Abstract
Introduced Phragmites (Phragmites australis australis (Cav.) Trin. Ex Steud.) is one of the most invasive plants in North America. To supplement existing management tools, a classical biological control program began in Canada in 2019 using two host-specific stem-boring moths, Archanara neurica (Hübner) and Lenisa geminipuncta (Haworth) (Lepidoptera: Noctuidae). In this article, we summarize the first three years of monitoring data for L. geminipuncta and A. neurica as biological control agents for introduced Phragmites. First, we assess agent presence and activity in the initial years post-release based on feeding damage from long-term monitoring data across 30 release sites initiated between 2019 and 2023. Second, we investigate the within-site distribution of agent feeding damage to improve future monitoring and agent collection from nurse sites. Third, we report the results of an experiment to determine optimal release densities of A. neurica larvae. We found agent feeding damage at 92% of initial release sites in the first year and agent activity persisted at all of these sites into years two and three post-release. Patterns of agent feeding damage suggest that the agents disperse quickly through the patch following release, favouring the interior area over the edges of introduced Phragmites stands. Finally, releasing intermediate densities of 40 A. neurica larvae per release point was more efficient than releasing either units of 20 or 80 larvae. The results of the first three years of monitoring are highly encouraging for the introduced Phragmites biological control program. Insights from these early monitoring results will be used to refine optimal release strategies, improve our ability to locate egg-bearing stems at nurse sites to facilitate the collection and redistribution of agents to new release locations, and inform protocols for longer-term monitoring of impacts on the target weed once agents are established.
Introduction
Common reed (Phragmites australis australis (Cav.) Trin. Ex Steud.)–hereafter referred to as “introduced Phragmites”–is one of the most invasive plants in North America. Arriving from Eurasia in the late 19th century, the weed is now widespread along the Atlantic, Pacific, and Gulf Coastal regions [1, 2], with the potential for significant further range expansion [3]. Introduced Phragmites is a perennial grass that forms dense stands in wetland and riverine habitats, as well as in roadside ditches [4, 5]. These dense stands cause significant reductions in native plant diversity [6], with related impacts on communities of birds [7], fish [8], and turtles [9]. Along with a cascade of other negative ecological impacts [10–12], introduced Phragmites presents a major threat to the native North American subspecies of common reed (P. australis americanus Saltonstall, P.M. Peterson & Soreng) through both competition [2, 13] and hybridization [14, 15].
Conventional control methods for introduced Phragmites have seen limited success [16–18]. The use of herbicides is prohibited in many invaded sites due to threats to biodiversity, human health, and water quality [19–22], while physical control methods such as cutting and burning are highly labour-intensive and are only practical at relatively small scales. There is a clear need for additional management options for introduced Phragmites. Classical biological control represents a scalable and sustainable tool to be used as part of an integrated management approach [23].
The biological control program for introduced Phragmites began in 1998 with assessments of the weed as an appropriate target and a search for candidate agents [24–26]. Two European stem-boring moths, Archanara neurica (Hübner) and Lenisa geminipuncta (formerly Archanara geminipuncta) (Haworth) (Lepidoptera: Noctuidae), were identified as promising candidates by the Centre for Agriculture and Biosciences International (CABI) in Delémont, Switzerland. Host-range testing conducted between 2005 and 2018 demonstrated that both species are specific to introduced Phragmites [27, 28]. Archanara neurica and L. geminipuncta share similar life histories with a few key differences in phenology and feeding behaviour. Both species are univoltine, overwintering as eggs laid under the leaf sheaths of introduced Phragmites [29, 30]. Larvae emerge in the spring and enter a young stem in which they feed on the tissue above the growing meristem [31]. Larvae typically feed on three shoots as they move through four instars (A. neurica) or four shoots through five instars (L. geminipuncta), inflicting varying degrees of damage on each host stem [29, 32]. Young stems attacked by early instars generally die, while older stems attacked by later instars suffer wilted stem tips and rarely produce flowers [32, 33]. During early instars, as many as ten L. geminipuncta larvae can be found feeding in a single stem, while A. neurica are strictly solitary feeders [32]. Mature larvae pupate in a new shoot and adults emerge after approximately 26 days (A. neurica) or 39 days (L. geminipuncta) [32]. Lenisa geminipuncta is the most common pest of Phragmites australis in Europe and has been observed to damage up to 90% of stems leading to reductions in above-ground biomass of 20 to 60% [34].
A petition to release both agents in Canada and the USA was submitted in 2018 [35]. In the USA, the insects were recommended for release by the USDA-APHIS Technical Advisory Group and are currently at a subsequent review step in the US regulatory process. In Canada, the Canadian Food Inspection Agency issued a release letter in 2019 and the first field releases were conducted in Ontario, Canada the same year. Since 2019, operational release protocols have been developed for eggs and larvae, prioritizing efficient release techniques that minimize the loss of agents to predation and buffer against phenological mismatches between the agents and the host weed [36]. Using these release methods, a total of 23,400 insects have been released across 30 sites in Ontario from 2019 to 2023.
In this article, we summarize the results of the first three years of L. geminipuncta and A. neurica releases in Canada, presenting the data in three main sections. First, we provide an overview of long-term monitoring data of agent feeding damage across the 30 release sites initiated between 2019 and 2023. These monitoring data can be used to assess initial agent release success, agent persistence over multiple years through overwintering and reproduction, changes in agent activity over time, and eventual long-term establishment. Second, we investigate the within-site distribution of agent feeding damage during the initial years post-release. We used the monitoring data to compare feeding damage intensity and distribution within sites and to compare agent feeding between introduced Phragmites edge and interior habitats. Understanding these within-site patterns of agent feeding damage will help improve monitoring protocols and facilitate the collection and redistribution of agents from established nurse sites. Third, we report the results of a larval release density experiment conducted across eight sites in 2023. The experiment sought to identify optimal release densities that would efficiently use limited biological control agents to maximize damage to the weed while minimizing agent loss through presumed intraspecific competition. Because this biological control program is in the early stages, the availability of the two agents for releases varied greatly year-to-year. Given this and the very similar life histories of A. neurica and L. geminipuncta, we have combined monitoring data from all sites for both agents for the purposes of this study.
Materials and methods
Release methods and sites in Ontario
From 2019 to 2023, A. neurica and L. geminipuncta have been released into the field using seven release methods spanning all life stages (i.e., eggs, larvae, pupae, and adults). Descriptions of the basic release techniques and the number of times that they have been used to date are provided (Table 1). Egg and larval release methods are described in greater detail elsewhere [36], with egg cups and stem larvae currently recommended as the best and most frequently used release techniques. Relatively few releases have been conducted with A. neurica or L. geminipuncta pupae or adults due to the additional rearing and field logistics required (Fig 1).
Table 1. Overview of methods used for releasing Archanara neurica and Lenisa geminipuncta from 2019 to 2023 in Ontario, Canada.
| Release method | General description | Number of releases |
|---|---|---|
| Stem larvae | Hatching larvae are inoculated into cut Phragmites stems and transferred into the field in spring (~May to early June). | 24 |
| Egg cups | Eggs are placed in a closed cup with a screened bottom to exclude predators and staked in the field (in December or April to May). | 10 |
| Open pupae | Pupae are placed in open air vermiculite-filled containers and transferred to the field in summer (~June). | 4 |
| Caged pupae | Pupae are placed in open air vermiculite-filled containers and transferred to the field in mesh-enclosed cages in summer (~June). | 2 |
| Egg cards | Eggs are glued to paper cards secured to dead standing stems in the field in early spring (~April to early May). | 2 |
| Loose larvae | Larvae are scattered directly into the field amongst emerging stems within 24 h of hatching in spring (~May). | 1 |
| Caged moths | Moths are placed in portable mesh cages and transferred to larger mesh cage enclosures in the field in summer (~June). | 1 |
Release methods are organized by frequency of use. Number of releases indicates how many times each method has been used to date at a release site.
