sessions Intervention duration: average of 22 treatment sessions (range: 5 to 43) over 10 weeks. Average number of session per week 2.9 (range 0.7 to 4.6; median 2.8). Length of sessions: 30 to 45 minutes. Outcome measures: change in FOIS level Timing of outcome measures: pre‐ and post‐treatment. Post‐treatment: VFSS performed on average 0.9 months following the last NMES session (range: ‐0.03 to 15.5 months) Control group: VFSS separated by an average of 2.9 months (range: 1.3 to 4.2 months) Results: FOIS levels improved in both groups (P < 0.01), but difference between groups not significant (P = 0.11). Children in the treatment group with acquired dysphagia demonstrated greater improvements than controls (P = 0.007) Further limitations: Retrospective Difficult to replicate methodology owing to lack of detail |
| DeMatteo 2002 | Not an RCT (single case study with randomised Latin square cross‐over design) Characteristics of the study Participants: Participant 1: 12 years old (female), MVA, recruited 41 days post admission Participant 2: 14 years old (female), MVA, recruited 56 days post admission Participant 3: 3 years old (female), near drowning accident, recruited 33 days post admission Inclusion criteria: severe ABI; receiving ward level care; level II minimum on the Rancho Scale of Cognitive Function; intact swallow, gag, and cough reflexes Intervention type: food textures: puréed, minced and soft food textures randomly allocated to each of 3 daily meals for each participant. Each meal lasted 30 minutes. Nasogastric feeds provided after the child attempted to eat Intervention duration: participant 1: 11 days; participant 2: 7 days; participant 3: 4 days Outcome measures: Amount of food intake (grams) Oral motor movement patterns measured by the Behavioral Assessment of Oral Functions in Feeding. Raters blinded to texture provided to child Timing of outcome measures: not clear ‐ every meal? Results: food intake variable across all participants. Increases noted for participants 1 and 3. Effect of texture on intake significant for participants 2 and 3 (greater intake achieved with puréed meals). Quality of oral‐motor function influenced by feeder for 2 participants. No correlation between intake and oral motor performance. Further limitations: No follow‐up Difficult to replicate methodology owing to lack of detail |
| Ganz 1987 | Not an RCT (single subject ABA design) Characteristics of the study Participants: 8‐year‐old female with CP and profound intellectual disability Intervention type: neuromotor and sensory facilitation techniques to inhibit abnormal oral and postural reflexes, improve muscle tone and decrease hypersensitivity in and around the mouth Intervention duration: 14 weeks Outcome measures: oral motor function (Pre‐Speech Assessment Scale). Frequency of tongue thrusting after each bite of food. Presence of tonic bite Timing of outcome measures: baseline, intervention phase, return to baseline phase (2 weeks following treatment) Results: significant decrease in tongue thrusting (P < 0.0001). Post‐treatment, this improvement remained stable for semi‐solids foods but not for solids. No change observed in the number of tonic bites. Positive oral motor changes: emergence of tongue lateralisation and improved lip seal. Negative oral motor changes: decrease in tongue elevation with jaw separation Further limitations: Outcome assessors not blinded? No long‐term follow‐up Difficult to replicate methodology owing to lack of detail |
| Gisel 1996a | No randomisation Characteristics of the study Participants: group A: 7 male children aged 5.4 ± 2.7 years demonstrating signs of aspiration during a VFSS. Group NA: 20 children (13 female) aged 4.6 ± 2.3 with no signs of aspiration. All children diagnosed with moderate‐severe CP (majority spastic quadriplegic) Intervention type: oral sensorimotor treatment focusing on: 1. tongue lateralisation, 2. lip control, 3. vigour of chewing (see Gisel 1996a for details) Intervention duration: all children followed their school feeding routine for 10 weeks, followed by 10 weeks of sensorimotor treatment (5 days a week for 5 to 7 minutes prior to lunch or snack) Outcome measures: weight, skinfold thickness (triceps and subscapular), oral motor ability: modified version of the Functional Feeding Assessment subtest of the Multidisciplinary Feeding Profile (Kenny et al.) administered while children ate, VFSS, eating‐associated drooling Timing of outcome measures: baseline, 10 and 20 weeks; VFSS completed at baseline for all children and at 20 weeks for children who aspirated. Results: VFSS: at week 20, 5 children showed penetration (the remaining 2 children did not undergo a second VFSS). Group A demonstrated significantly reduced oral motor skills and lower skinfold thickness compared to group NA. No significant difference between groups in the amount of weight gained. Drooling decreased slightly in group NA and did not change in group A Further limitations: Outcome assessor not blinded? Difficult to replicate methodology owing to lack of detail |
