Abstract
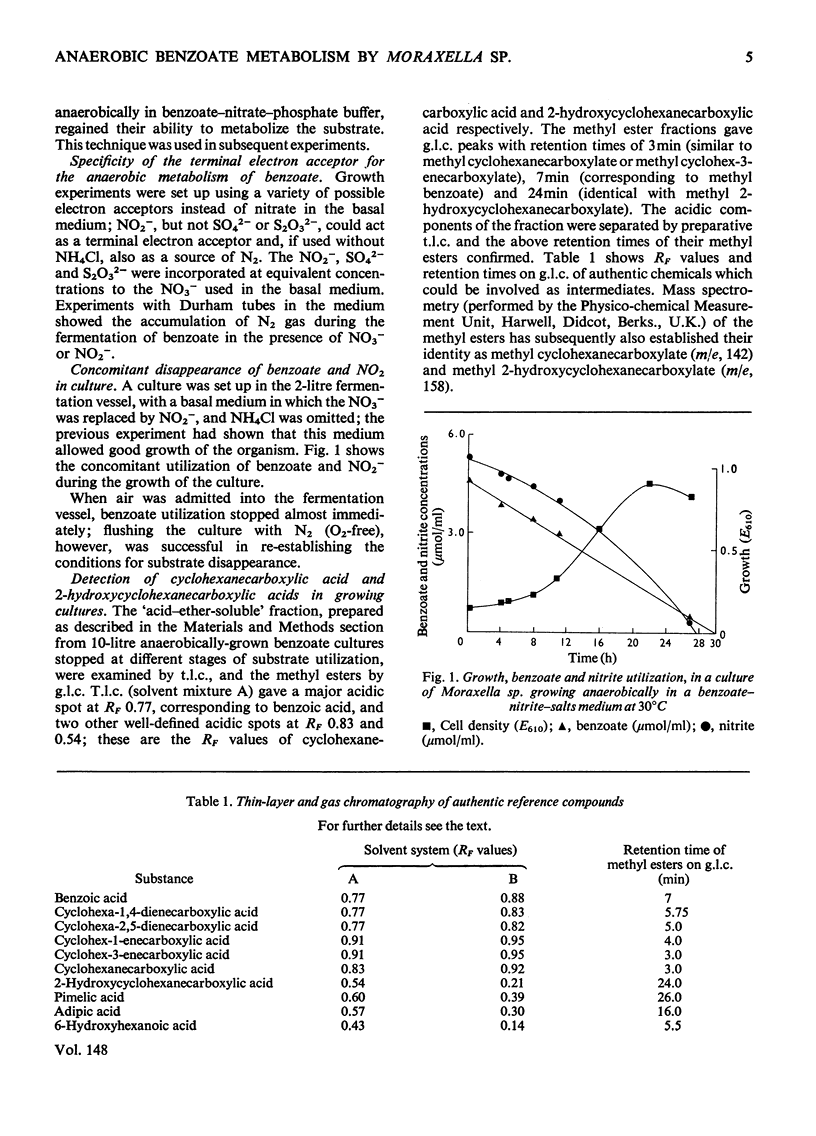
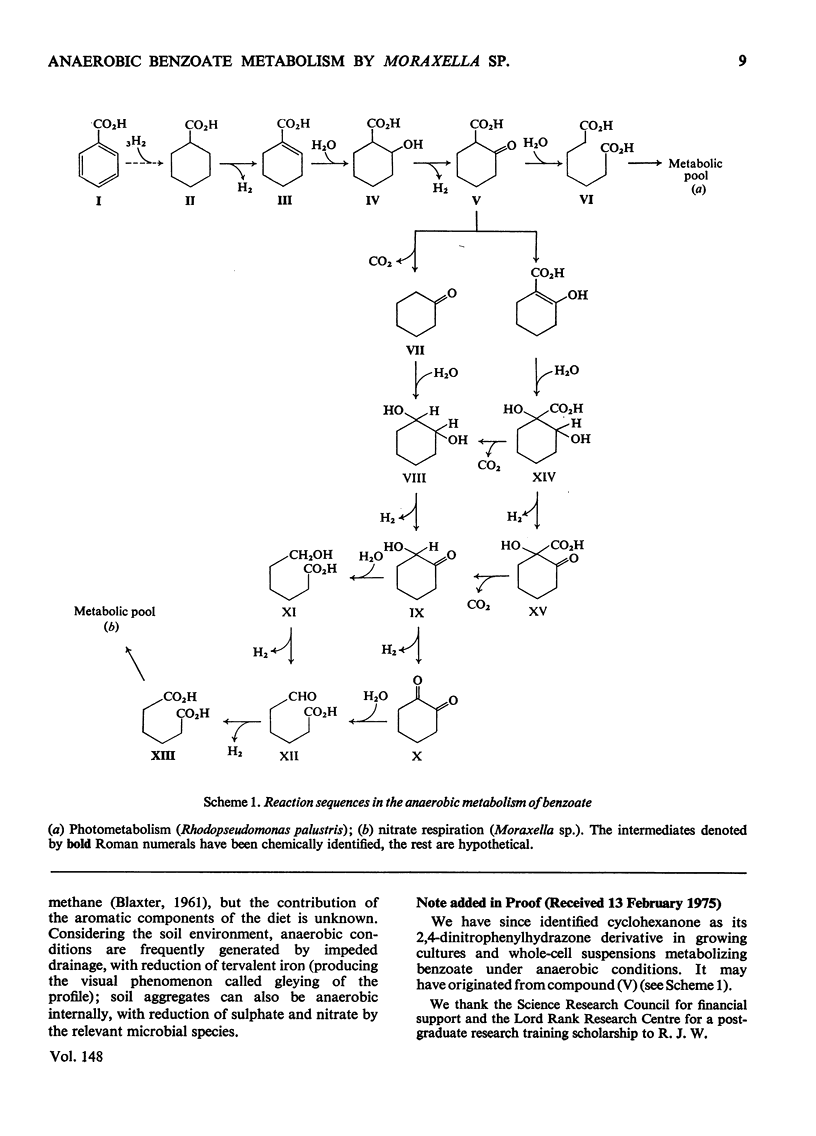
Moraxella sp. isolated from soil grows anaerobically on benzoate by nitrate respiration; nitrate or nitrite are obligatory electron acceptors, being reduced to molecular N2 during the catabolism of the substrate. This bacterium also grows aerobically on benzoate. 2. Aerobically, benzoate is metabolized by ortho cleavage of catechol followed by the beta-oxoadipate pathway. 3. Cells of Moraxella grown anaerobically on benzoate are devoid of ortho and meta cleavage enzymes; cyclohexanecarboxylate and 2-hydroxycyclohexanecarboxylate were detected in the anaerobic culture fluid. 4. [ring-U-14C]Benzoate, incubated anaerobically with cells in nitrate-phosphate buffer, gave rise to labelled 2-hydroxycyclohexanecarboxylate and adipate. When [carboxy-14C]benzoate was used, 2-hydroxycyclohexanecarboxylate was radioactive but the adipate was not labelled. A decarboxylation reaction intervenes at some stage between these two metabolites. 5. The anaerobic metabolism of benzoate by Moraxella sp. through nitrate respiration takes place by the reductive pathway (Dutton & Evans, 1969). Hydrogenation of the aromatic ring probably occurs via cyclohexa-2,5-dienecarboxylate and cyclohex-1-enecarboxylate to give cyclohexanecarboxylate. The biochemistry of this reductive process remains unclear. 6. CoA thiol esterification of cyclohexanecarboxylate followed by beta-oxidation via the unsaturated and hydroxy esters, would afford 2-oxocyclohexanecarboxylate. Subsequent events in the Moraxella culture differ from those occurring with Rhodopseudomonas palustris; decarboxylation precedes hydrolytic cleavage of the alicyclic ring to produce adipate in the former, whereas in the latter the keto ester undergoes direct hydrolytic fission to pimelate.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAGLEY S., EVANS W. C., RIBBONS D. W. New pathways in the oxidative metabolism of aromatic compounds by microorganisms. Nature. 1960 Nov 12;188:560–566. doi: 10.1038/188560a0. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Evans W. C. The metabolism of aromatic compounds by Rhodopseudomonas palustris. A new, reductive, method of aromatic ring metabolism. Biochem J. 1969 Jul;113(3):525–536. doi: 10.1042/bj1130525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Oxidation of phenol and benzoic acid by some soil bacteria. Biochem J. 1947;41(3):373–382. doi: 10.1042/bj0410373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. J., Patel J. C. The non-oxidative decarboxylation of p-hydroxybenzoic acid, gentisic acid, protocatechuic acid and gallic acid by Klebsiella aerogenes (Aerobacter aerogenes). Antonie Van Leeuwenhoek. 1969;35(3):325–343. doi: 10.1007/BF02219153. [DOI] [PubMed] [Google Scholar]