Fig 1. Additional release methods for Archanara neurica and Lenisa geminipuncta pupae and adults.
Pupae can be placed in vermiculite in the bottom of containers with open side panels and fiberglass/metal mesh surfaces to allow emerging moths to climb up to exit (A) and then hung out in the open (B) or in mesh cages (C). Adults can be transported to the field and released directly into mesh cages (C).
As of fall 2023, 8,317 A. neurica and 12,401 L. geminipuncta eggs, larvae, pupae, and adults have been released using these methods at 30 locations across southern Ontario, Canada (Table 2). Site permits were obtained as required (e.g., for lands managed by conservation authorities) but were not needed for all sites (e.g., private lands, public roadsides) (S1 Table). Release sites are numerically labelled in approximate order of release date. Releases ranged from small, single plot releases designed solely to establish the biocontrol agents on the landscape, to large-scale manipulative experiments testing various operational and biological questions of interest. In most cases, a single set of releases was performed concurrently at each site, with the exceptions of several early release sites (P02, P03, P04, P05, P06) that received a second year of releases. Both species were initially released in 2020 at P02 and P03, but no eggs hatched during the 2020 season (see [36] for additional details) so agents were re-released in 2021. Because no hatch was ever observed in 2020, releases in that year were not counted towards the total numbers released. Agents were also released in 2021 at sites P02, P04, and P05, and again in 2022 for smaller experimental tests of the release methods. However, because the majority of agents at these sites were released in 2021, this was considered the baseline release year for monitoring purposes. Finally, because no activity was observed following the open pupal release at P06 in 2021, agents were re-released in 2023 and this was considered the baseline year for monitoring.
Table 2. Summary of Archanara neurica and Lenisa geminipuncta biological control releases from 2019 to 2023 in Ontario, Canada.
| Site | Release year(s) | Release method(s) | Archanara neurica | Lenisa geminipuncta | Additional analyses |
|---|---|---|---|---|---|
| P01: Davern | 2019 | Caged pupae | 42 | - | - |
| P02: Aurora | 2020, 2021*, 2022 | Egg cards, egg cups, loose larvae, stem larvae | - | 3285 | Y1-2, Y2-3, DD (Y2, Y3), EI (Y3) |
| P03: Wainfleet | 2020, 2021* | Egg cards, egg cups, open pupae | - | 52 | Y2-3, DD (Y2, Y3), EI (Y3) |
| P04: Sinclair Campbell | 2021*, 2022 | Egg cups, stem larvae, caged pupae | 68 | 1995 | Y1-2, Y2-3, DD (Y2, Y3) |
| P05: Oshawa | 2021*, 2022 | Egg cups, stem larvae | - | 2538 | Y1-2, Y2-3, DD (Y2, Y3), EI (Y3) |
| P06: Koffler | 2021, 2023* | Stem larvae, open pupae | 420 | 52 | LD |
| P07: Aultsville | 2021 | Open pupae | - | 52 | Y2-3, DD (Y2, Y3) |
| P08: Madoc | 2021 | Open pupae | - | 52 | Y2-3, DD (Y3) |
| P09: Scarborough | 2022 | Egg cups, stem larvae | - | 1275 | Y1-2, DD (Y2), EI (Y2) |
| P10: Zoo | 2022 | Egg cups, stem larvae | 450 | - | Y1-2, DD (Y2), EI (Y2) |
| P11: Waterloo | 2022 | Egg cups, stem larvae | 1450 | 1450 | Y1-2, DD (Y2), EI (Y2) |
| P12: rare | 2022 | Egg cups, stem larvae | - | 1650 | Y1-2, DD (Y2), EI (Y2) |
| P13: Dunnville | 2022 | Caged moths | 180 | - | - |
| P14: Cranberry | 2023 | Egg cups, stem larvae | 1900 | - | - |
| P15: Mac Coutts | 2023 | Egg cups | 780 | - | - |
| P16: Collavino | 2023 | Stem larvae | 840 | - | LD |
| P17: Cooper | 2023 | Stem larvae | 420 | - | LD |
| P18: Whitby | 2023 | Stem larvae | 140 | - | LD |
| P19: Brickworks | 2023 | Stem larvae | 280 | - | LD |
| P20: St. Lukes | 2023 | Stem larvae | 560 | - | LD |
| P21: Brimblecombe | 2023 | Stem larvae | 280 | - | LD |
| P22: North Bay | 2023 | Stem larvae | 149 | - | LD |
| P23: Garrard | 2023 | Stem larvae | 40 | - | - |
| P24: Nichol | 2023 | Stem larvae | 40 | - | - |
| P25: Victoria | 2023 | Stem larvae | 40 | - | - |
| P26: Gordon | 2023 | Stem larvae | 40 | - | - |
| P27: Lakeridge | 2023 | Stem larvae | 40 | - | - |
| P28: Cochrane | 2023 | Stem larvae | 40 | - | - |
| P29: Brooklin | 2023 | Stem larvae | 40 | - | - |
| P30: Donkey | 2023 | Stem larvae | 78 | - | - |
In sites with multiple release years, “*” indicates the initial baseline year that was used for monitoring feeding damage by the agents. Totals include the overall number of eggs / larvae / pupae / adults released for each species. The following codes indicate which sites were included in additional analyses beyond basic descriptive statistics, including the year(s) of monitoring (Y), if relevant: Y1-2 (comparison of feeding damage from year 1 to year 2); Y2-3 (comparison of feeding damage from year 2 to year 3); DD (damage distribution analyses, including comparison of damage density and coverage, and VMR); EI (comparison of feeding damage and coverage between patch edge and interior); and LD (larval density release trial).
Overview of release site monitoring
The primary goal of this study was to assess the presence and relative activity level of biological control agents through feeding damage as a means of evaluating release success, agent persistence, and eventual establishment at release sites. The program is also developing additional monitoring protocols to track longer-term agent demographics, dispersal, impacts on introduced Phragmites, and plant community responses that are beyond the scope of this study. We determined that the most practical and informative primary indicator of overall agent activity to monitor across all sites was summer feeding damage by A. neurica and L. gemininpuncta. Larval stem-mining by both species produces dead or wilted stems with one or two bore holes [31]. Stem damage is most obvious in June to early July when the brown wilted stems stand out against the young green introduced Phragmites stems (personal observation). As a result, larval feeding damage provides an informative measure of overall biological control agent presence and activity, and it can be measured non-destructively with high confidence and low disturbance to the Phragmites stand and the developing biological control agents. As larvae typically remain within ~ 3 m from the point of hatching whereas moths may disperse to find new oviposition sites throughout and between patches of introduced Phragmites [31], we developed different monitoring protocols for assessing agent feeding damage in year one and in subsequent years; year one focuses on feeding damage immediately around release points, while monitoring in year two onwards focuses on an overall assessment of feeding damage across an entire patch of introduced Phragmites. Both protocols produce the same unit of measurement (i.e., number of damaged stems per m2) but represent differ areas of coverage (i.e., year one is only the area immediately around release points, year two is the whole patch). For both the plot and patch-level monitoring protocols, we decided to express feeding damage as a function of areas rather than as an attack rate (i.e., % of stems with feeding damage). Counts of damaged stems provide an easy to measure and interpret assessment of agent presence and activity, while stem attack rates can vary based on underlying stem density and require additional measurements that are not practically scalable to this particular patch-level monitoring protocol. However, stem attack rates may be an important component of future monitoring protocols designed to measure agent impacts on the target weed.