| Gisel 2001 | No randomisation Characteristics of the study Participants: 17 children (10 female) aged 6.6 to 15.4 years (mean age 10.2 ± 3.0 years). Group A: 9 children, Group B: 8 children. All children diagnosed with moderate spastic tetraparesis CP Intervention type: intra‐oral appliance therapy. Children assigned randomly to: group A: children continued wearing the ISMAR from a previous study; group B: children ceased wearing the ISMAR intervention duration: children were followed 1 year after receiving 1 year of ISMAR therapy Outcome measures: anthropometric measurements: height, weight, skinfold thickness. Functional Feeding Assessment Timing of outcome measures: 12, 18 and 24 months Results: Maturation equally effective as ISMAR therapy. No significant differences in functional feeding skills across both groups. No significant differences in weight and skinfold thickness between groups. Height: equivalent to typically developing children |
| Haberfellner 2001 | Not an RCT Characteristics of the study Participants: 20 children (11 female) aged 4.2 to 13.1 years (mean age 8.3 ± 0.9 years). All children diagnosed with moderate CP (tetraparesis). 1 child withdrew from the study Intervention type: intraoral appliance therapy. Children assigned randomly to: group A (n = 10): immediate ISMAR treatment; group B (n = 10): control group (children received ISMAR treatment after a 6‐month control period). First 6 months of treatment focused on jaw stabilisation (phase I), followed by 6 months of oral structure mobilisation (phase II) Intervention duration: 1 year (appliance recommended to be worn daily) Outcome measures: anthropometric measurements: weight, height, skinfold thickness. Modified version of the Functional Feeding Assessment Timing of outcome measures: start of control period (group B), commencement of phase I and II, end of treatment Results: significant improvement in functional feeding skills (e.g. spoon‐feeding, biting, cup drinking, chewing and swallowing) during phase I. Chewing improved further during phase II No differences in weight gain between groups. Weight gain resulted in improvements in height for all children Further limitations: No follow‐up Difficult to replicate methodology owing to lack of detail |
| Iammatteo 1990 | Not focused on dysphagia outcome (focused on saliva control) |
| Kiger 2006 | Age range was 18 years and over |
| Korbmacher 2004 | Non‐neurological population |
| Ottenbacher 1983 | Not an RCT (multiple baseline across‐subjects design) Characteristics of the study Participants: 3 children with severely or profound intellectual disability. Participant 1: aged 12 years; 7 years with translocation of chromosome 22; participant 2: aged 8 years; 9 years with multiple congenital anomalies; participant 3: male aged 10; 11 years with Sturge‐Weber syndrome. Intervention type: programme of oral motor habilitation: 1. inhibition of abnormal oral and postural reflexes: jaw control manipulation (upwards pressure on mandible) to reduce suck reflex; similar techniques used to inhibit rooting and bite reflex; 2. facilitation of normal muscle tone: manual guidance of the musculature through the target movement; 3. Desensitisation of the oral region: gradually increased tactile stimulation in the oral region and in the oral cavity. Participants were randomly assigned to treatment order Intervention duration: 30 minutes a day for 5 days a week. Participant 1: 2 weeks at baseline, 10 weeks of treatment; participant 2: 4 weeks at baseline, 8 weeks of treatment; participant 3: 8 weeks of baseline, 4 weeks of treatment. Intervention introduced at 2 week intervals throughout the 12 weeks of the study Outcome measures: weight of child (pounds) ‐ assessors blinded to the study’s hypothesis and whether the child was in the baseline or treatment phase. Oral motor evaluation adapted from the Vulpe Assessment Battery (completed weekly by OT), measuring 4 oral reflexes, tongue position at resent, presence of drooling and 21 feeding skills Timing of outcome measures: oral motor evaluation completed weekly, administered on the same day each week and at approximately the same time Results: mixed results reported. Weight increased for participants 2 (P = 0.004) and 3 (P = 0.06). Weight decreased for participant 1 (P = 0.001). Oral motor performance improved for participants 1 (P value not calculated) and 3 (P = 0.06). Performance decreased for participant 2 (P = 0.004) Further limitations: ? blinding outcome assessor No long‐term follow‐up Difficult to replicate methodology owing to lack of detail |
