Abstract
The cytokine gamma interferon (IFN-γ) plays a major role in the control of Mycobacterium avium infections. We assessed whether the progressive growth of virulent strains of M. avium was associated with alterations in the production of this cytokine as evaluated by reverse transcription-PCR and detection of immunoreactive cytokine in the serum and in spleen homogenates. We found that IFN-γ was induced during infection by a virulent strain of M. avium to similar or even higher extents than the levels found during infections by a less virulent strain whose growth was controlled. IFN-γ produced during infection by both mycobacterial strains was partly derived from T cells and led to activation of macrophages, namely, those that were infected. Concomitant with the development of the infection with the virulent strain of M. avium there was an extensive depletion of lymphocytes in the spleen. Thymectomy alone promoted the proliferation of the virulent, but not of the less virulent, strain of M. avium. Our data indicate that virulent strains of M. avium resist the antimicrobial mechanisms of IFN-γ-activated macrophages and raise the possibility that a second, T-cell-dependent signal is required for the effective control of mycobacterial replication inside macrophages.
Mycobacterium avium infections are mostly found in immunocompromised human patients, such as patients with AIDS who have low CD4+ T-cell counts (16). M. avium infections are also found in human patients free of human immunodeficiency virus infection (23) or in veterinary contexts (32). This species can be isolated from environmental sources, and it is believed that environmental contact underlies the infection of human beings (31). The ability of different isolates to grow in target organs of experimentally infected animals such as mice can give us information regarding the relative virulence among the clinical and environmental isolates (21). Such studies have revealed an extraordinary variation in the virulence of different strains. Also, it has long been known that phenotypic variation, apparent as the emergence of morphologically different colonies of mycobacteria growing on solid media, is related to dramatic changes in the ability of a particular strain of M. avium to grow and infect the host (21). The molecular basis of virulence in M. avium is still not determined, nor do we understand the relationship between virulence (as a microbial quality) and the host response, namely, the immune response. We therefore compared two strains of M. avium with distinct virulence properties: one strain (strain 2447) can grow for a limited period of time, but its growth is arrested by the emergence of a series of responses from both the innate and the adaptive mechanisms of immunity; on the other hand, strain 25291 proliferates extensively and eventually kills the infected mouse. The ability of the latter strain to proliferate could be due to its capacity either to downmodulate the immune response of the host or, alternatively, to resist the antimicrobial mechanisms induced. It is widely accepted that resistance to mycobacteria requires the secretion of gamma interferon (IFN-γ), which, by activating the mononuclear phagocytes, leads to the control of the infection (1, 9, 13, 14, 27). We describe here the ability of the highly virulent strain of M. avium to induce an immune response characterized by high levels of secretion of IFN-γ but to resist the antimicrobial activity of the IFN-γ-activated macrophages. Concomitant with the progression of the infection, there is an extensive loss of the lymphoid population, which may suggest that a second signal, associated with the T cells, is lost, accounting for the susceptibility to infection.
MATERIALS AND METHODS
Reagents and antibodies.
Bacterial culture media were purchased from Difco (Detroit, Mich.). Tween 80, oleic acid, bovine serum albumin, phorbol myristate acetate (PMA), Triton X-100, cytochrome c, and Escherichia coli lipopolysaccharide (LPS) were purchased from Sigma (St. Louis, Mo.). Dulbecco’s modified Eagle tissue culture medium, HEPES buffer, Hank’s balanced salt solution, fetal calf serum (FCS), and protein G columns were purchased from Gibco Life Technologies (Paisley, Scotland). The hybridomas GK1.5 (secreting anti-CD4 immunoglobulin G2a [IgG2a]; American Type Culture Collection [ATCC], Manassas, Va.), R4-6A2 (secreting anti-IFN-γ IgG1 [ATCC]), and AN18 (secreting anti-IFN-γ IgG1; DNAX, Palo Alto, Calif.) were grown in ascites fluid in Harlan Sprague-Dawley nude mice to produce the monoclonal antibodies. All of the antibodies were purified by protein G-agarose affinity chromatography followed by dialysis against phosphate-buffered saline (PBS) before being used. Fluorescein isothiocyanate-conjugated rat anti-mouse CD4, phycoerythrin (PE)-conjugated rat anti-mouse CD8, and PE-conjugated rat anti-mouse CD3 were purchased from Pharmingen, San Diego, Calif.
Mice.
Specific-pathogen-free C57BL/6 and BALB/c female mice were purchased from Gulbenkian Institute of Science, Oeiras, Portugal. Female C.B17 mice with severe combined immunodeficiency (SCID) were purchased from Bommice (Ry, Denmark) and were screened for leakiness by confirming the lack of serum immunoglobulin. Mice with the IFN-γ gene disrupted (IFN-γ−/−) were bred at our facilities in HEPA filter-bearing cages from breeding pairs obtained from D. Dalton (10). Outbred Harlan Sprague-Dawley nude mice were purchased from the Gulbenkian Institute of Science. The animals were kept under sterilized conditions, in HEPA filter-bearing cages, fed autoclaved commercial chow, and given autoclaved drinking water ad libitum. The animals were used at 5 to 12 weeks of age.
Bacteria.
M. avium strains 2447 (an AIDS isolate obtained from F. Portaels, Institute of Tropical Medicine, Antwerp, Belgium) and ATCC 25291 (an animal isolate), both growing as smooth transparent colonies, were grown until mid-log phase in Middlebrook 7H9 medium plus 0.04% Tween 80 at 37°C. The bacteria were harvested by centrifugation, suspended in a small volume of saline, and sonicated with a Branson (Danbury, Conn.) sonifier for 15 s at 50 W to disrupt bacterial clumps. This suspension was then diluted, frozen in aliquots, and kept at −70°C until use. Before inoculation, bacterial aliquots were thawed at 37°C and diluted in saline to the desired concentration.