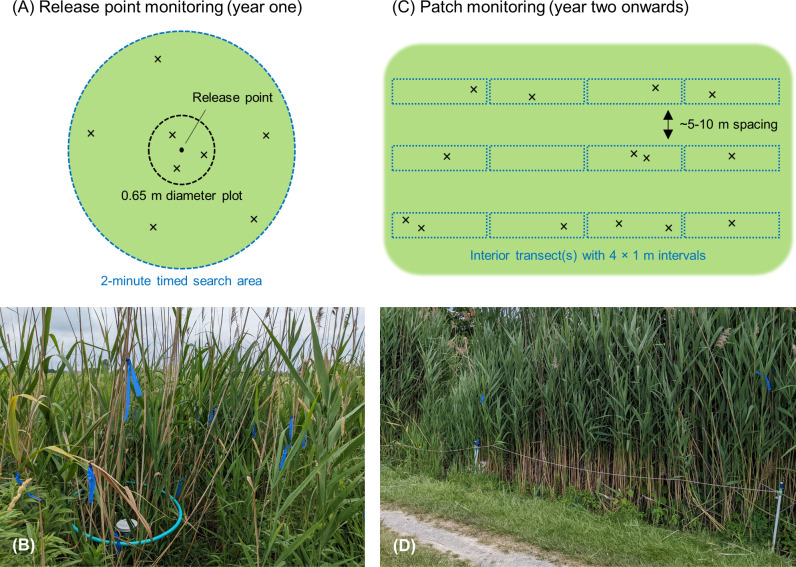
Initial release point monitoring was used for the first year in which eggs or larvae were released at a site to determine that amount of larval feeding damage immediately around each release point. Initial feeding damage could not be assessed for releases using pupae or moths. A 0.65 m-diameter plot was established around each release point and a count was made of the number of damaged stems per m2. Beginning in 2022, we determined that initial release point monitoring could be enhanced by measuring the total count of damaged stems in the 0.65 m plot and found during a 2-min timed search in a 3-m radius area around the plots (Fig 2A and 2B). While we recommend continued use of this combined plot and timed search damage estimate for initial release point monitoring going forward, because timed searches were not used for release sites from 2019 to 2021, for this analysis only we report only the number of damaged stems per m2 from the 0.65 m plots as initial release point monitoring data.
Fig 2. Release point and patch level monitoring protocols to assess feeding damage by released biocontrol agents.
Schematic and photographic representations of monitoring protocols for (A, B) release point monitoring of new egg or larval releases in year one, and (C, D) patch monitoring of damage in subsequent years. The green background area depicts introduced Phragmites and “×” denotes hypothetical agent-damaged stems that would be documented by the monitoring). The number, arrangement, and length of transects were determined on a site-by-site basis to provide practical and representative coverage based on the size and shape of the introduced Phragmites patch.
Patch monitoring was used in subsequent years after an adult flight period had occurred and agents had a chance to disperse further within a patch of introduced Phragmites. This protocol was intended as an overall assessment of agent feeding throughout a patch. Agent-damaged stems were quantified in 4-m2 intervals (4 × 1 m) run contiguously along one or more transects spaced across the patch (Fig 2C and 2D). The number, arrangement, and length of transects were determined on a site-by-site basis to provide practical and representative coverage based on the size and shape of the introduced Phragmites patch. To the extent that was practical in the field, transects were run parallel with ~5- to 10-m spacing between them. Once determined for a site, transect configuration was maintained in subsequent monitoring years for within-site comparability. Of the sites that received patch level monitoring between 2022 and 2023, sites were documented using a range of 1 to 6 transects and 14 to 66 4-m2 monitoring intervals per site (S2 Table). Counts of damaged stems from the 4-m2 intervals were used to calculate mean feeding damage density (number of damaged stems per m2) at the site level, and damage coverage (% of 4-m2 intervals with one or more damaged stems).
As of 2023, the release point monitoring protocol was used at all sites where eggs or larvae were released. Patch-level monitoring was conducted at all 30 sites, annually, beginning one-year post-release. In total, release point monitoring in year one and patch monitoring in years two and three were collected from 2019 to 2023 for 25, 11, and 7 sites respectively (the changing numbers reflect the availability of sites in each category from newest to oldest and the necessary omission of releases using pupae or adults from the year one release point monitoring). These monitoring data were used to generate basic descriptive statistics characterizing agent feeding damage from the first several years of evaluation of releases. We also assessed whether feeding damage observed in a given year was a reliable predictor of damage in the subsequent year. To do this, we tested for an association between mean site-level release point year one feeding damage (number of damaged stems per m2 around the release point) with mean patch level year two feeding damage (number of damaged stems per m2 from 4 × 1 m monitoring intervals) (n = 7 sites). We then similarly tested for an association between mean patch level damage between years two and three (n = 6 sites).
Feeding damage distribution within sites
To determine the relationship between feeding damage intensity and how it was distributed across a site, we tested for an association between site-level mean damage density and damage coverage from patch level monitoring data pooled from years two (n = 9 sites) and three (n = 6 sites). For those same 15 data collections, we also calculated average variance to mean ratio (VMR or Fisher’s Index of Aggregation) as a simple measure of spatial aggregation of feeding damage density across the 4 × 1 m monitoring intervals at each site. VMR < 1 suggests a uniform distribution, VMR = 1 suggests a random distribution, and VMR > 1 suggests aggregation.
We also used patch level monitoring data from sites in 2023 to determine whether A. neurica/L. geminipuncta feeding damage was more common in the interior or edge habitats of introduced Phragmites at the release sites. Of the sites that were monitored for patch-level feeding damage in 2023, we selected seven with larger introduced Phragmites patches that could be used to clearly delineate monitoring intervals placed either along the perimeter of the patch (i.e., edge habitat) or into the middle of the patch a minimum of 5 m away from the edge (i.e., interior habitat). Four were in year two of monitoring (P09, P10, P11, P12) and three were in year three (P02, P03, P05). Sites consisting of long, narrow corridors of introduced Phragmites were excluded because their configuration did not create a clear difference patch edges and interiors. Additional perimeter transects were placed around the introduced Phragmites patches at these sites as needed and monitored using the same patch-level protocol (see S2 Table for an overview of the interior and edge transects and number of monitoring intervals per site). Mean density of feeding damage and damage coverage were compared between the edge and interior transects of each site. We also tested whether the damage density or damage coverage at the edge of an introduced Phragmites patch could predict the amount of damage density or coverage in the interior.
Effects of larval density on initial release success
An experiment was conducted to determine the effectiveness of releasing different densities of larval-inoculated stems (20, 40, or 80 A. neurica larvae per release). Forty stems were chosen as a practical release density previously used for releases in 2022, and 20 and 80 stems were selected as release rates half and twice as high respectively. Blocks of larvae-inoculated stems were prepared using standard methods [36]. Blocks with 20 or 40 larvae-inoculated stems or two blocks of 40 stems for 80 treatments were released across eight sites from 9 to 25 May 2023. Each site received one to six replicates of each density treatment based on the size of the site (P06 × 3 replicates, P16 × 6, P17 × 3, P18 × 1, P19 × 2, P20 × 4, P21 × 2, and P22 × 1), resulting in n = 22 replicates for the three density treatments and 66 releases in total. Stem blocks were placed ~1–5 m into the edge of each patch and separated at least 5 m between plots. At the time of the release, the density of living stems and the mean height of living stems in a circular quadrat around the release point (0.65 m diameter) were measured. Plots were monitored for initial feeding damage between June 27 and July 11, 2023 using the standard release point protocol.
Statistical analyses
To analyze the overall site-level monitoring data, mean feeding damage from release point and patch level monitoring were calculated for all sites and summary statistics of feeding density were calculated for all three years of monitoring data. We used linear models to assess relationships between release point damage in year one and patch-level damage in year two, and between patch-level damage in years two and three. For all statistical models, unless otherwise noted, assumptions of data or residual normality and heteroscedasticity were assessed by visual inspection of quantile-quantile plots from ggpubr [37] and boxplots respectively. Different transformations were used on the predictor and response variables and the best fitted models were selected using AIC.