| Pinnington 1999 | No randomisation (AB within subjects design) Characteristics of the study Participants: 20 children (8 female) aged 7 to 17 years with moderate to severe motor impairment (spastic or spastic‐athetoid) and severe neurological impairment. 17 children diagnosed with CP, 2 with TBI and 1 with Lesch‐Nylan syndrome. 4 children withdrawn from the study Intervention type: consistent presentation of food delivered by an assistive feeding device (Handy‐1 Robotic Aid to Eating) compared to hand delivered presentation of food Intervention duration: unclear whether 3 or 9 months (device used during 1 meal per day) Outcome measures: eating efficiency (percentage of food lost from the mouth), amount of energy and protein consumed, duration of meals and rate of eating, and height and growth. Timing of outcome measures: pre‐treatment and during treatment, no post‐treatment follow‐up Results: assistive feeding device resulted in reduced eating efficacy and increased duration of mealtimes. No differences in weight and growth, and the amount of energy and protein consumed Further limitations: No control group No post‐treatment follow‐up Difficult to replicate methodology owing to lack of detail |
| Pinnington 2000 | No randomisation (ABA within subjects design) Characteristics of the study Participants: 16 children aged 7 to 17 years with moderate to severe motor impairment (spastic or spastic‐athetoid) and severe neurological impairment. 13 children diagnosed with CP, 2 with ABI and 1 with Lesch‐Nylan syndrome. Intervention type: consistent presentation of food delivered by an assistive feeding device (Handy‐1 Robotic Aid to Eating) compared to hand‐delivered presentation of food. Intervention duration: 3 months (device used 5 days a week, 1 meal per day). Outcome measures: oral motor behaviour and postural control as measured by the Schedule for Oral Motor Assessment (modified by the authors). Timing of outcome measures: beginning, middle, and end of the pre‐treatment and treatment phases, and post‐treatment Results: assistive feeding device resulted in improved oral motor performance and postural control during feeding (reduction in postural control observed post‐treatment) Further limitations: Blinding of outcome assessors unclear No control group Difficult to replicate methodology owing to lack of detail |
| Sobsey 1984 | No randomisation (reversal design) Characteristics of the study Participants: 4 children (3 female) aged 3, 4, 7 and 12 years with spastic quadriplegia (n = 2), spastic biparetic (n = 1) and severe disability (n = 1) Intervention type: Developed facilitation procedures:
Intervention duration: 1 session per day for 80 days. Session length: 10 to 15 minutes approximately 15 minutes prior to lunch Outcome measures: lip closure, rotary chews per bite, spills per bite of food, and spills per drink of fluid Timing of outcome measures: unclear, seemingly pre‐ and post‐treatment Results: all participants demonstrated improvements in lip closure although variability noted in participants 2 and 4. Improvements in rotary jaw movements only observed in participants 2 and 4. Spills per bite of food decreased during treatment for participants 1, 3 and 4 and did not change for participant 2. Spills per drink decreased for participants 1 and 3 and did not change in participant 2 (no data available for participant 4) Further limitations: No follow‐up No control participants Difficult to replicate methodology owing to lack of detail |
AB: assessment, baseline; ABA: assessment, baseline, assessment; ABI: acquired brain injury; CP: cerebral palsy; DS: Down's syndrome; FOIS: Functional Oral Intake Scale; ISMAR: Innsbruck Sensorimotor Activator and Regulator; MVA: motor vehicle accident; NMES: neuromuscular electrical stimulation; OT: occupational therapist; RCT: randomised controlled trial; SD: standard deviation; TBI: traumatic brain injury; VFSS: videofluroscopic swallowing study.
Differences between protocol and review
Some minor edits to Background and Methods sections were made.
Exclusion criteria were tightened to exclude children who were treated for drooling without direct intervention also being applied for oropharyngeal dysphagia.