In vivo infections.
The mice were infected intravenously with 106 CFU of M. avium through the lateral tail vein. Infected mice were sacrificed at different time points of infection, and the organs were aseptically collected and homogenized in a 0.04% Tween 80 solution in distilled water. The number of CFU of M. avium in the livers, spleens, and lungs of the infected mice was determined by serial dilution and plating the tissue homogenates into 7H10 agar medium supplemented with oleic acid-albumin-dextrose-catalase (OADC). In some cases, the mice were thymectomized 3 weeks prior to infection. The depletion of CD4+ T cells was achieved by the intraperitoneal administration to the thymectomized mice of 0.2 mg of anti-CD4 monoclonal antibody (GK1.5) per animal, 3 days and 1 day before infection and every 10 days during the course of infection.
Detection of immunoreactive IFN-γ.
Blood was collected from infected mice at different time points of infection and incubated for 30 min at 37°C followed by incubation at 4°C to allow clot formation and retraction. After centrifugation, serum was collected and frozen at −70°C. The spleen homogenates were incubated with 1% Triton X-100 for 2 h at 4°C. After centrifugation, the supernatants were collected and frozen at −70°C. Quantification of IFN-γ in the serum and spleen homogenates was done by enzyme-linked immunosorbent assay with the IFN-γ-specific monoclonal antibodies R4-6A2 as the coating antibody and biotin-conjugated AN18 as the secondary antibody.
Semiquantitative RT-PCR.
Total mRNA from portions of the spleens, livers, and lungs of infected mice was obtained by using guanidinium thiocyanate-phenol-chloroform purification and stored at −70°C until processed further. Reverse transcription (RT) was performed with p(dT)12-18 oligonucleotides (Pharmacia Biotech, Uppsala, Sweden) and Superscript reverse transcriptase (Gibco Life Technologies) in the presence of 10 U of RNase inhibitor (Promega, Madison, Wis.). cDNA was amplified with Taq polymerase (Oncor Appligene, Gaithersburg, Md.) in the presence of a specific pair of primers for the housekeeping gene coding for hypoxanthine phosphoribosyl transferase (HPRT) or for IFN-γ (sequences are described in reference 19) in a Gene Amp PCR System 9600 (Perkin-Elmer) for 30 cycles. The amplification products were generated under conditions of linear correlation with the amount of cDNA and standardized for similar HPRT mRNA. The amplification products were run in parallel with a titration of both HPRT and IFN-γ cDNA from internal standards in a 1.4% agarose gel, transferred to a nitrocellulose membrane (Hybond N+; Amersham, Buckinghamshire, United Kingdom), and hybridized with specific [α-32P]deoxyCTP-labeled probes. The membranes were exposed, and the photographic plates were read with the aid of a computer-assisted scanner. The values of the amplified product for IFN-γ were corrected for the amount of HPRT in each sample, taking into account the titration of both HPRT and IFN-γ cDNA. All samples from the same time points of infection were run and blotted in parallel with the titrations and exposed to the same photographic plates to ensure a correct comparison of the signals generated.
Analysis of the state of activation of peritoneal macrophages.
Peritoneal cells were obtained from mice at different time points of infection by washing the peritoneal cavities of the infected mice with PBS. The cells were washed, resuspended in Dulbecco’s modified Eagle medium containing 10 mM HEPES buffer and supplemented with 10% FCS, and cultured in triplicate at a density of 3 × 106 per well in a 24-well tissue culture plate. After a 2-h incubation at 37°C in a 7% CO2 atmosphere, nonadherent cells were removed by extensive washing with prewarmed Hank’s balanced salt solution. Adherent cells were incubated for 3 days in the presence of 1 μg of E. coli LPS/ml for the detection of nitrite secretion or incubated for 90 min at 37°C in a 7% CO2 atmosphere with a cytochrome c solution for the detection of superoxide secretion as described elsewhere (4). The concentration of NO2− in the supernatants was measured with the Griess reagent as described elsewhere (4). Cell monolayers were lysed by three cycles of freezing and thawing, and the amount of protein in each well was determined by the Lowry method.
Immunohistochemistry.
Liver biopsy samples were fixed in formaldehyde and embedded in paraffin. Sections were deparaffinated and placed in 10 mM sodium citrate buffer (pH 6.0) followed by pressure cooking for exactly 1 min. For pressure cooker pretreatment, a normal household pressure cooker was filled with enough 10 mM sodium citrate buffer to cover the slides. The buffer was brought to a boil before the slides were submerged. The lid was closed and the sections were boiled at top pressure for exactly 1 min. The tissue sections were then covered with carbol fuchsin (Merck, Darmstadt, Germany) and heated until they were steaming. After they had cooled for 5 min, a solution containing 70% ethanol and 0.5% hydrochloric acid was applied for differentiation, followed by thorough rinsing. After being blocked for 20 min in 1% H2O2, the slides were stained for acid-fast bacteria and then with a rabbit anti-mouse inducible nitric oxide synthase (iNOS) (Genzyme-Virotech, Russelsheim, Germany) in Tris-buffered saline with 10% FCS for 30 min in a humid chamber. As a bridging antibody, appropriately diluted goat anti-rabbit IgG-peroxidase (Dianova, Hamburg, Germany) was used, and as a tertiary antibody, diluted rabbit anti-goat IgG-peroxidase (Dianova) was used, in sequential incubations of 30 min each.
Fluorescence-activated cell sorter analysis.