To assess within-site patterns of agent feeding damage, we created linear mixed models with lme4 [38] to characterize the relationship between patch-level damage density and damage coverage, with follow-up monitoring year as an additional fixed effect and site as a random effect. We also used paired t-tests to compare damage density and damage coverage between edge and interior transects across the seven sites. Linear regression was then applied to model the relationship between edge/interior damage and coverage. Different transformations were used on the predictor variables and the best-fitted models were selected using AIC.
For the larval release density experiment, we assessed the effects of larval release density on total damage using linear mixed models with the lme4 package [38]. Larval release density and the density and height of living Phragmites stems at the time of release were included as fixed factors and site as a random factor. Early versions of the models included interactions between all fixed effects, but these terms were not significant and were subsequently removed (data not shown). Pairwise post-hoc comparisons between larval density treatments were performed on estimated marginal means of significant models with a Tukey adjustment using emmeans [39]. Spearman’s correlation was assessed between living stem density and total damage to account for lack of bimodal normality using agricolae [40].
Unless otherwise stated, all statistical analyses were conducted using R [41] and R Studio [42] at α = 0.05. Data can be accessed online through the Zenodo repository [43].
Results
Overview of release site monitoring
The results of site-level release point and patch-level monitoring of A. neurica and L. geminipuncta feeding damage are presented by site (Table 3) and as summary statistics by monitoring year (Table 4). Detection of damage caused by agent feeding was high across all monitoring periods, with damage detected at all but four locations as of the most recent monitoring period in 2023. Sites with no current evidence of activity by the biological control agents include one site that received an experimental caged pupal release (P01), a municipal roadside site that was mown and destroyed post-release (P25), and two municipal roadside sites that experienced partial disturbance from mowing and had received releases of a small number of older larvae (P27, P29). Mean site-level feeding damage remained similar or increased across most sites from year two to three. Feeding damage was also initially undetected at one release site that received an open pupal release (P08) but subsequently appeared at low levels (0.1 ± 0.1 stems per m2) the following year.
Table 3. Release-point and patch-level monitoring of feeding damage caused by biological control agents released across 30 sites in Ontario, Canada between 2019 and 2023.
| Site | Year 1 [release point] | Year 2 [patch level] | Year 3 [patch level] |
|---|---|---|---|
| P01: Davern | N/A | 0.0 ± 0.0 (n = 14) | 0.0 ± 0.0 (n = 14) |
| P02: Aurora | 12.3 ± 16.0 (n = 11) | 2.0 ± 2.4 (n = 38) | 3.4 ± 2.0 (n = 39) |
| P03: Wainfleet | N/A | 0.1 ± 0.2 (n = 18) | 0.1 ± 0.2 (n = 18) |
| P04: Sinclair Campbell | 18.5 ± 16.8 (n = 14) | 5.2 ± 4.5 (n = 20) | 4.7 ± 3.5 (n = 16) |
| P05: Oshawa | 3.7 ± 8.2 (n = 9) | 0.1 ± 0.3 (n = 53) | 0.8 ± 1.0 (n = 42) |
| P07: Aultsville | N/A | 0.1 ± 0.2 (n = 15) | 0.5 ± 0.8 (n = 16) |
| P08: Madoc | N/A | 0.0 ± 0.0 (n = 17) | 0.1 ± 0.1 (n = 16) |
| P09: Scarborough | 1.4 ± 3.9 (n = 11) | 0.2 ± 0.3 (n = 20) | - |
| P10: Zoo | 8.7 ± 15 (n = 3) | 0.3 ± 0.4 (n = 14) | - |
| P11: Waterloo | 14.1 ± 12.4 (n = 38) | 3.3 ± 2.2 (n = 66) | - |
| P12: rare | 12.9 ± 14.2 (n = 11) | 1.8 ± 2.6 (n = 55) | - |
| P06: Koffler | 28.1 ± 12.4 (n = 9) | - | - |
| P13: Dunnville | 0.2 ± 0.2 (n = 6) | - | - |
| P14: Cranberry | 11.0 ± 10.3 (n = 30) | - | - |
| P15: Mac Coutts | 13.4 ± 8.2 (n = 9) | - | - |
| P16: Collavino | 33.5 ± 20.3 (n = 18) | - | - |
| P17: Cooper | 25.4 ± 13.1 (n = 9) | - | - |
| P18: Whitby | 9.0 ± 8.0 (n = 3) | - | - |
| P19: Brickworks | 15.6 ± 10.3 (n = 6) | - | - |
| P20: St. Lukes | 26.1 ± 12.6 (n = 12) | - | - |
| P21: Brimblecombe | 15.1 ± 12.9 (n = 6) | - | - |
| P22: North Bay | 6.0 ± 10.4 (n = 3) | - | - |
| P23: Garrard | 6.0 (n = 1) | - | - |
| P24: Nichol | 6.0 (n = 1) | - | - |
| P25: Victoria | (Site destroyed) | - | - |
| P26: Gordon | 9.0 (n = 1) | - | - |
| P27: Lakeridge | 0.0 (n = 1) | - | - |
| P28: Cochrane | 3.0 (n = 1) | - | - |
| P29: Brooklin | 0.0 (n = 1) | - | - |
| P30: Donkey | 1.5 ± 2.1 (n = 2) | - | - |
Table 4. Summary of monitoring of feeding damage by biological control agents by year.
| Monitoring period | Number of sites | Sites with damage (%) | Mean damage ± SD (stems per m2) | Mean damage coverage ± SD (% of intervals with damaged stems) |
|---|---|---|---|---|
| Year 1 | 25 | 92 | 11.2 ± 9.3 | N/A |
| Year 2 | 11 | 82 | 1.2 ± 1.7 | 50 ± 38 |
| Year 3 | 7 | 86 | 1.4 ± 1.9 | 51 ± 40 |
Note that year one damage is measured at the release point scale while year two onwards is measured at patch scale. Damage coverage cannot be measured during year one monitoring.
Feeding damage (number of damaged stems per m2) is presented as mean ± standard deviation with the number of release point plots (year one) or monitoring intervals (years two and three) monitored for each site. “N/A” indicates sites for which release point larval feeding damage could not be monitored during the release year because pupae or moths were released. Dashes (“-”) indicate sites that were not old enough for data to have been collected.
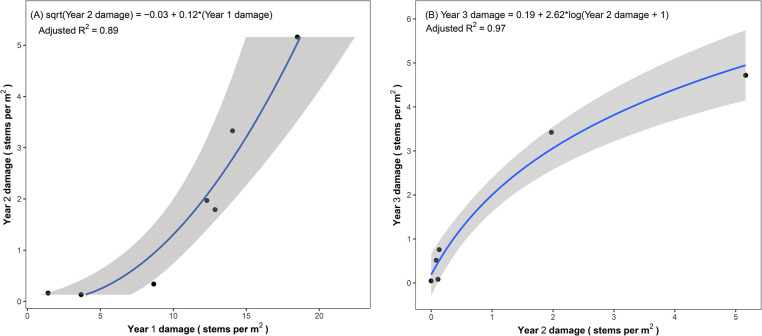
Initial feeding damage observed during the release year was a strong predictor of the amount of feeding damage observed in year two (F1,5 = 47.62, p < 0.001, adjusted R2 = 0.89) (Fig 3A), which in turn was a strong predictor of the amount of feeding damage observed in the third year (F1,4 = 176, p < 0.001, adjusted R2 = 0.97) (Fig 3B).
Fig 3. Feeding damage caused by the biological control agents in a given year predicted feeding damage in subsequent years.
Scatterplots with regression lines depicting the relationships of the amount of agent feeding damage (number of damaged stems per m2) observed between (a) release point monitoring in year one and patch level monitoring in year two (n = 7 release sites), and (b) between the second and third years of patch level monitoring (n = 6 release sites). The shaded area depicts a 95% confidence interval.