- HAYAISHI O., KATAGIRI M., ROTHBERG S. Studies on oxygenases; pyrocatechase. J Biol Chem. 1957 Dec;229(2):905–920. [PubMed] [Google Scholar]
- Jamieson W. D., Taylor A., Mathur D. K., Blackwood A. C. Identification of an intermediate of phloroglucinol degradation by mass spectroscopic analysis. Can J Biochem. 1970 Feb;48(2):215–216. doi: 10.1139/o70-036. [DOI] [PubMed] [Google Scholar]
- Norris D. B., Trudgill P. W. The metabolism of cyclohexanol by Nocardia globerula CL1. Biochem J. 1971 Feb;121(3):363–370. doi: 10.1042/bj1210363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M., Hegeman G. D. Metabolism of benzoic acid by bacteria. Accumulation of (-)-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid by mutant strain of Alcaligenes eutrophus. Biochemistry. 1971 Jun 22;10(13):2530–2536. doi: 10.1021/bi00789a017. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of benzoic acid by bacteria: 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid is an intermediate in the formation of catechol. J Bacteriol. 1971 Oct;108(1):89–94. doi: 10.1128/jb.108.1.89-94.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W., Evans W. C. Oxidative metabolism of phthalic acid by soil pseudomonads. Biochem J. 1960 Aug;76(2):310–318. doi: 10.1042/bj0760310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER D., TAGGART J. V. Benzoyl coenzyme A and hippurate synthesis. J Biol Chem. 1953 Aug;203(2):925–934. [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Taylor B. F., Campbell W. L., Chinoy I. Anaerobic degradation of the benzene nucleus by a facultatively anaerobic microorganism. J Bacteriol. 1970 May;102(2):430–437. doi: 10.1128/jb.102.2.430-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Heeb M. J. The anaerobic degradation of aromatic compounds by a denitrifying bacterium. Radioisotope and mutant studies. Arch Mikrobiol. 1972;83(2):165–171. doi: 10.1007/BF00425023. [DOI] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUGARI Y. Metabolism of cyclohexane-diol-(1,2)-trans by a soil bacterium. Biken J. 1961 Sep;4:197–207. [PubMed] [Google Scholar]