Single-cell suspensions from spleens of control and infected mice were prepared by teasing portions of the spleen through a fine-mesh screen in PBS containing 3% FCS. Erythrocytes were lysed by incubation of the cell suspensions with hemolytic buffer (155 mM NH4Cl, 10 mM KHCO3, pH 7.2) for 5 min at room temperature and thoroughly washed. For immunofluorescence staining, 106 cells were incubated in a microtiter plate with fluorescein isothiocyanate-conjugated anti-CD4 antibody (dilution, 1:100) and PE-conjugated anti-CD8 antibody (dilution, 1:100) or with PE-conjugated anti-CD3 antibody (dilution, 1:50). The cells were washed twice with PBS–3% FCS, and propidium iodide was added to the cells at a final concentration of 1 μg/ml to allow the exclusion of dead cells. The analysis of the cell populations was based on the acquisition of 10,000 events in a Becton Dickinson FACSort equipped with PCLysis II software.
Statistical analysis.
Student’s t test was used to compare data.
RESULTS
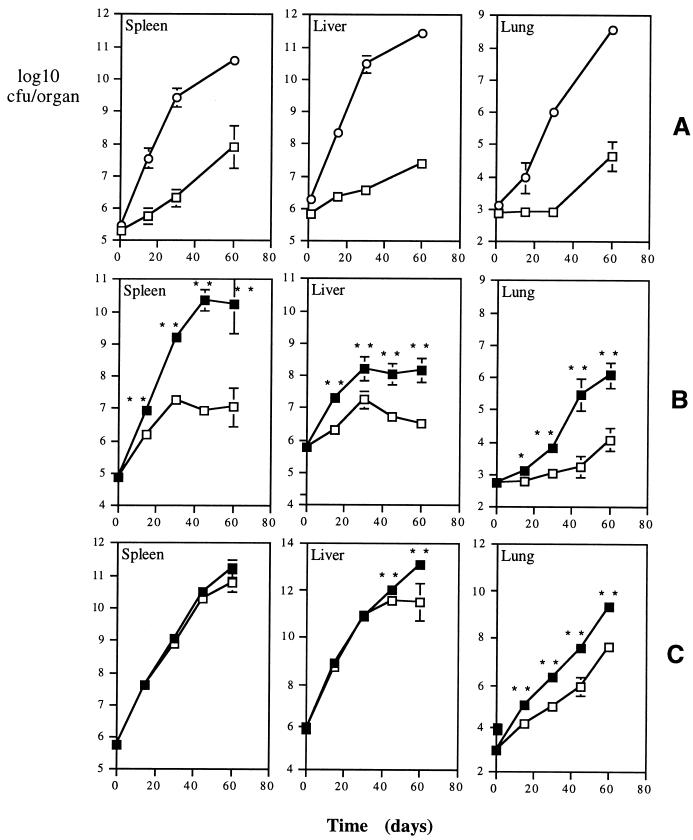
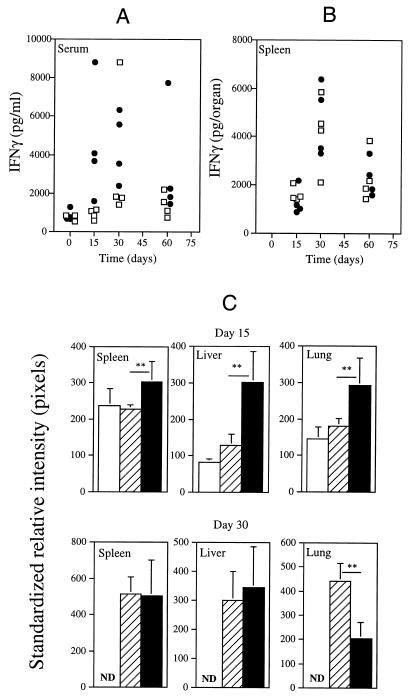
In Fig. 1A we present the proliferation of the two isolates of M. avium in the organs of intravenously infected C57Bl/6 mice. The animals were inoculated with similar infectious doses of either strain 2447 or strain 25291. Whereas the growth of the former was slow, in part due to IFN-γ-induced antimycobacterial mechanisms (1), strain 25291 proliferated extensively with no sign of any restriction by the host. To confirm the role of IFN-γ in the restriction of growth of strain 2447 and to know whether this cytokine could still be involved in some degree of control of the more virulent 25291 strain, we infected mice with the IFN-γ gene disrupted, as well as the respective controls, which were heterozygous animals with similar genetic background, i.e., BALB/c. As shown in Fig. 1B, IFN-γ-deficient mice allowed an enhanced proliferation of strain 2447 compared to the respective controls, confirming previous findings in studies with neutralizing antibodies (1). Despite the absence of IFN-γ, the knockout animals evidenced the ability to restrict mycobacterial proliferation late in infection, especially in their livers and spleens. No differences in proliferation of strain 25291 were observed in the spleens of IFN-γ-deficient mice compared with their controls (Fig. 1C). Increased mycobacterial proliferation was observed in the lungs of the knockout animals compared to the control heterozygotes during the latter infection as well as later in infection, in the liver, suggesting that IFN-γ was being produced, at least in the lungs of control mice infected with the virulent strain of M. avium. The lack of an exacerbation of mycobacterial growth observed in the spleens and livers following disruption of the IFN-γ gene could be due to a lack of production of this cytokine during infection of the wild-type animals by the virulent strain. Therefore, we analyzed IFN-γ production during infection of C57Bl/6 mice by either of the two strains of M. avium. As shown in Fig. 2A, high levels of immunoreactive IFN-γ were detectable in the sera of the mice infected with the highly virulent strain of M. avium. In fact, the levels of the cytokine were even higher than the ones found in mice infected with the low-virulence strain. Similar results were obtained when the presence of immunoreactive IFN-γ was analyzed in spleen homogenates (Fig. 2B), showing that the highly virulent strain of M. avium was able to induce a prominent IFN-γ response in one of the target organs although it was unable to induce the restriction of mycobacterial proliferation. The kinetics of IFN-γ production was also evaluated by performing RT-PCR analysis on samples taken from all the organs studied. As shown in Fig. 2C, expression of IFN-γ was found throughout the course of both infections. Densitometric analysis revealed that the expression of this cytokine on day 15 was, on the whole, higher in the organs of the mice infected with strain 25291 than in those of mice infected with the less virulent strain 2447 (Fig. 2C). This situation was reversed by day 30 of infection, the time point of peak expression of IFN-γ, when similar levels of expression were observed in the spleen and the liver and an opposite relationship was observed in the lungs. A similar study showed that levels of another cytokine, tumor necrosis factor alpha (TNF-α) correlated well with the levels of IFN-γ (data not shown). The levels of immunoreactive IFN-γ detected in the serum at days 15 and 30 correlated with the levels of expression of this cytokine in the liver (r2 = 0.69).
