Abstract
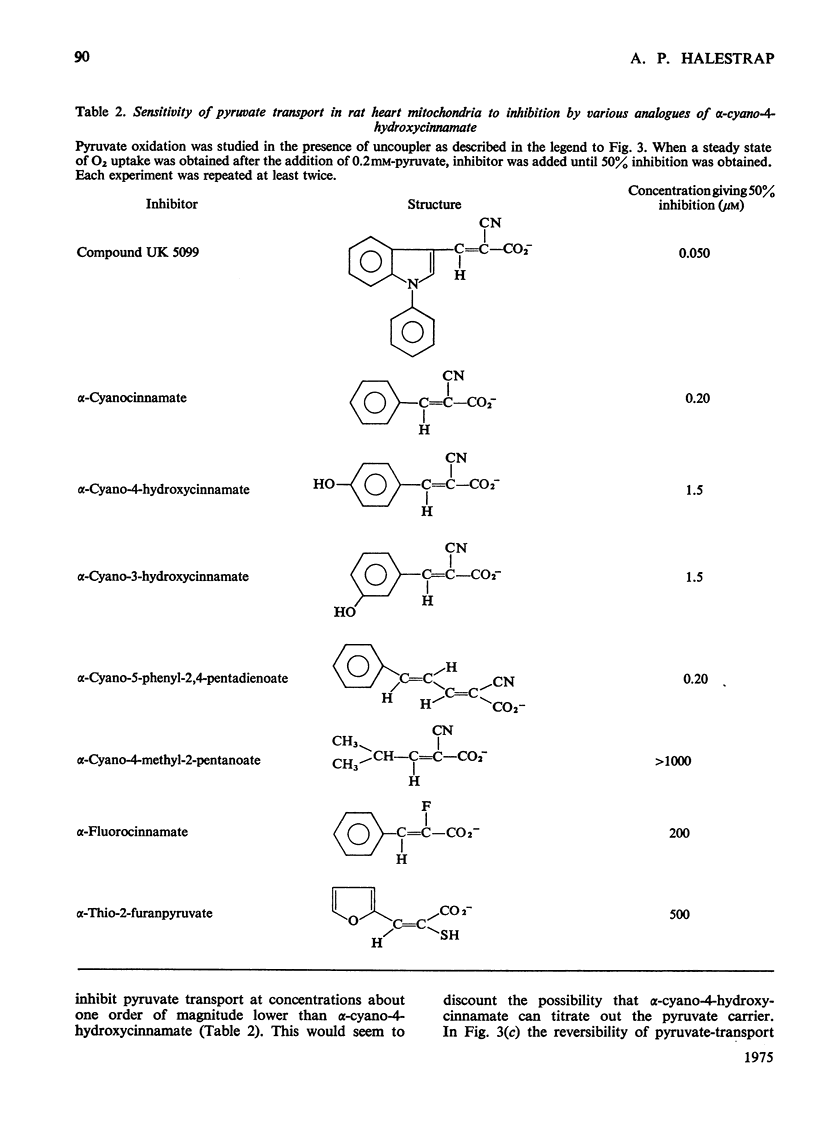
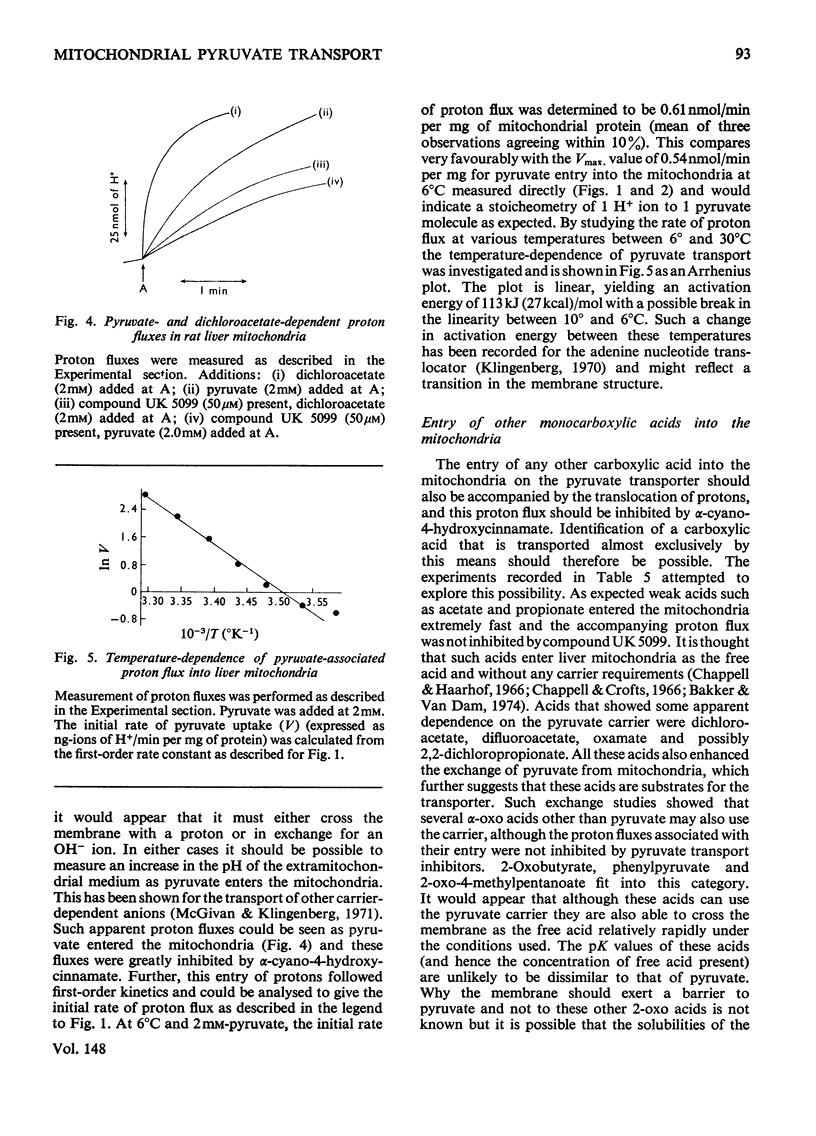
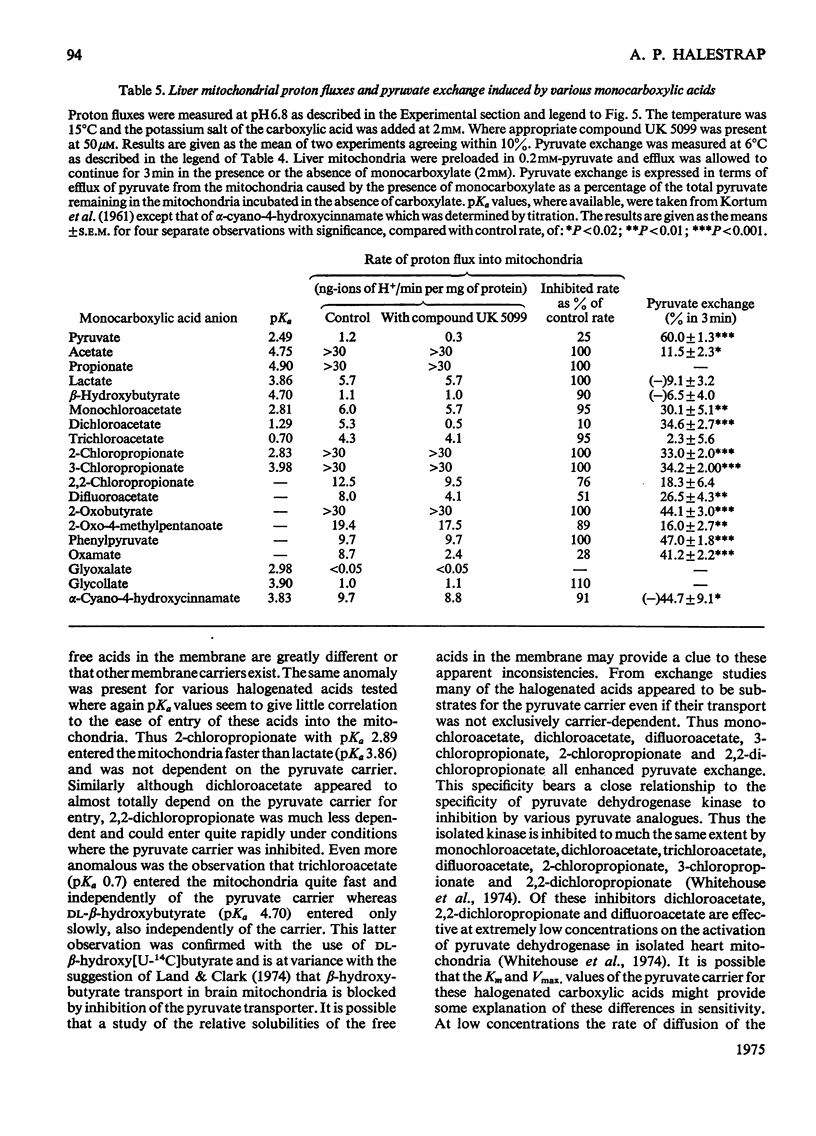
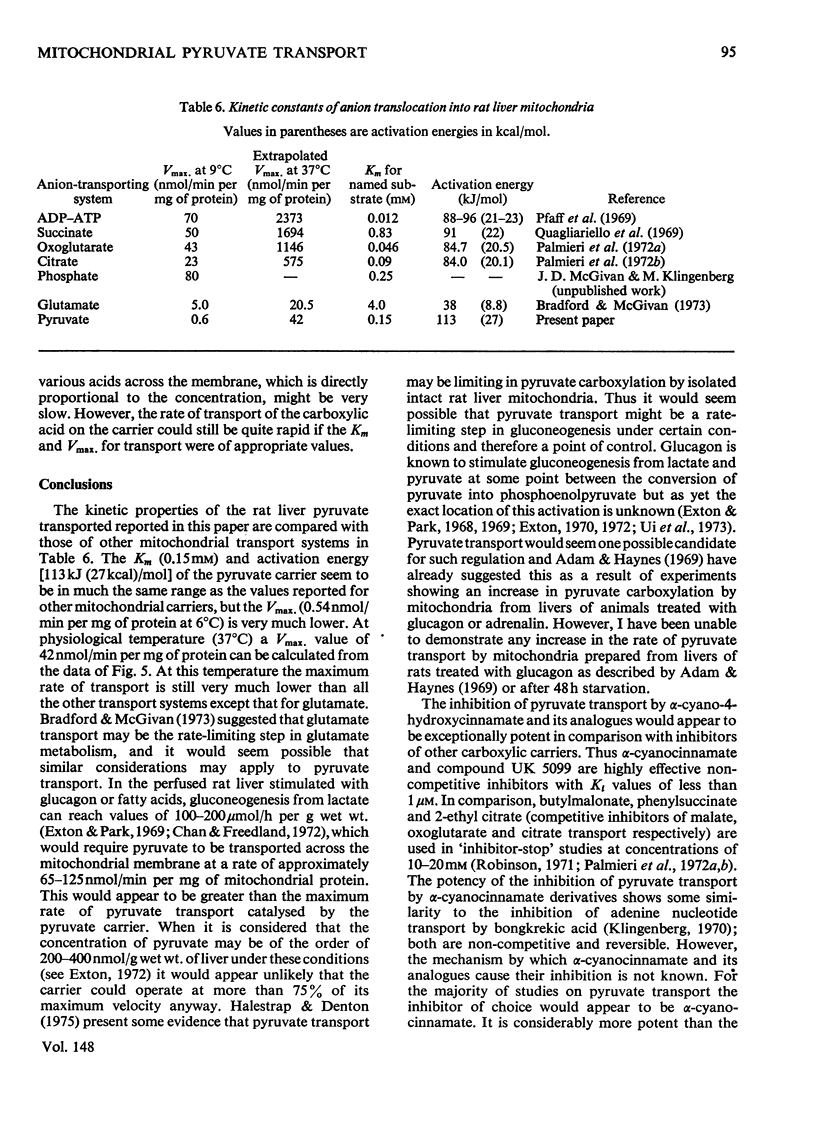
1. Studies on the kinetics of pyruvate transport into mitochondria by an 'inhibitor-stop' technique were hampered by the decarboxylation of pyruvate by mitochondria even in the presence of rotenone. Decarboxylation was minimal at 6 degrees C. At this temperature the Km for pyruvate was 0.15 mM and Vmax. was 0.54nmol/min per mg of protein; alpha-cyano-4-hydroxycinnamate was found to be a non-competitive inhibitor, Ki 6.3 muM, and phenyl-pyruvate a competitive inhibitor, Ki 1.8 mM. 2. At 100 muM concentration, alpha-cyano-4-hydroxycinnamate rapidly and almost totally inhibited O2 uptake by rat heart mitochondria oxidizing pyruvate. Inhibition could be detected at concentrations of inhibitor as low as 1 muM although inhibition took time to develop at this concentration. Inhibition could be reversed by diluting out the inhibitor. 3. Various analogues of alpha-cyano-4-hydroxycinnamate were tested on rat liver and heart mitochondria. The important structural features appeared to be the alpha-cyanopropenoate group and the hydrophobic aromatic side chain. Alpha-Cyanocinnamate, alpha-cyano-5-phenyl-2,4-pentadienoate and compound UK 5099 [alpha-cyano-beta-(2-phenylindol-3-yl)acrylate] were all more powerful inhibitors than alpha-cyano-4-hydroxycinnamate showing 50% inhibition of pyruvate-dependent O2 consumption by rat heart mitochondria at concentrations of 200, 200 and 50 nM respectively. 