The secondary outcome measure "level of reliance on supplementary feeding, for example, nasogastric or gastrostomy tube feeding" was removed because this item should have been originally included under the primary outcome measure of diet consistency a child is able to consume, as a possible indicator of oral and pharyngeal skills (that is, whether the child can manage a developmentally appropriate oral diet, or if the texture/consistency of foods and fluids must be modified, or if supplementary feeding is required, such as nasogastric or gastrostomy tube feeding).
Data extraction methodology was altered from all three review authors abstracting the data to one author only (AM) abstracting the data and the abstractions being independently verified by the remaining two authors (LW, PD). This protocol change occurred for reasons of efficiency for the review authors.
Contributions of authors
All authors contributed to the development of this protocol. AM and EW drafted the original version of the protocol, with input from PD. AM and EW devised the original search strategy with support from Margaret Anderson. AM, PD and EW screened the abstracts and titles and AM retrieved potentially eligible papers. AM, PD and EW reviewed the papers and made decisions about eligibility. AM extracted the data. AM drafted the full review with input from PD and EW.
Sources of support
Internal sources
Centre for Evidence‐Based Intervention, University of Oxford, UK.
External sources
-
National Health and Medical Research Council, Australia.
Angela Morgan is supported by a National Health and Medical Research Council Career Development Award (#607315)
Declarations of interest
Angela T Morgan ‐ none known.
Elizabeth C Ward ‐ none known.
Pamela Dodrill ‐ none known.
New
References
References to studies included in this review
Gisel 1996b {published and unpublished data}
- Gisel EG. Effect of oral sensorimotor treatment on measures of growth and efficiency of eating in the moderately eating‐impaired child with Cerebral Palsy. Dysphagia 1996;11:48‐58. [DOI] [PubMed] [Google Scholar]
Ottenbacher 1981 {published data only (unpublished sought but not used)}
- Ottenbacher K, Scoggins A, Wayland J. The effectiveness of a program of oral sensory motor therapy with the severely and profoundly disabled. Occupational Therapy Journal of Research 1981;1(2):147‐60. [Google Scholar]
Sjogreen 2010 {published and unpublished data}
- Sjogreen L, Tulinius M, Kiliaridis S, Lohmander A. The effect of lip strengthening exercises in children and adolescents with myotonic dystrophy type 1. International Journal of Pediatric Otorhinolaryngology 2010;74:1126–34. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alacam 2007 {published data only}
- Alacam A, Kolcuoǧlu N. Effects of two types of appliances on orofacial dysfunctions of disabled children. The British Journal of Developmental Disabilities (BJDD) 2007;35(2):111‐23. [Google Scholar]
Arvedson 2010 {published data only}
- Arvedson J, Clark H, Lazarus C, Schooling T, Frymark T. The effects of oral‐motor exercises on swallowing in children: an evidence‐based systematic review. Developmental Medicine and Child Neurology 2010;19(4):321‐40. [DOI] [PubMed] [Google Scholar]
Christiaanse 2011 {published data only}
- Christiaanse ME, Mabe B, Russell G, Long Simeone T, Fortunato J, Rubin B. Neuromuscular electrical stimulation is no more effective than usual care for the treatment of primary dysphagia in children. Pediatric Pulmonology 2011;46:559–65. [DOI] [PubMed] [Google Scholar]
DeMatteo 2002 {published data only}
- DeMatteo C, Law M, Goldsmith C. The effect of food textures on intake by mouth and the recovery of oral motor function in the child with a severe brain injury. Physical & Occupational Therapy in Pediatrics 2002;22(3/4):51‐71. [PubMed] [Google Scholar]
Ganz 1987 {published data only}
- Ganz SF. Decreasing tongue thrusting and tonic bite reflex through neuromotor and sensory facilitation techniques. Physical and Occupational Therapy in Pediatrics 1987;7(4):57‐75. [Google Scholar]