FIG. 1.
Proliferation of two M. avium strains in the organs of C57Bl/6 mice (A) or IFN-γ−/− mice and their heterozygous controls (B and C). (A) C57Bl/6 mice were infected with similar inoculum doses of strain 25291 (circles) or of strain 2447 (squares), and viable counts were done at the indicated time points in the spleens, livers, and lungs. (B and C) IFN-γ−/− (solid symbols) and (IFN-γ−/− × BALB/c)F1 (open symbols) mice were infected with strains 2447 (B) or 25291 (C), and viable counts were done in the same organs as for panel A at the indicated time points. Statistically different values are indicated by ∗ for P < 0.01 and ∗∗ for P < 0.05. Each time point represents the geometric mean of CFU values from four mice ± 1 standard deviation.
FIG. 2.
Amounts of immunoreactive IFN-γ in the sera (A) and spleen homogenates (B) of C57Bl/6 mice infected at the indicated time points with M. avium strains 2447 (open squares) or 25291 (solid circles). Each point represents the determination of IFN-γ from one animal. (C) Analysis by RT-PCR of IFN-γ gene expression in the spleens, livers, and lungs of C57Bl/6 mice infected with M. avium strains 2447 or 25291 at days 15 and 30 of infection and of uninfected control mice. Southern blotting of the PCR products for HPRT and IFN-γ was performed with 32P-labeled probes, and the photographic plates were scanned and analyzed. The data are presented as the means ± 1 standard deviation of the corrected results (standardized for the HPRT message) in terms of the intensity of the reading in pixels. The open bars represent the signal for uninfected mice, the hatched bars represent those of strain 2447-infected mice, and the solid bars represent those of strain 25291-infected mice. Independent analysis of the results for days 15 and 30 was done, and therefore, exposure of the photographic plates differed between those time points, leading to different readings of the uninfected material. ND, not detected. Note that linear increases in intensity (in pixels) correspond to exponential increases in PCR product under the conditions used here. Statistically significant differences according to Student’s t test are labeled ∗ (P < 0.05) or ∗∗ (P < 0.01).
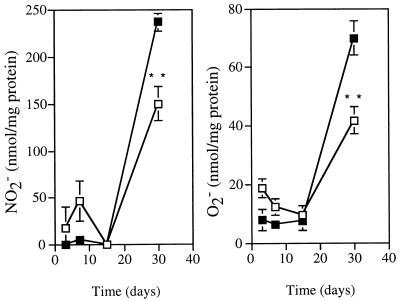
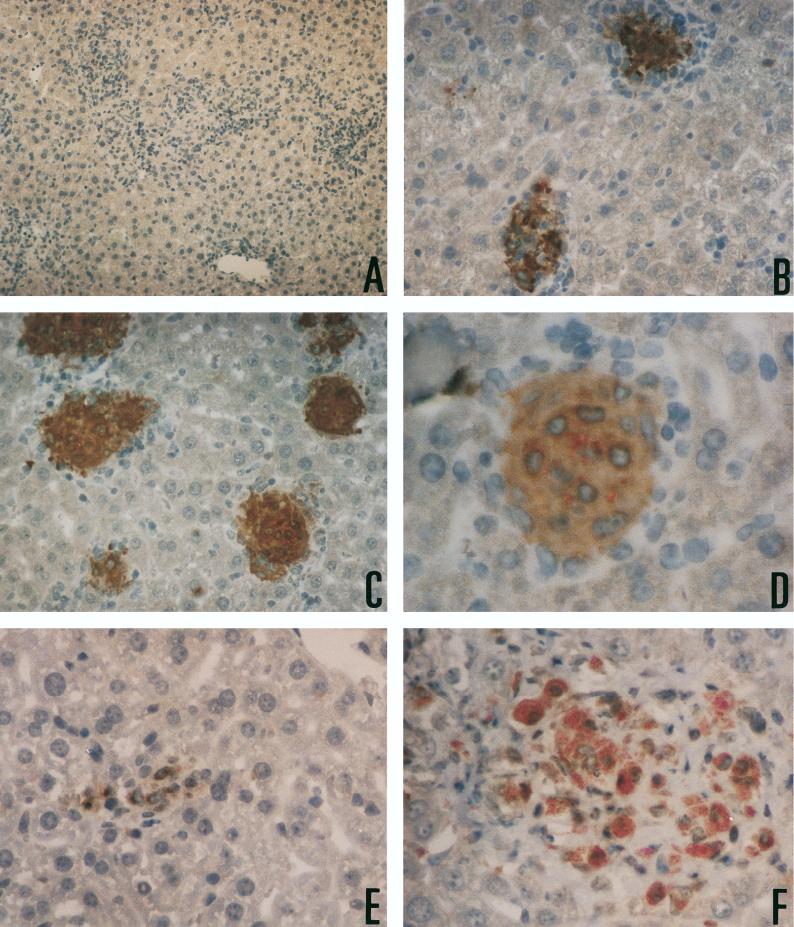
One straightforward explanation for the ineffectiveness of IFN-γ in inducing stasis of M. avium 25291 would be a failure of the cytokine to activate macrophages. We therefore isolated peritoneal macrophages from the infected mice to look at the role of the IFN-γ produced during infection in modulating macrophage functions. Since both the induction of iNOS and the priming of the respiratory burst enzymes are mostly dependent on IFN-γ, we measured the ability of the isolated macrophages to secrete nitrite or superoxide in response to LPS or PMA as their respective agonistic triggers. Macrophage activation, as evaluated by the two chosen markers, was found to occur among peritoneal cells of mice infected with either of the two mycobacterial strains (Fig. 3). The degree of activation was higher in macrophages from mice infected with the M. avium strain with the higher virulence than in the macrophages isolated from the animals infected with the low-virulence strain. Therefore, IFN-γ produced during the response to infection was acting on target macrophages, leading to their activation in a dose-response-related manner. A trivial explanation for the inability of IFN-γ to control the growth of strain 25291 could be that the infected macrophages (a minority of the macrophages isolated from these mice and studied in vitro) were themselves unresponsive to the cytokine and therefore not undergoing the activation pathways. In such a scenario, the overall activation of the macrophage population as a whole would mask a potential unresponsiveness of a small subpopulation, albeit the most important one for the control of the infection. To be able to study macrophage activation on a single-cell basis, we chose to detect iNOS protein by immunohistochemistry. At the same time, acid-fast bacteria were visualized