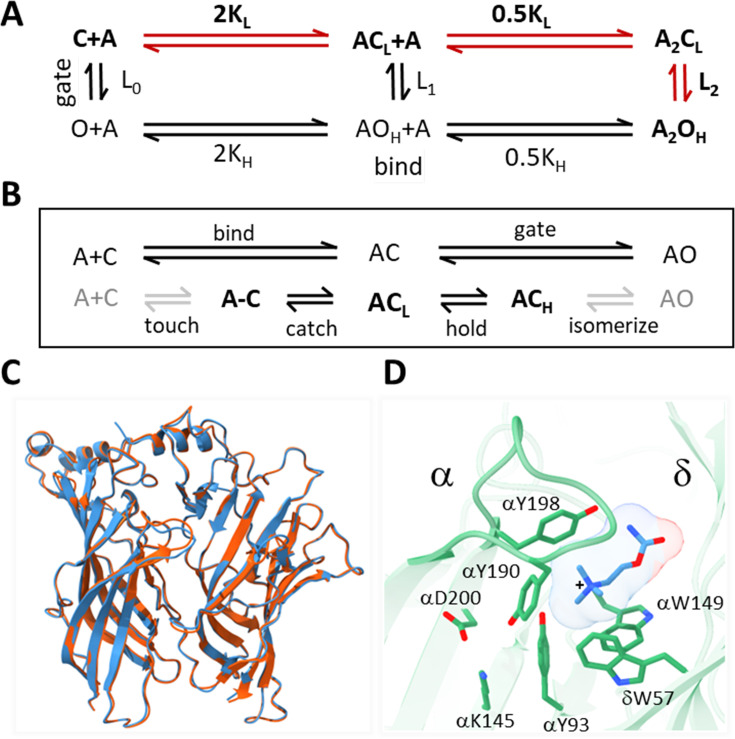
Figure 1. Acetylcholine receptor (AChR) activation.
(A) Traditional scheme. Vertical is ‘gating’ and horizontal is ‘binding;’ red, the main physiological pathway. The isomerization between closed-channel/low-affinity (CL) and open-channel/high-affinity (OH) conformations occurs with or without agonists (equilibrium constant Ln; n, number of bound ligands), is spontaneous (depends only on temperature), and global (on a ~μs time scale). Agonists (A) bind weakly to CL equilibrium association constant KL, free energy change ΔGL and strongly to OH (KH, ΔGH). The two orthosteric sites of adult AChRs are approximately equivalent and independent and there is no significant external energy, so by microscopic reversibility L2/L0 = (KH/KL)2 (Nayak and Auerbach, 2017). (B) Expansions of binding (top, ends with catch) and gating (bottom, starts with hold). The agonist diffuses to and contacts the target ('touch') to form an encounter complex (A–C); a local 'catch' rearrangement establishes the low affinity complex (ACL); a local ‘hold’ rearrangement establishes the high-affinity complex (ACH); the remaining protein domains rearrange (‘isomerize’) without a further change in affinity, to generate a conducting channel (AOH). Gray arrows, are steps that incur the same energy change for all agonists used in this study; black arrows, agonist-dependent free energy changes occur in catch (ΔGL) and in hold (ΔGH-ΔGL). (C) α-δ subunit extracellular domains; red, after toxin removal (6UWZ.pdb) and blue, apo (7QKO.pdb). There are no major deviations (Cα RMSD = 0.3 Å). (D) Closeup of the desensitized Torpedo α-δ subunit neurotransmitter site occupied by carbamylcholine (CCh, blue) (7QL6.pdb; Zarkadas et al., 2022). In this is H conformation, three aromatic groups in the α subunit (149-190-198) surround the agonist’s cationic center (+) together and provide most of the ACh binding energy (Purohit et al., 2014); the agonist’s tail points away from the α subunit (trans orientation).