Gisel 1996a {published data only}
- Gisel EG, Applegate‐Ferrante T, Benson J, Bosma JF. Oral‐motor skills following sensorimotor therapy in two groups of moderately dysphagic children with cerebral palsy: aspiration vs nonaspiration. Dysphagia 1996;11:59‐71. [DOI] [PubMed] [Google Scholar]
Gisel 2001 {published data only}
- Gisel EG. Impact of oral appliance therapy: are oral skills and growth maintained one year after termination of therapy?. Dysphagia 2001;16:296‐307. [DOI] [PubMed] [Google Scholar]
Haberfellner 2001 {published data only}
- Haberfellner H, Schwartz S, Gisel E. Feeding skills and growth after one year of intraoral appliance therapy in moderately dysphagic children with cerebral palsy. Dysphagia 2001;16:83‐96. [DOI] [PubMed] [Google Scholar]
Iammatteo 1990 {published data only}
- Iammatteo PA, Trombly C, Luecke L. The effect of mouth closure on drooling and speech. The American Journal of Occupational Therapy 1990;44(8):686‐91. [DOI] [PubMed] [Google Scholar]
Kiger 2006 {published data only}
- Kiger M, Brown CS, Watkins L. Dysphagia management: an analysis of patient outcomes using VitalStim therapy compared to traditional swallow therapy. Dysphagia 2006;21(4):243‐53. [DOI] [PubMed] [Google Scholar]
Korbmacher 2004 {published data only}
- Korbmacher HM, Schwan M, Berndsen S, Bull J, Kahl‐Nieke B. Evaluation of a new concept of myofunctional therapy in children. International Journal of Orofacial Myology 2004;30:39‐52. [PubMed] [Google Scholar]
Ottenbacher 1983 {published data only}
- Ottenbacher K, Hicks J, Roark A, Swinea J. Oral sensorimotor therapy in the developmentally disabled: a multiple baseline study. American Journal of Occupational Therapy 1983;37:541‐7. [DOI] [PubMed] [Google Scholar]
Pinnington 1999 {published data only}
- Pinnington L, Hegarty J. Effects of consistent food presentation on efficiency of eating and nutritive value of food consumed by children with severe neurological impairment. Dysphagia 1999;14:17‐26. [DOI] [PubMed] [Google Scholar]
Pinnington 2000 {published data only}
- Pinnington L, Hegarty J. Effects of consistent food presentation on oral‐motor skill acquisition in children with severe neurological impairment. Dysphagia 2000;15:213–23. [DOI] [PubMed] [Google Scholar]
Sobsey 1984 {published data only}
- Sobsey R, Orelove FP. Neurophysiological facilitation of eating skills in children with severe handicaps. Journal of the Association for Persons with Severe Handicaps 1984;9(2):98‐110. [Google Scholar]
Additional references
Arrowsmith 2006
- Arrowsmith FE, Allen JR, Gaskin KJ, Gruca MA, Clarke SL, Briody JN, et al. Reduced body protein in children with spastic quadriplegic cerebral palsy. The American Journal of Clinical Nutrition 2006;83:613‐8. [DOI] [PubMed] [Google Scholar]
Arvedson 1994
- Arvedson J, Rogers B, Buck G, Smart P, Msall M. Silent aspiration prominent in children with dysphagia. International Journal of Pediatric Otorhinolaryngology 1994;28:173‐81. [DOI] [PubMed] [Google Scholar]
Arvedson 2008
- Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Developmental Disabilities Research Reviews 2008;14:118‐27. [DOI] [PubMed] [Google Scholar]
Ball 2012
- Ball SL, Panter SG, Redley M, Proctor CA, Byrne K, Clare IC, et al. The extent and nature of need for mealtime support among adults with intellectual disabilities. Journal of Intellectual Disability Research 2011;56(4):382‐401. [DOI] [PubMed] [Google Scholar]
Calis 2008
- Calis EAC, Veugelers R, Sheppard JJ, Tibboel D, Evenhuis HM, Penning C. Dysphagia in children with severe generalized cerebral palsy and intellectual disability. Developmental Medicine & Child Neurology 2008;50(8):625‐30. [DOI] [PubMed] [Google Scholar]
Chadwick 2003
- Chadwick DD, Jolliffe J, Goldbart J. Adherence to eating and drinking guidelines for adults with intellectual disabilities and dysphagia. American Journal of Mental Retardation 2003;108(3):202‐11. [DOI] [PubMed] [Google Scholar]
Cherney 2012
- Cherney L. Aphasia treatment: intensity, dose parameters, and script training. International Journal of Speech‐Language Pathology July 2012;E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 1989
- Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, et al. Opening mechanisms of the human upper esophageal sphincter. The American Journal of Physiology 1989;257:G748‐59. [DOI] [PubMed] [Google Scholar]
Cornwell 2003
- Cornwell PL, Murdoch BE, Ward EC, Morgan AT. Dysarthria and dysphagia as long‐term sequelae in a child treated for recurrent posterior fossa tumour. Pediatric Rehabilitation (now known as Developmental Neurorehabilitation) 2003;6(2):67‐75. [DOI] [PubMed] [Google Scholar]
Farmer 2001
- Farmer JE, Muhlenbruch L. Telehealth for children with special health care needs: promoting comprehensive systems of care. Clinical Pediatrics 2001;40(2):93‐8. [DOI] [PubMed] [Google Scholar]
Faulks 2007
- Faulks D, Collado V, Mazille MN, Veyrune JL, Hennequin M. Masticatory dysfunction in persons with Down's syndrome. Part 1: aetiology and incidence. Journal of Oral Rehabilitation 2008;35(11):854‐62. [DOI] [PubMed] [Google Scholar]
Gallander 1979
- Gallander D. Eating handicaps: illustrated techniques for feeding disorders.. Eating Handicaps: Illustrated Techniques For Feeding Disorders. Springfield, Illinois: Charles CThomas Pub Ltd, 1979. [Google Scholar]
Gallander 1980
- Gallander D. Teaching Eating and Toileting Skills to the Multi‐handicapped in the School Setting. Springfield, Illinois: Charles C Thomas Pub Ltd, 1980. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (Editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huckabee 1999
- Huckabee ML, Cannito MP. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: a retrospective evaluation. Dysphagia 1999;14:93‐109. [DOI] [PubMed] [Google Scholar]
Jennett 2003
- Jennett PA, Affleck Hall L, Hailey D, Ohinmaa A, Anderson C, Thomas R, et al. The socio‐economic impact of telehealth: a systematic review. Journal of Telemedicine and Telecare 2003;9(6):311‐20. [DOI] [PubMed] [Google Scholar]
Jones 2012
- Jones CJ, Bryant‐Waugh R. Development and pilot of a group skills‐and‐support intervention for mothers of children with feeding problems. Appetite 2012;58(2):450‐6. [DOI] [PubMed] [Google Scholar]
Kleim 2008
- Kleim JA, Jones TA. Principles of experience‐dependent neural plasticity: implications for rehabilitation after brain damage. Journal of Speech, Language and Hearing Research 2008;51(1):S225‐39. [DOI] [PubMed] [Google Scholar]
Larnert 1995
- Larnert G, Ekberg O. Positioning improves the oral and pharyngeal swallowing function in children with cerebral palsy. Acta Paediatrica 1995;84(6):689‐92. [DOI] [PubMed] [Google Scholar]
Lazarus 1993
- Lazarus C, Logemann JA, Rademaker AW, Kahrilas PJ, Pajak T, Lazar R, et al. Effects of bolus volume, viscosity and repeated swallows in nonstroke subjects and stroke patients. Archives of Physical Medicine and Rehabilitation 1993;74:1066‐70. [DOI] [PubMed] [Google Scholar]
Lazzara 1986
- Lazzara G, Lazarus C, Logemann JA. Impact of thermal stimulation on the triggering of the swallow reflex. Dysphagia 1986;1:73‐7. [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheinmer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Logemann 1991
- Logemann J. Approaches to management of disordered swallowing. Bailliere's Clinical Gastroenterology 1991;5(2):269‐80. [DOI] [PubMed] [Google Scholar]
Logemann 1993
- Logemann JA. Noninvasive approaches to deglutitive aspiration. Dysphagia 1993;8(4):331‐3. [DOI] [PubMed] [Google Scholar]
Logemann 1995
- Logemann JA, Pauloski BR, Colangelo L, Lazarus C, Fujiu M, Kahrilas PJ. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. Journal of Speech and Hearing Research 1995;383:556‐63. [DOI] [PubMed] [Google Scholar]
Loughlin 1989
- Loughlin GM. Respiratory consequences of dysfunctional swallowing and aspiration. Dysphagia 1989;3:126‐30. [DOI] [PubMed] [Google Scholar]
Morgan 2003
- Morgan AT, Ward E, Murdoch B, Kennedy B, Murison R. Incidence, characteristics and predictive factors for dysphagia following paediatric traumatic brain injury. Journal of Head Trauma Rehabilitation 2003;18(3):239‐51. [DOI] [PubMed] [Google Scholar]