4. The specificity of the carrier for its substrate was studied by both influx and efflux experiments. Oxamate, 2-oxobutyrate, phenylpyruvate, 2-oxo-4-methyl-pentanoate, chloroacetate, dichloroacetate, difluoroacetate, 2-chloropropionate, 3-chloropropionate and 2,2-dichloropropionate all exchanged with pyruvate, whereas acetate, lactate and trichloroacetate did not. 5. Pyruvate entry into the mitochondria was shown to be accompanied by the transport of a proton (or by exchange with an OH-ion). This proton flux was inhibited by alpha-cyano-4-hydroxycinnamate and allowed measurements of pyruvate transport at higher temperatures to be made. The activation energy of mitochondrial pyruvate transport was found to be 113 kJ (27 kcal)/mol and by extrapolation the rate of transport of pyruvate at 37 degrees C to be 42 nmol/min per mg of protein. The possibility that pyruvate transport into mitochondria may be rate limiting and involved in the regulation of gluconegenesis is discussed. 6. The transport of various monocarboxylic acids into mitochondria was studied by monitoring proton influx. The transport of dichloroacetate, difluoroacetate and oxamate appeared to be largely dependent on the pyruvate carrier and could be inhibited by pyruvate-transport inhibitors. However, many other halogenated and 2-oxo acids which could exchange with pyruvate on the carrier entered freely even in the presence of inhibitor.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam P. A., Haynes R. C., Jr Control of hepatic mitochondrial CO2 fixation by glucagon, epinephrine, and cortisol. J Biol Chem. 1969 Dec 10;244(23):6444–6450. [PubMed] [Google Scholar]