Feeding damage distribution within sites
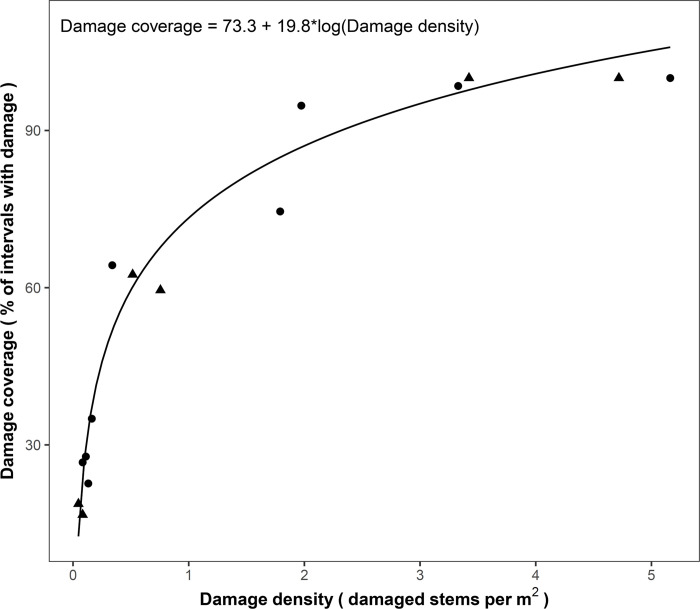
Analyzing 15 data points of patch-level monitoring from years two and three (Table 2), the damage density was a significant predictor of damage coverage (F1,12.02 = 332.04, p < 0.001) and damage coverage increased linearly with the natural logarithm of damage density (Fig 4). The relationship between damage density and coverage did not differ between monitoring years two and three (F1,5.13 = 0.47, p = 0.52). Across the same 15 data collections, mean VMR was 1.4 ± 1.2 (± SD) (range 0.22 to 3.99) suggesting stem damage was clumped or aggregated within sites.
Fig 4. Density of feeding damage and damage coverage caused by the biological control agents was related.
Scatterplot of the density of agent feeding damage (number of damaged stems per m2) and coverage (% of monitoring intervals with ≥ 1 damaged stems) during follow-up monitoring (total n = 15 sites, circles: year two, triangles: year three). The regression line was produced from a linear mixed-effects model of damage coverage as a function of the natural logarithm of damage density with monitoring year as a fixed effect (non-significant) and site as a random effect.
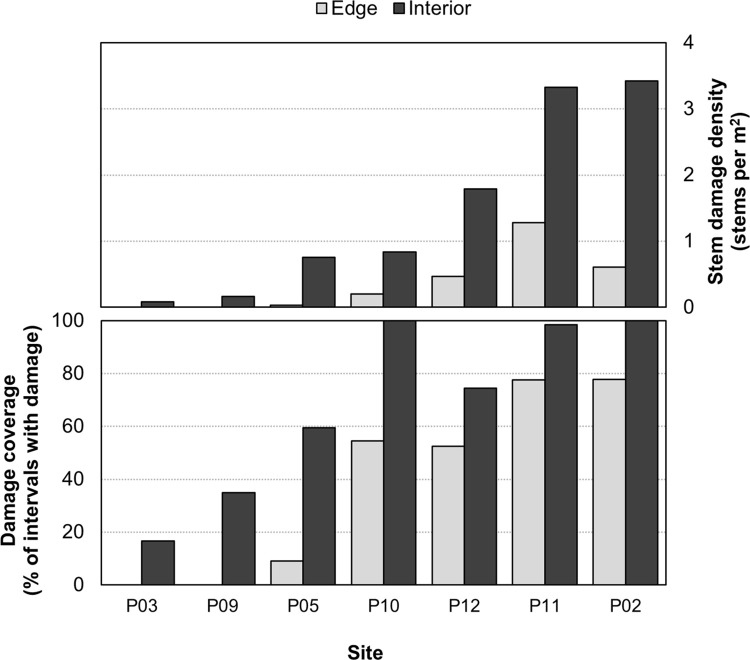
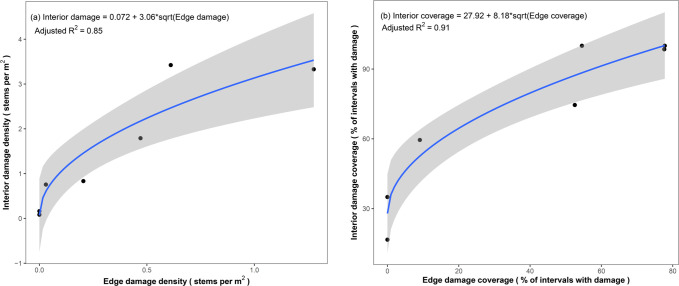
At the seven biological control sites assessed in summer 2023 (Table 2), interior transects had higher rates of biological control agent feeding damage (t = 2.90, df = 6, p = 0.03) and damage coverage (t = 6.03, df = 6, p = 0.001) compared to edge transects. Interior damage was 4-fold higher than edge damage and interior coverage 30% higher than edge coverage (Fig 5). Edge damage was also a significant predictor of interior damage (F1,5 = 34.16, p = 0.002, adjusted R2 = 0.85), and edge coverage was a significant predictor of interior coverage (F1,5 = 59.97, p < 0.001, adjusted R2 = 0.91) (Fig 6).
Fig 5. Density of feeding damage and damage coverage caused by released biological control agents were higher in patch interiors compared to edges of Phragmites plots.
Comparison of (A) mean stem damage density (number of damaged stems per m2 of monitoring transect) and (B) stem damage coverage (% of 4 × 1 m monitoring transects with ≥ 1 damaged stems) between edge (light grey) and interior (dark grey) habitats at seven biological control release sites for introduced Phragmites.
Fig 6. Relationship of feeding damage and damage coverage caused by released biological control agents between patch edges and interior areas of Phragmites plots.
Scatterplots with regression lines of edge and interior (A) stem-damage density (number of damaged stems per m2 of monitoring transect) and (B) stem damage coverage (% of 4 × 1 m monitoring transects with ≥ 1 damaged stems) at seven biological control release sites for introduced Phragmites. The shaded area depicts a 95% confidence interval.
Effects of larval density on initial release success
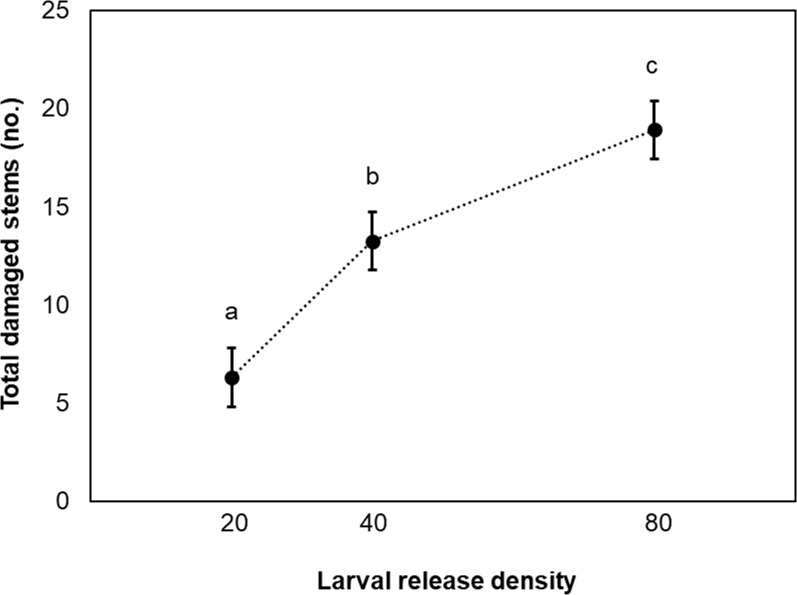
Larval release density affected the number of damaged stems around release points (F2,55.44 = 25.77, p < 0.001), as did the density of living introduced Phragmites stems at the time of release (F1,33.20, p = 0.004, β = 0.11, SE = 0.04). Based on the estimated marginal means of the model, damage increased with the number of larvae released. However, the amount of damage increased 2.1-fold when doubling the release density from 20 to 40 larvae but only increased by 1.4-fold when doubling again from 40 to 80 larvae (Fig 7). Independent of larval release density, the total number of damaged stems increased moderately with the density of living introduced Phragmites stems (rs(64) = 0.39, p = 0.001). Mean stem height at the time of release was 50 ± 14 cm and was not a significant predictor of feeding damage (F1,32.74 = 2.92, p = 0.10).