Morgan 2004
- Morgan A, Ward E, Murdoch B. A case study of the resolution of paediatric dysphagia following brainstem injury: clinical and instrumental assessment. Journal of Clinical Neuroscience 2004;11(2):182‐90. [DOI] [PubMed] [Google Scholar]
Morgan 2010a
- Morgan AT. Dysphagia in childhood traumatic brain injury: a reflection on the evidence and its implications for practice. Developmental Neurorehabilitation 2010;13(3):192‐203. [DOI] [PubMed] [Google Scholar]
Morgan 2010b
- Morgan AT, Mageandran SD, Mei C. Incidence and clinical presentation of dysarthria and dysphagia in the acute setting following paediatric traumatic brain injury. Child: Care, Health and Development 2010;36(1):44‐53. [DOI] [PubMed] [Google Scholar]
Morton 1997
- Morton RE, Bonas R, Minford J, Kerr A, Ellis RE. Feeding ability in Rett syndrome. Developmental Medicine and Child Neurology 1997;39(5):331‐5. [DOI] [PubMed] [Google Scholar]
Ohmae 1996
- Ohmae Y, Logemann JA, Kaiser P, Hanson DG, Kahrilas PJ. Effects of two breath‐holding maneuvers on oropharyngeal swallow. Annals of Otology, Rhinology and Laryngology 1996;105:123‐31. [DOI] [PubMed] [Google Scholar]
Packman 2012
- Packman A, Onslow M. Investigating optimal intervention intensity with the Lidcombe Program of early stuttering intervention. International Journal of Speech‐Language Pathology July 2012;E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
Patrick 1990
- Patrick J, Gisel EJ. Nutrition for the feeding impaired child. Journal of Neurology & Rehabilitation 1990;4:115‐9. [Google Scholar]
Philpot 1999
- Philpot J, Bagnall A, King C, Dubowitz V, Muntoni F. Feeding problems in merosin deficient congenital muscular dystrophy. Archives of Disability in Childhood 1999;80(6):542‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redstone 2004
- Redstone F, West JF. The importance of postural control for feeding. Pediatric Nursing 2004;30(2):97‐100. [PubMed] [Google Scholar]
Reilly 1996
- Reilly S, Skuse D, Poblete X. Prevalence of feeding problems and oral motor dysfunction in children with cerebral palsy: a community survey. Journal of Pediatrics 1996;129(6):877‐82. [DOI] [PubMed] [Google Scholar]
Robbins 2007
- Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, et al. The effects of lingual exercise in stroke patients with dysphagia. Archives of Physical Medicine Rehabilitation 2007;88(2):150‐8. [DOI] [PubMed] [Google Scholar]
Rosenbloom 1996
- Rosenbloom L, Sullivan P, editors. Feeding the Disabled Child. Clinics in Developmental Medicine. Vol. 140, London: Mac Keith Press, 1996. [Google Scholar]
Stallings 1993
- Stallings VA, Charney EB, Davies JC, Cronk CE. Nutrition related growth failure of children with quadriplegic cerebral palsy. Developmental Medicine and Child Neurology 1993;35:126‐38. [DOI] [PubMed] [Google Scholar]
Thommessan 1991
- Thommessan M, Heiberg A, Kase BF, Larsen S, Riis G. Feeding problems, height and weight in different groups of disabled children. Acta Paediatrica Scandinavica 1991;80(5):527‐33. [DOI] [PubMed] [Google Scholar]
Veness 2008
- Veness C, Reilly S. Mealtime interaction patterns between young children with cerebral palsy and their mothers: characteristics and relationship to feeding impairment. Child Care Health Development 2008;34(6):815‐24. [DOI] [PubMed] [Google Scholar]
Vulpe 1977
- Vulpe SG. Vulpe Assessment Battery. 2nd Edition. Toronto: Canadian Association for Retarded Citizens, 1977. [Google Scholar]
Wade 2012
- Wade VA, Eliott JA, Hiller JE. A qualitative study of ethical, medico‐legal and clinical governance matters in Australian telehealth services. Journal of Telemedicine and Telecare 2012;18(2):109‐14. [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization (WHO). International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization 2001.
Williams 2012
- Williams AL. Intensity in phonological intervention: is there a prescribed amount?. International Journal of Speech‐Language Pathology July 2012;E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]