- Bakker E. P., van Dam K. The movement of monocarboxylic acids across phospholipid membranes: evidence for an exchange diffusion between pyruvate and other monocarboxylate ions. Biochim Biophys Acta. 1974 Mar 15;339(2):285–289. doi: 10.1016/0005-2736(74)90325-3. [DOI] [PubMed] [Google Scholar]
- Bradford N. M., McGivan J. D. Quantitative characteristics of glutamate transport in rat liver mitochondria. Biochem J. 1973 Aug;134(4):1023–1029. doi: 10.1042/bj1341023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. M., Freedland R. A. The effect of propionate on the metabolism of pyruvate and lactate in the perfused rat liver. Biochem J. 1972 Apr;127(3):539–543. doi: 10.1042/bj1270539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Gluconeogenesis. Metabolism. 1972 Oct;21(10):945–990. doi: 10.1016/0026-0495(72)90028-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. 3. Effects of L-lactate, pyruvate, fructose, glucagon, epinephrine, and adenosine 3',5'-monophosphate on gluconeogenic intermediates in the perfused rat liver. J Biol Chem. 1969 Mar 25;244(6):1424–1433. [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. II. Effects of glucagon, catecholamines, and adenosine 3',5'-monophosphate on gluconeogenesis in the perfused rat liver. J Biol Chem. 1968 Aug 25;243(16):4189–4196. [PubMed] [Google Scholar]
- Halestrap A. P., Brand M. D., Denton R. M. Inhibition of mitochondrial pyruvate transport by phenylpyruvate and alpha-ketoisocaproate. Biochim Biophys Acta. 1974 Oct 10;367(1):102–108. doi: 10.1016/0005-2736(74)90140-0. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974 Feb;138(2):313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. The specificity and metabolic implications of the inhibition of pyruvate transport in isolated mitochondria and intact tissue preparations by alpha-Cyano-4-hydroxycinnamate and related compounds. Biochem J. 1975 Apr;148(1):97–106. doi: 10.1042/bj1480097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Kinetic study of the tricarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1972 Apr 24;26(4):587–594. doi: 10.1111/j.1432-1033.1972.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Land J. M., Clark J. B. Inhibition of pyruvate and beta-hydroxybutyrate oxidation in rat brain mitochondria by phenylpyruvate and alpha-ketoisocaproate. FEBS Lett. 1974 Aug 30;44(3):348–351. [PubMed] [Google Scholar]
- Martin B. R., Denton R. M., Pask H. T., Randle P. J. Mechanisms regulating adipose-tissue pyruvate dehydrogenase. Biochem J. 1972 Sep;129(3):763–773. doi: 10.1042/bj1290763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivan J. D., Klingenberg M. Correlation between H+ and anion movement in mitochondria and the key role of the phosphate carrier. Eur J Biochem. 1971 Jun 11;20(3):392–399. doi: 10.1111/j.1432-1033.1971.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Papa S., Francavilla A., Paradies G., Meduri B. The transport of pyruvate in rat liver mitochondria. FEBS Lett. 1971 Jan 30;12(5):285–288. doi: 10.1016/0014-5793(71)80200-4. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Heldt H. W., Klingenberg M. Adenine nucleotide translocation of mitochondria. Kinetics of the adenine nucleotide exchange. Eur J Biochem. 1969 Oct;10(3):484–493. doi: 10.1111/j.1432-1033.1969.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M. Adenine nucleotide translocation of mitochondria. 