in the same tissues to assess the colocalization of iNOS and the mycobacteria. As shown in Fig. 4, iNOS expression in the livers of infected mice was induced by infection with either strain of M. avium and was more marked in the organs of the animals infected with the highly virulent strain than in those from mice infected with the low-virulence strain. Such upregulation of iNOS was observed in the infected as well as the apparently noninfected macrophages in the granuloma with no obvious differences in intensity of staining. Although the data are presented for BALB/c mice, similar results were obtained with C57Bl/6 animals. It can also be seen from Fig. 4 that granuloma formation, as well as activation (at least for iNOS), is severely reduced in the absence of endogenous IFN-γ, as judged from the lesions in mice with the IFN-γ gene disrupted.
FIG. 3.
Kinetic analysis of macrophage activation. Peritoneal cells from infected animals were collected at different time points of infection, cultured, and used to assess their ability to secrete nitrite in response to LPS (A) or superoxide in response to PMA (B). The open symbols represent the results from mice infected with strain 2447, and the closed symbols represent those from mice infected with strain 25291. Each value represents the mean ± 1 standard deviation of triplicates from a pool of cells from four mice. ∗, P < 0.05; ∗∗, P < 0.01.
FIG. 4.
Immunohistological evidence of macrophage activation in hepatic granulomas. (A) Liver sections were stained simultaneously with a polyclonal antibody for the expression of iNOS, with reactivity revealed through a peroxidase-conjugated secondary antibody, and for the presence of acid-fast bacilli. Sections from mice with the iNOS gene disrupted that were infected for 2 months with M. avium 25291 were used as a negative control for specificity (magnification, ×600) (B and C) Extensively labeled granulomas were observed in BALB/c mice infected for 1 month with strain 2447 (B) (magnification, ×1,200) or strain 25291 (C) (magnification, ×1,200). (D) Note the presence of acid-fast bacilli in the granuloma induced by strain 25291 (magnification, ×2,400) despite the marked reaction for iNOS. (E and F) IFN-γ gene-deficient mice on a BALB/c background infected with strain 2447 (E) (magnification, ×1,700) or strain 25291 (F) (magnification, ×1,700) showed reduced granuloma formation as well as reduced reactivity for iNOS. Note the extensive proliferation of strain 25291 inside macrophages of IFN-γ−/− mice in panel F.
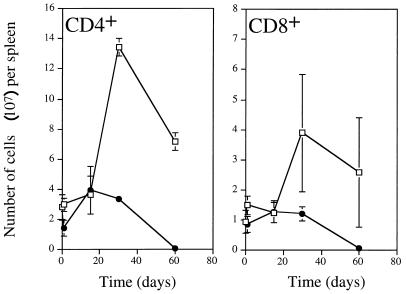
The lack of control of the proliferation of strain 25291 even in the presence of high levels of IFN-γ could suggest a complete resistance of this strain to the antimycobacterial mechanisms induced by this cytokine. However, we have been able to induce some restriction of proliferation of this strain in vitro by activating macrophages with IFN-γ (3). Also, data from Fig. 1 suggest that IFN-γ can induce some growth restriction of strain 25291 in the lung. Thus, an alternative explanation is that for in vivo control of M. avium proliferation there is a need for a second signal in addition to that provided by IFN-γ and possibly also by TNF-α. That second signal would be dependent on T cells, as the control of growth of strain 2447 is lost upon in vivo depletion of CD4+ T cells (1). To assess this hypothesis, we followed the number of T cells in the spleen during the course of infection by either of the strains of M. avium. As shown in Fig. 5, T cells expanded during infection with strain 2447, peaking at day 30 of infection. This increase in T cells was mostly due to CD4+ T cells, although some expansion of CD8+ T cells could also be found. In contrast, the number of T cells of both subsets failed to increase in response to infection by the virulent strain 25291. In the latter infection, T cells sharply decreased in number after day 30 of infection.
FIG. 5.
Kinetics of the effect of infection on numbers of CD4+ and CD8+ cells in the spleens of mice infected with strains 2447 (open symbols) or 25291 (solid symbols).