Fig 7. Damage of Phragmites stems by released biological control agents increased with higher larval release densities, albeit with diminishing returns.
Total damaged stems around Archanara neurica release plots (i.e., the sum of damaged stems found in a 0.65-m diameter centered on the release points and during a 2-min timed search around the plots) receiving different densities of released larvae (20, 40, 80) (n = 22 plots per release density). Dots depict estimated marginal means from a mixed effects model of larval density, living stem density, living stem height, and site, error bars denote standard error, and letters indicate post-hoc groupings (means that do not share a letter are statistically significantly different).
Discussion
Initial monitoring demonstrates release and overwintering success
Long-term monitoring is essential to evaluate the success of biological control programs and invasive weed management [19, 44]. Immediate post-release and subsequent annual monitoring to assess agent presence and activity have therefore been a priority for the program targeting introduced Phragmites in Ontario, Canada. Initial release-point monitoring of 25 sites where A. neurica and L. geminipuncta were released revealed detectable Phragmites damage in 92% of the sites during the year of agent release. Those sites without detectable release-point damage experienced unplanned site disturbances or did not use the currently recommended egg and larval release techniques [36]. At all sites where damage was observed in the year of release, agent activity was also observed in subsequent years, with the density of damage often increasing between years two and three. The amount of damage caused by agent feeding observed immediately post-release was strongly predictive of the amount of damage that would be seen in the subsequent year of monitoring, which in turn was predictive of the following year. The combination of dedicated release point and patch-scale monitoring protocols was effective and efficient at assessing agent feeding damage across a growing number of large weed populations. This practical and scalable approach could be adapted to monitor the progress of other weed and biological control systems [44].
These initial results are highly encouraging for the Phragmites biological control program. First, they underline that successful release methods have been developed for A. neurica and L. geminipuncta [36]. Second, they confirm successful reproduction and overwintering of both A. neurica and L. geminipuncta in their introduced range, suggesting broad climatic compatibility [45] and phenological synchrony across southern Ontario [46, 47]. Finally, our results indicate that releases of even relatively small numbers of agents are sufficient to initiate a population, and that achieving significant damage in the year of release also tends to yield a proportionate increase in damage the following year. The predictive capacity of initial monitoring results will be useful to decide whether a site with low initial damage post-release may benefit from supplementary releases the following year to improve chances of agent establishment.
Patterns of feeding damage to inform monitoring and agent recollection
Release sites with multiple years of monitoring data reveal several patterns of within-site agent feeding damage that can improve monitoring and harvesting of agents from nurse sites. Monitoring results from the second and third years after release suggest that the biological control agents are highly mobile, with feeding damage found in 100% of monitoring intervals at several sites as early as two- or three-years post-release. While there was a positive correlation between stem feeding damage density and damage coverage, even sites with low densities of feeding demonstrated relatively high dispersal throughout sites. Additionally, although the majority of agents were released within 1 to 5 m of patch edges, damage density and coverage were generally higher in patch interiors, indicating the efficient spread of agents throughout Phragmites infestations.
These findings also suggest larval or oviposition preferences for microsites in the interior of patches, or differential larval/egg survival associated with interior locations. These differences may be due to within patch variation in factors such as stem density, stem diameter, predation, or wind disturbance. A priority for future research will be to investigate within-site agent habitat selection, survival, and dispersal. Because most initial larval feeding damage is observed within ~ 3 m of the release points (McTavish, unpublished data), adult flight may be the principal contributor to within patch dispersal of A. neurica and L. geminipuncta. While adult flight is currently expected to be the primary means of dispersal of A. neurica and L. geminipuncta, many stem-boring lepidopteran larvae can disperse relatively long distances by crawling or “ballooning” [48–50]. Identifying the primary mode of within and between patch spread will contribute to understanding population growth patterns that will become evident during longer-term impact assessments.
These initial insights into the within-site patterns of agent feeding damage will greatly enhance the introduced Phragmites biological control program. First, damage density and coverage can be monitored at the edges of less accessible sites as a potential metric of overall site condition. Second, these results will help identify where to best collect agents for redistribution from potential biocontrol nurse sites to help grow the program to a landscape scale [51, 52]. Until now, all releases of A. neurica and L. geminipuncta in Canada have been insects reared in laboratories either at CABI, Switzerland or the University of Toronto. Understanding within site habitat selection and dispersal could help guide harvesting of field-adapted agent eggs from the field, reducing rearing costs and potentially preventing declines in agent fitness associated with long-term captive rearing [53–55].
Intermediate larval densities as an optimal release strategy
Given that we found larvae to be the leading release strategy for A. neurica and L. geminipuncta [36], in 2023 we used A. neurica to refine an optimal density for releasing larvae. While releasing more larvae consistently produced more feeding damage immediately post-release, intermediate release densities proved to be the most efficient use of larvae. Our results indicated that a single release of 40 A. neurica larvae produced slightly more damage than two releases of 20 larvae. However, a single release of 80 larvae did not generate as much damage as two releases of 40 larvae. Based on these results, releases of 40 larvae appear to be optimal, providing sufficient insects to presumably survive various sources of mortality such as predation [25, 29, 32] while limiting losses to suspected intraspecific competition [56].
Results of our larval density release experiment also suggested that greater damage will occur when more introduced Phragmites stems are available in a 0.65-m diameter quadrat centered on the release point. Given that stem phenology during the early season can influence release success [36], these findings further demonstrate the importance of releasing an appropriate density of insects into a microsite with a sufficient number of host stems at appropriate maturity. Surprisingly, the stem height of introduced Phragmites at the time of agent release, however, was not a significant predictor of post-release feeding damage. This suggests that the releases were successfully conducted at times when there was a sufficient density of phenologically appropriate introduced Phragmites stems, or that the agents can be released into patches with a broader range of stem heights than expected with good results. Because of its strict solitary feeding and cannibalism, we speculate that the performance of A. neurica may be more constrained by intraspecific competition for resources than that of L. geminipuncta [56], and therefore it will be more sensitive to higher release densities. We therefore recommend that future experiments investigate the sensitivity of both species to phenological variation in introduced Phragmites stem density and height at the time of release.
Future research and recommendations
Our results document an extremely encouraging start to the Phragmites biological control program in Canada, with both A. neurica and L. geminipuncta inflicting detectable feeding damage on introduced Phragmites that persists through the first three years of monitoring post-release. Annual monitoring of agent feeding activity will be continued at all sites to evaluate whether agents continue to persist and increase in activity at release locations. With agents now persisting across release sites for multiple years, additional standardized monitoring protocols are being developed and implemented to evaluate long-term agent demographics, agent dispersal, impacts on introduced Phragmites populations, and plant community responses to biological control. Going forward, another key area of study will be the ability of the two agents to establish and thrive across the wide range of conditions in which introduced Phragmites grows in North America. This will include study of the agents’ response to variation in introduced Phragmites densities, stem sizes, and hydrological conditions, as well as the potential to integrate biocontrol with other management tools. While most of the releases in Canada to date have been a single species, future releases will also investigate the potential interactive impacts and performance of releasing one or both species together under a range of environmental conditions.