1. Specificity and control. Eur J Biochem. 1968 Oct 17;6(1):66–79. doi: 10.1111/j.1432-1033.1968.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Quagliariello E., Palmieri F., Prezioso G., Klingenberg M. Kinetics of succinate uptake by rat-liver mitochondria. FEBS Lett. 1969 Aug;4(4):251–254. doi: 10.1016/0014-5793(69)80247-4. [DOI] [PubMed] [Google Scholar]
- Robinson B. H. Transport of phosphoenolpyruvate by the tricarboxylate transporting system in mammalian mitochondria. FEBS Lett. 1971 May 20;14(5):309–312. doi: 10.1016/0014-5793(71)80287-9. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Williams G. R., Halperin M. L., Leznoff C. C. Factors affecting the kinetics and equilibrium of exchange reactions of the citrate-transporting system of rat liver mitochondria. J Biol Chem. 1971 Sep 10;246(17):5280–5286. [PubMed] [Google Scholar]
- Ui M., Claus T. H., Exton J. H., Park C. R. Studies on the mechanism of action of glucagon on gluconeogenesis. J Biol Chem. 1973 Aug 10;248(15):5344–5349. [PubMed] [Google Scholar]
- Walter P., Stucki J. W. Regulation of pyruvate carboxylase in rat liver mitochondria by adenine nucleotides and short chain fatty acids. Eur J Biochem. 1970 Feb;12(3):508–519. doi: 10.1111/j.1432-1033.1970.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., Cooper R. H., Randle P. J. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974 Sep;141(3):761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahlten R. N., Hochberg A. A., Stratman F. W., Lardy H. A. Pyruvate uptake in rat liver mitochondria: Transport or adsorption? FEBS Lett. 1972 Mar;21(1):11–13. doi: 10.1016/0014-5793(72)80150-9. [DOI] [PubMed] [Google Scholar]