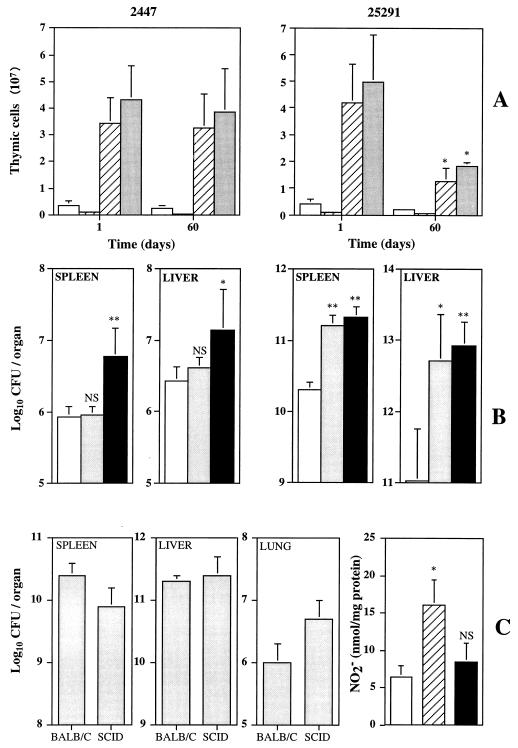
On prolonged observation of mice infected with strain 25291, thymic atrophy was found. Therefore, we analyzed the populations in the thymus of mice infected for 1 or 60 days with either strain of M. avium. As shown in Fig. 6A, infection by strain 25291 (but not by strain 2447) induced a decrease in the numbers of all subsets of thymic T cells. This phenomenon was not studied here in terms of mechanisms, but we speculated whether it could represent an attempt by the host to replenish the T-cell populations of the peripheral organs infected with strain 25291. We therefore compared the effects of thymectomy on resistance to either strain 2447 or strain 25291. As shown in Fig. 6B, thymectomy on its own did not affect the number of viable mycobacteria in the organs of mice infected with the less virulent strain, whereas the use of depleting anti-CD4 monoclonal antibodies increased mycobacterial proliferation. In contrast, mice that were only thymectomized became more susceptible to strain 25291 to an extent similar to that of CD4-depleted animals.
FIG. 6.
Involvement of the thymus in systemic infection by M. avium 2447 or 25291. (A) Cellularity of the thymus in terms of numbers of CD4+ CD8− (open bars), CD4− CD8+ (solid bars), CD4+ CD8+ (hatched bars), or total cells (shaded bars) of the thymi of mice infected for 1 or 60 days with strains 2447 (left) or 25291 (right). Results are means ± 1 standard deviation (SD). (B) Effects of thymectomy alone (shaded bars) or thymectomy followed by CD4+-T-cell depletion (solid bars) on the proliferation of M. avium 2447 (left) or 25291 (right) compared to that in untreated mice (open bars) at 40 days of infection. The results are shown as means ± 1 SD (n = 5). (C) Infection of SCID mice with M. avium 25291. The growth of M. avium was evaluated in the spleens, livers, and lungs of BALB/c and SCID mice after 30 days of infection. The results are shown as the geometric means ± 1 SD of the CFU values from three or four mice. The degree of macrophage activation of the peritoneal cells from the same animals as evaluated from the production of LPS-stimulated nitrite secretion is shown on the right. In vitro production of NO2− by peritoneal macrophages from uninfected BALB/c mice (open bar), infected BALB/c mice (hatched bar), or infected SCID mice (solid bar) is shown. The data represent means ± 1 SD of triplicates from a pool of three or four mice. ∗, P < 0.05; ∗∗, P < 0.01.
The data presented so far would argue that during infection by the most virulent strain of M. avium, there was a T-cell response leading to secretion of IFN-γ, but as the mycobacteria resisted the mechanisms induced in macrophages by the cytokine, a progressive loss of T cells took place, causing the lack of the required putative second signal. One could argue that the IFN-γ response to strain 25291, unlike that to strain 2447, which is dependent on T cells (1, 2), could rely on T-cell-independent mechanisms. We therefore infected SCID and BALB/c mice with strain 25291 and studied mycobacterial growth as well as macrophage activation. As shown in Fig. 6C, the highly virulent strain proliferated to similar numbers in both mouse strains, confirming previous findings (1). However, in the absence of T cells, macrophage activation in SCID mice was significantly lower than that in control BALB/c animals (Fig. 6C).
DISCUSSION
For mycobacteria in general and M. avium in particular it is believed that IFN-γ mediates resistance to infection through its ability to activate the macrophage’s antimycobacterial machinery. The ability of different isolates or strains of M. avium to proliferate in mice varies extensively, ranging from those that are efficiently eliminated to those that grow progressively and eventually kill the animal (21). Such virulence properties in M. avium have not yet been studied in detail. Here we have analyzed the relationship of virulence to the induction of immune responses. In general terms, the inability of the host to control the proliferation of a strain of M. avium with high virulence could be due to either a downmodulation of the IFN-γ response or resistance to the antimycobacterial mechanisms induced by the cytokine. We found that the infection of mice with a highly virulent strain of M. avium was associated with the in vivo induction of IFN-γ in the infected host. Such production of the cytokine was accompanied by macrophage activation, as evaluated by the priming of the oxidative response and NO synthesis. Furthermore, macrophage activation took place in those macrophages that were infected, showing that the virulent mycobacteria were not turning down the activation pathways of the infected macrophages. The IFN-γ response was dependent, at least to a great extent, on the induction of T cells, as SCID mice exhibited lower levels of macrophage activation. The production of IFN-γ in response to infection by the virulent strain was similar to or even greater than that found during infection by a strain of lower virulence whose growth came under control by mechanisms at least partly dependent on IFN-γ. Therefore, it appears that virulent mycobacteria survive in the host not by turning down the IFN-γ response but rather by adopting strategies to survive within activated macrophages. Data from Fig. 1 show that virulent mycobacteria resist IFN-γ-mediated mechanisms in vivo better than less virulent strains but also that, in the absence of IFN-γ, virulent M. avium already is endowed with a greater proliferation potential.