Finally, there are recommendations for the ongoing expansion of the Phragmites biological control program based on the early years of monitoring. First, the data support a combination of large and small releases of A. neurica and L. geminipuncta. Large releases will help achieve the high initial damage and growth required to generate robust “nurse sites” for further agent redistribution, while small releases are expected to be sufficient to establish a broader network of inoculated sites across the landscape. Second, we recommend that A. neurica larval releases use iterations of 40 larvae per release point as this was an effective and efficient release density. Finally, efforts to collect eggs from “nurse sites” should focus on stems from patch interiors, where agent activity is highest. Transferring agents between weed infestations has been used to great effect in past successful weed biological control programs including purple loosestrife [57], leafy spurge [58], and knapweed [59]. Our study has demonstrated that we can get the Phragmites biocontrol agent insects to persist on the landscape. Developing efficient methods to locate and recollect the insects from nurse sites is the next key step for biological control of introduced Phragmites in North America.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We thank Carla Timm, Lauren Janke, Jasmine Carpick, Adonis Doherty, Jolie Nguyen, and Ayumi Akimoto (University of Toronto) for laboratory support, Patrick Häfliger (CABI) for expertise and recommendations related to the biological control agents, and Claire Schon and Rebecca Rooney (University of Waterloo) for collecting release data from three of the release sites (P11, P12, and P15).
Data Availability
Data are available from the Zenodo repository (https://zenodo.org/records/10637131).
Funding Statement
Funding for this work was provided by Agriculture and Agri-Food Canada (RB, https://agriculture.canada.ca/en), the University of Toronto (SS, https://www.utoronto.ca/), Ducks Unlimited Canada (MM, IJ, https://www.ducks.ca/), the Invasive Species Centre (SS, https://www.invasivespeciescentre.ca/), and the Ontario Ministry of Natural Resources and Forestry (SS, https://www.ontario.ca/page/ministry-natural-resources-and-forestry). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Marks M, Lapin B, Randall J. Phragmites australis (P. communis): threats, management and monitoring. Natural Areas Journal. 1994;14(4):285–94. [Google Scholar]
- 2.Saltonstall K. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci U S A. 2002. Feb 19;99(4):2445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catling PM, Mitrow G. The recent spread and potential distribution of Phragmites australis subsp. australis in Canada. Can Field-Nat. 2011. Jun;125(2):95–104. [Google Scholar]
- 4.Lambert AM, Saltonstall K, Long R, Dudley TL. Biogeography of Phragmites australis lineages in the southwestern United States. Biol Invasions. 2016. Sep;18(9):2597–617. [Google Scholar]
- 5.Packer JG, Meyerson LA, Skálová H, Pyšek P, Kueffer C. Biological flora of the British Isles: Phragmites australis. Journal of Ecology. 2017;105(4):1123–62. [Google Scholar]
- 6.Crocker EV, Nelson EB, Blossey B. Soil conditioning effects of Phragmites australis on native wetland plant seedling survival. Ecology and Evolution. 2017;7(15):5571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer SW, Badzinski SS, Petrie SA, Ankney CD. Seasonal abundance and species richness of birds in common reed habitats in Lake Erie. Journal of Wildlife Management. 2010. Sep;74(7):1559–67. [Google Scholar]
- 8.Able KW, Hagan SM. Impact of common reed, Phragmites australis, on essential fish habitat: Influence on reproduction, embryological development, and larval abundance of mummichog (Fundulus heteroclitus). Estuaries. 2003. Feb 1;26(1):40–50. [Google Scholar]
- 9.Bolton RM, Brooks RJ. Impact of the seasonal invasion of Phragmites australis (common reed) on turtle reproductive success. Chelonian Conservation and Biology. 2010. Dec;9(2):238–43. [Google Scholar]
- 10.Chambers RM. Porewater chemistry associated with Phragmites and Spartina in a Connecticut tidal marsh. Wetlands. 1997. Sep 1;17(3):360–7. [Google Scholar]
- 11.Rooth JE, Stevenson JC, Cornwell JC. Increased sediment accretion rates following invasion by Phragmites australis: The role of litter. Estuaries. 2003;26(2):475–83. [Google Scholar]
- 12.Wails CN, Baker K, Blackburn R, Del Vallé A, Heise J, Herakovich H, et al. Assessing changes to ecosystem structure and function following invasion by Spartina alterniflora and Phragmites australis: a meta-analysis. Biol Invasions [Internet]. 2021. Apr 24 [cited 2021 Jun 7]; Available from: 10.1007/s10530-021-02540-5 [DOI] [Google Scholar]
- 13.Bhattarai GP, Meyerson LA, Cronin JT. Geographic variation in apparent competition between native and invasive Phragmites australis. Ecology. 2017;98(2):349–58. [DOI] [PubMed] [Google Scholar]
- 14.Meyerson LA, Viola DV, Brown RN. Hybridization of invasive Phragmites australis with a native subspecies in North America. Biol Invasions. 2010. Jan;12(1):103–11. [Google Scholar]
- 15.Williams J, Lambert AM, Long R, Saltonstall K. Does hybrid Phragmites australis differ from native and introduced lineages in reproductive, genetic, and morphological traits? American Journal of Botany. 2019;106(1):29–41. [DOI] [PubMed] [Google Scholar]
- 16.Hazelton ELG, Mozdzer TJ, Burdick DM, Kettenring KM, Whigham DF. Phragmites australis management in the United States: 40 years of methods and outcomes. AoB PLANTS [Internet]. 2014. Jan 1 [cited 2019 Jul 29];6. Available from: http://academic.oup.com/aobpla/article/doi/10.1093/aobpla/plu001/155942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin LJ, Blossey B. The runaway weed: Costs and failures of Phragmites australis management in the USA. Estuaries and Coasts. 2013. May;36(3):626–32. [Google Scholar]
- 18.Quirion B, Simek Z, Dávalos A, Blossey B. Management of invasive Phragmites australis in the Adirondacks: a cautionary tale about prospects of eradication. Biol Invasions. 2018. Jan 1;20(1):59–73. [Google Scholar]
- 19.Blossey B. A framework for evaluating potential ecological effects of implementing biological control of Phragmites australis. Estuaries. 2003;26(2):607–17. [Google Scholar]
- 20.Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, et al. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. The Lancet Oncology. 2015. May 1;16(5):490–1. doi: 10.1016/S1470-2045(15)70134-8 [DOI] [PubMed] [Google Scholar]
- 21.Kettenring KM, Adams CR. Lessons learned from invasive plant control experiments: a systematic review and meta-analysis. Journal of Applied Ecology. 2011;48(4):970–9. [Google Scholar]
- 22.Pollack A. Weed killer, long cleared, is doubted. The New York Times [Internet]. 2015. Mar 27 [cited 2019 Dec 16]; Available from: https://www.nytimes.com/2015/03/28/business/energy-environment/decades-after-monsantos-roundup-gets-an-all-clear-a-cancer-agency-raises-concerns.html [Google Scholar]
- 23.Blossey B, Endriss SB, Casagrande R, Häfliger P, Hinz H, Dávalos A, et al. When misconceptions impede best practices: evidence supports biological control of invasive Phragmites. Biol Invasions. 2020;22:873–83. [Google Scholar]
- 24.Casagrande RA, Häfliger P, Hinz HL, Tewksbury L, Blossey B. Grasses as appropriate targets in weed biocontrol: is the common reed, Phragmites australis, an anomaly? BioControl. 2018. Jun 1;63(3):391–403. [Google Scholar]
- 25.Schwarzländer M, Häfliger P. Shoot flies, gall midges, and shoot and rhizome mining moths associated with common reed in Europe and their potential for biological control. In: Proceedings of the X International Symposium on Biological Control of Weeds. Montana, USA; 2000. p. 397–420. [Google Scholar]