The in vivo data presented here contrast somewhat with our previous findings in in vitro macrophage cultures, where some bacteriostasis of strain 25291 could be induced in bone marrow-derived macrophages activated by IFN-γ with or without TNF-α (1, 3). Furthermore, the degree of growth restriction observed in vitro with IFN-γ-activated macrophages is always smaller than the restriction found in vivo with strain 2447 (our unpublished observations). Also, when resident peritoneal macrophages are used, there is very limited ability to induce bacteriostasis of M. avium, even strain 2447 (our unpublished observations). Together with the fact that IFN-γ can lead to a small but significant reduction in proliferation of strain 25291 in the lung (Fig. 1C), we find very likely the possibility that additional activating signals exist in vivo to lead to complete bacteriostasis of M. avium. These signals would require T cells, as T-cell depletion abrogates the control of the proliferation of strain 2447 (1). This assumption is supported by the fact that IFN-γ-deficient mice could start controlling the proliferation of strain 2447 in their spleens and livers at some point in the infection (Fig. 1B). The virulence of strain 25291 would therefore involve an enhanced resistance to the limited bacteriostatic effects of IFN-γ coupled with a concomitant downmodulation of the putative second signal provided by T cells. In line with these speculations, we found that those virulent strains, while able to resist the mechanisms induced on the macrophage by IFN-γ, caused a dramatic loss of T cells after having led to macrophage activation through IFN-γ secretion. This loss would therefore help the survival of the microbe by abolishing the second signal postulated above. Other groups have indeed found that T cells are required for the optimal induction of mycobacterial growth arrest or killing (7, 28, 29). Also, this hypothesis would explain why many groups failed to show any induction of antimycobacterial activity by IFN-γ in vitro, especially with human monocytes and macrophages (6, 12, 24, 26, 30), despite the very convincing literature describing the importance of IFN-γ in vivo in both mice and human beings (9, 14, 17, 18, 20, 22). Hanano and colleagues (15) have recently described an experimental model of Pneumocystis carinii infection where the pathogen can be controlled in the absence of IFN-γ or TNF-α signalling but not when T cells, particularly from the CD4+ subset, are nonfunctional. Curiously, the lack of control of P. carinii in the latter situation happened when evident macrophage activation is taking place. These data suggest that T-cell-dependent but IFN-γ- and TNF-α-independent resistance mechanisms exist. Whether unknown macrophage activation pathways are associated with those mechanisms cannot be answered at present. In the case of the strains of M. avium used here, we can exclude TNF-α as the second signal, as this cytokine is as efficiently induced by the virulent strain as by the low-virulence strain and, more important, the lack of the p55 receptor in mice with disrupted genes did not affect the course of the mycobacterial proliferation of either strain (our unpublished data).
The mechanism(s) leading to the demise of the T cells is still unclear. However, one could speculate, as was already done several years ago by Collins (8), that a subclinical M. avium infection in human beings in the initial phase of human immunodeficiency virus infection could be an accessory factor in the development of immunodeficiency by triggering macrophage activation and the subsequent elimination of locally recruited CD4+ T cells.
The molecular mechanisms responsible for the expression of mycobacteriostasis in M. avium-infected macrophages are still not known. It is clear that reactive oxygen species play a very limited role in restriction of growth of a very few isolates of M. avium, mostly those that trigger the secretion of high amounts of TNF-α by the infected macrophage and that show a limited ability to proliferate inside those cells both in vivo and in vitro (25). Also, activation of the infected macrophages with IFN-γ and other cytokines induces an enhanced antimycobacterial activity on those cells, which is independent of the generation of reactive oxygen species (3). Nitric oxide and related antimicrobial molecules are also not involved in the restriction of the growth of M. avium inside activated macrophages (3, 5, 11, 33). Here we have evaluated the degree of macrophage activation by measuring the production of either of the reactive species. This analysis was done to test whether IFN-γ had exerted its effects on the macrophage and not to evaluate antimycobacterial mechanisms. As we do not know what macrophage functions restrict the growth of M. avium, we could not evaluate such functions here in either type of infection.
In summary, we showed that virulent strains of M. avium stimulate IFN-γ production in vivo, namely, through activation of T cells. The reason for their resistance to these effector mechanisms is not clear, but we speculate that such strains downmodulate a second, T-cell-derived signal by promoting T-cell death, namely, through nitric oxide-mediated pathways. Therefore, despite the activation of macrophage functions, such as free radical production, virulent strains would escape control by still-unidentified effector mechanisms of the macrophage. These data stress the need to identify new correlates of protective immunity in mycobacteriosis.
ACKNOWLEDGMENTS
This work was partially supported by contracts AI-41922 from the NIH and P/SAU 58/96 from the PRAXIS XXI program (Lisbon). M.F. and A.S.G. are recipients of fellowships from the PRAXIS XXI program.