- 26.Tewksbury L, Casagrande R, Blossey B, Häfliger P, Schwarzländer M. Potential for biological control of Phragmites australis in North America. Biological Control. 2002. Feb;23(2):191–212. [Google Scholar]
- 27.Blossey B, Häfliger P, Tewksbury L, Dávalos A, Casagrande R. Host specificity and risk assessment of Archanara geminipuncta and Archanara neurica, two potential biocontrol agents for invasive Phragmites australis in North America. Biological Control. 2018. Oct;125:98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blossey B, Häfliger P, Tewksbury L, Dávalos A, Casagrande R. Complete host specificity test plant list and associated data to assess host specificity of Archanara geminipuncta and Archanara neurica, two potential biocontrol agents for invasive Phragmites australis in North America. Data in Brief. 2018. Aug 1;19:1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel R, Tscharntke T. Ursachen der Populationsdichteschwankungen von Schmetterlingen im Ökosystem Schilf (Phragmites australis Trin.). Mitteilungen der Deutschen Gesellschaft für Allgemeine und Angewandte Entomologie. 1993;8:511–5. [Google Scholar]
- 30.Mook JH, van der Toorn J. Delayed response of common reed Phragmites australis to herbivory as a cause of cyclic fluctuations in the density of the moth Archanara geminipuncta. Oikos. 1985;44(1):142–8. [Google Scholar]
- 31.Tscharntke T. Fluctuations in abundance of a stem-boring moth damaging shoots of Phragmites australis: Causes and effects of overexploitation of food in a late-successional grass monoculture. Journal of Applied Ecology. 1990;27(2):679–92. [Google Scholar]
- 32.Häfliger P, Schwarzländer M, Blossey B. Comparison of biology and host plant use of Archanara geminipuncta, Archanara dissoluta, Archanara neurica, and Arenostola phragmitidis (Lepidoptera: Noctuidae), potential biological control agents of Phragmites australis (Arundineae: Poaceae). Annals of the Entomological Society of America. 2006;99(4):683–96. [Google Scholar]
- 33.Häfliger P, Schwarzländer M, Blossey B. Impact of Archanara geminipuncta (Lepidoptera: Noctuidae) on aboveground biomass production of Phragmites australis. Biological Control. 2006. Sep;38(3):413–21. [Google Scholar]
- 34.Tscharntke T. Insects on common reed (Phragmites australis): community structure and the impact of herbivory on shoot growth. Aquatic Botany. 1999. Sep;64(3–4):399–410. [Google Scholar]
- 35.Blossey B, Casagrande R, Tewksbury L, Hinz HL, Häfliger, Patrick, et al. A petition for open-field releases of Archanara geminipuncta and Archanara neurica, potential biological control agents of invasive Phragmites australis in North America. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McTavish MJ, Jones IM, Häfliger P, Smith SM, Bourchier RS. Field tests of egg and larval release methods of biological control agents (Archanara neurica, Lenisa geminipuncta) for introduced Phragmites australis australis (Cav.) trin. Ex Steud. Biological Control. 2024. Jan 1;188:105414. [Google Scholar]
- 37.Kassambara A. ggpubr: “ggplot2” based publication ready plots [Internet]. 2023. Available from: https://CRAN.R-project.org/package=ggpubr [Google Scholar]
- 38.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015. Oct 1;67(1):1–48. [Google Scholar]
- 39.Lenth RV. emmeans: Estimated Marginal Means, aka Least-Squares Means [Internet]. 2023. Available from: https://CRAN.R-project.org/package=emmeans [Google Scholar]
- 40.de Mendiburu F. agricolae: Statistical procedures for agricultural research [Internet]. 2021. Available from: https://CRAN.R-project.org/package=agricolae [Google Scholar]
- 41.R Core Team. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2023. Available from: https://www.R-project.org/ [Google Scholar]
- 42.Posit team. RStudio: Integrated development for R [Internet]. Boston, MA: Posit Software; 2023. Available from: http://www.posit.co/
- 43.McTavish MJ, Jones IM, Smith SM, Bourchier RS. Data for McTavish et al. Current status of biological control of introduced Phragmites in Canada [Internet]. Zenodo; 2024. [cited 2024 Feb 8]. Available from: https://zenodo.org/records/10637131 [DOI] [PubMed] [Google Scholar]
- 44.Before Blossey B., during and after: The need for long-term monitoring in invasive plant species management. Biological Invasions. 1999;1:301–11. [Google Scholar]
- 45.Stiling P. Why do natural enemies fail in classical biological control programs? American Entomologist. 1993. Jan 1;39(1):31–7. [Google Scholar]
- 46.Grevstad FS, Wepprich T, Barker B, Coop LB, Shaw R, Bourchier RS. Combining photoperiod and thermal responses to predict phenological mismatch for introduced insects. Ecological Applications. 2022;32(3):e2557. doi: 10.1002/eap.2557 [DOI] [PubMed] [Google Scholar]
- 47.Clewley GD, Eschen R, Shaw RH, Wright DJ. The effectiveness of classical biological control of invasive plants. Journal of Applied Ecology. 2012;49(6):1287–95. [Google Scholar]
- 48.Dethier VG. Food-plant distribution and density and larval dispersal as factors affecting insect populations. The Canadian Entomologist. 1959. Sep;91(9):581–96. [Google Scholar]
- 49.Lasack PM, Pedigo LP. Movement of stalk borer larvae (Lepidoptera: Noctuidae) from noncrop areas into corn. Journal of Economic Entomology. 1986. Dec 1;79(6):1697–702. [Google Scholar]
- 50.Meijden EVD. Changes in the distribution pattern of Tyriajacobaeae during the larval period. Netherlands Journal of Zoology. 1975. Jan 1;26(1):136–61. [Google Scholar]
- 51.Ireson J, Leighton S, Holloway R, Chatterton W. Establishment and redistribution of Longitarsus flavicornis (Stephens) (Coleoptera: Chrysomelidae) for the biological control of ragwort (Senecio jacobaea L.) in Tasmania. Australian Journal of Entomology. 2000;39(1):42–6. [Google Scholar]
- 52.Malecki RA, Blossey B, Hight SD, Schroeder D, Kok LT, Coulson JR. Biological control of purple loosestrife. BioScience. 1993;43(10):680–6. [Google Scholar]
- 53.Bertin A, Pavinato VAC, Parra JRP. Fitness-related changes in laboratory populations of the egg parasitoid Trichogramma galloi and the implications of rearing on factitious hosts. BioControl. 2017. Aug 1;62(4):435–44. [Google Scholar]
- 54.Hoffmann AA, Hallas R, Sinclair C, Partridge L. Rapid loss of stress resistance in Drosophila melanogaster under adaptation to laboratory culture. Evolution. 2001;55(2):436–8. [DOI] [PubMed] [Google Scholar]
- 55.Jones IM, Bourchier RS, Smith SM. Long-term captive-rearing affects oviposition behavior and nymphal survival of a weed biological control agent. Biological Control. 2021. Nov 1;162:104727. [Google Scholar]
- 56.Richardson ML, Mitchell RF, Reagel PF, Hanks LM. Causes and consequences of cannibalism in noncarnivorous insects. Annual Review of Entomology. 2010. Jan 7;55(Volume 55, 2010):39–53. doi: 10.1146/annurev-ento-112408-085314 [DOI] [PubMed] [Google Scholar]
- 57.Blossey B, Hunt TR. Mass rearing methods for Galerucella calmariensis and G. pusilla (Coleoptera: Chrysomelidae), biological control agents of Lythrum salicaria (Lythraceae). Journal of Economic Entomology. 1999. Apr 1;92(2):325–34. [DOI] [PubMed] [Google Scholar]
- 58.Setter CM, Lym RG. Change in leafy spurge (Euphorbia esula) density and soil seedbank composition 10 years following release of Aphthona spp. biological control agents. Invasive Plant Science and Management. 2013. Mar;6(1):147–60. [Google Scholar]
- 59.Wilson L, Randall CB. Biology and biological control of knapweed. USDA Forest Service / UNL Faculty Publications [Internet]. 2005. Jan 1; Available from: https://digitalcommons.unl.edu/usdafsfacpub/113 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
Data are available from the Zenodo repository (https://zenodo.org/records/10637131).