REFERENCES
- 1.Appelberg R, Castro A G, Pedrosa J, Silva R A, Orme I M, Minóprio P. The role of gamma interferon and tumor necrosis factor alpha during the T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg R, Pedrosa J. Induction and expression of protective T cells during Mycobacterium avium infections in mice. Clin Exp Immunol. 1992;87:379–385. doi: 10.1111/j.1365-2249.1992.tb03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelberg R, Orme I M. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology. 1993;80:352–359. [PMC free article] [PubMed] [Google Scholar]
- 4.Appelberg R, Orme I M, Sousa M I P, Silva M T. In vitro effects of interleukin-4 on interferon-γ-induced macrophage activation. Immunology. 1992;76:553–559. [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez L E. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin Exp Immunol. 1993;91:277–281. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard D K, Michelini-Norris M B, Djeu J Y. Interferon decreases the growth inhibition of Mycobacterium avium-intracellulare complex by fresh human monocytes but not by culture-derived macrophages. J Infect Dis. 1991;164:152–157. doi: 10.1093/infdis/164.1.152. [DOI] [PubMed] [Google Scholar]
- 7.Bonecini-Almeida M G, Chitale S, Boutsikakis I, Geng J, Doo H, He S, Ho J L. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for IFN-γ and primed lymphocytes. J Immunol. 1998;160:4490–4499. [PubMed] [Google Scholar]
- 8.Collins F M. Mycobacterium avium-complex infections and development of the acquired immunodeficiency syndrome: casual opportunist or causal cofactor? Int J Lepr Other Mycobact Dis. 1986;54:458–474. [PubMed] [Google Scholar]
- 9.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 11.Doi T, Ando M, Akaike T, Suga M, Sato K, Maeda H. Resistance to nitric oxide in Mycobacterium avium complex and its implication in pathogenesis. Infect Immun. 1993;61:1980–1989. doi: 10.1128/iai.61.5.1980-1989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douvas G S, Looker D L, Vatter A E, Crowle A J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985;50:1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flórido M, Appelberg R, Orme I M, Cooper A M. Evidence for a reduced chemokine response in the lungs of beige mice infected with Mycobacterium avium. Immunology. 1997;95:600–606. doi: 10.1046/j.1365-2567.1997.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanano R, Reifenberg K, Kaufmann S H E. Activated pulmonary macrophages are insufficient for resistance against Pneumocystis carinii. Infect Immun. 1998;66:305–314. doi: 10.1128/iai.66.1.305-314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horsburgh C R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 17.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J-F, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova J-L. Interferon-γ-receptor deficiency in an infant with fatal Bacille Calmette-Guérin infection. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 18.Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondanèche M-C, Tuerlinckx D, Blanche S, Emile J-F, Gaillard J-L, Schreiber R, Levin M, Fischer A, Hivroz C, Casanova J-L. Partial interferon-γ receptor 1 deficiency in a child with tuberculoid Bacillus Calmette-Guérin infection and a sibling with clinical tuberculosis. J Clin Investig. 1997;100:2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy E, Hieny S, Sher A, O’Garra A. Detection of in vivo expression of interleukin-10 using a semi-quantitative polymerase chain reaction method in Schistosoma mansoni infected mice. J Immunol Methods. 1993;162:211–223. doi: 10.1016/0022-1759(93)90386-l. [DOI] [PubMed] [Google Scholar]
- 20.Newport M J, Huxley C M, Huston S, Hawrylowicz C M, Oostra B A, Williamson R, Levin M. A mutation in the interferon-γ-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 21.Pedrosa J, Flórido M, Kunze Z M, Castro A G, Portaels F, McFadden J J, Silva M T, Appelberg R. Characterization of the virulence of Mycobacterium avium complex isolates in mice. Clin Exp Immunol. 1994;98:210–216. doi: 10.1111/j.1365-2249.1994.tb06127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierre-Audigier C, Jouanguy E, Lamhamedi S, Altare F, Rauzier J, Vincent V, Canioni D, Emile J-F, Fischer A, Blanche S, Gaillard J-L, Casanova J-L. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon γ receptor deficiency. Clin Infect Dis. 1997;24:982–984. doi: 10.1093/clinids/24.5.982. [DOI] [PubMed] [Google Scholar]
- 23.Prince D S, Peterson D D, Steiner R M, Gottlieb J E, Scott R, Israel H L, Figueroa W G, Fish J E. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321:863–866. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 24.Rook G A W, Steele J, Ainsworth M, Champion B R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986;59:333–338. [PMC free article] [PubMed] [Google Scholar]
- 25.Sarmento A M, Appelberg R. Involvement of reactive oxygen intermediates in the tumor necrosis factor-dependent bacteriostasis of Mycobacterium avium. Infect Immun. 1996;64:3224–3230. doi: 10.1128/iai.64.8.3224-3230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiratsuchi H, Johnson J L, Toba H, Ellner J J. Strain- and donor-related differences in the interaction of Mycobacterium avium with human monocytes and its modulation by interferon-γ. J Infect Dis. 1990;162:932–938. doi: 10.1093/infdis/162.4.932. [DOI] [PubMed] [Google Scholar]
- 27.Silva R A, Pais T F, Appelberg R. Evaluation of IL-12 in immunotherapy and vaccine design in experimental Mycobacterium avium infections. J Immunol. 1998;161:5578–5585. [PubMed] [Google Scholar]
- 28.Silver R F, Li Q, Boom W H, Ellner J J. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol. 1998;160:2408–2417. [PubMed] [Google Scholar]
- 29.Sypek J P, Jacobson S, Vorys A, Wyler D J. Comparison of gamma interferon, tumor necrosis factor, and direct cell contact in activation of antimycobacterial defense in murine macrophages. Infect Immun. 1993;61:3901–3906. doi: 10.1128/iai.61.9.3901-3906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toba H, Crawford J T, Ellner J J. Pathogenicity of Mycobacterium avium for human monocytes: absence of macrophage-activating factor activity of gamma interferon. Infect Immun. 1989;57:239–244. doi: 10.1128/iai.57.1.239-244.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Reyn C F, Maslow J N, Barber T W, Falkinham III J O, Arbeit R D. Persistent colonisation of potable water as a source of Mycobacterium avium infection in AIDS. Lancet. 1994;343:1137–1141. doi: 10.1016/s0140-6736(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 32.Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Resp Dis. 1979;119:107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- 33.Zhao B, Collins M T, Czuprynski C J. Effects of gamma interferon and nitric oxide on the interaction of Mycobacterium avium subsp. paratuberculosis with bovine monocytes. Infect Immun. 1997;65:1761–1766. doi: 10.1128/iai.65.5.1761-1766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]