Abstract
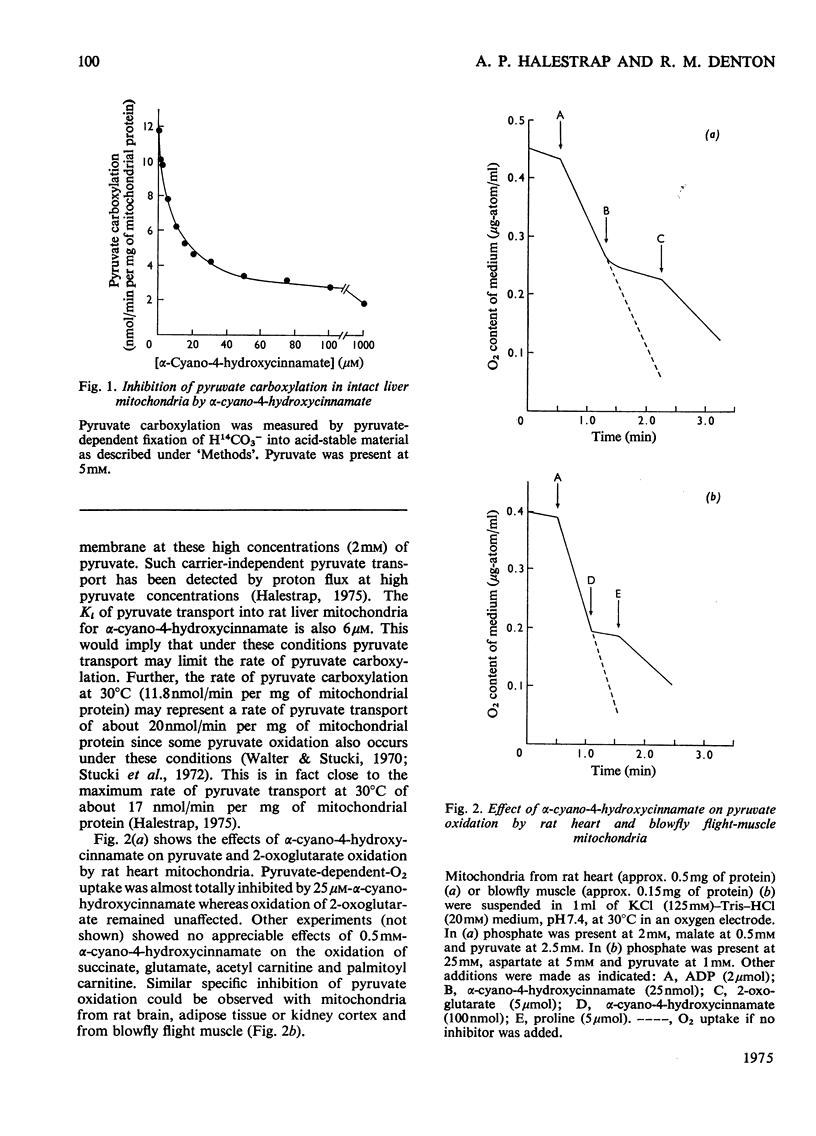
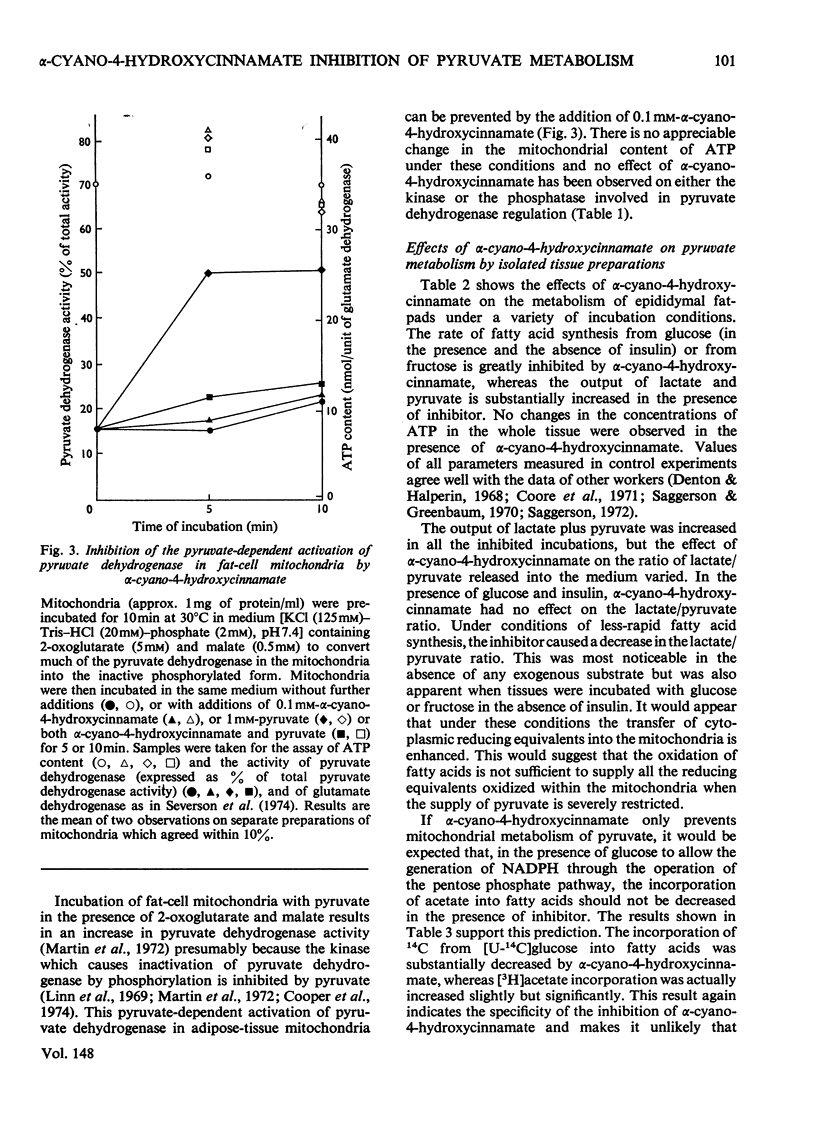
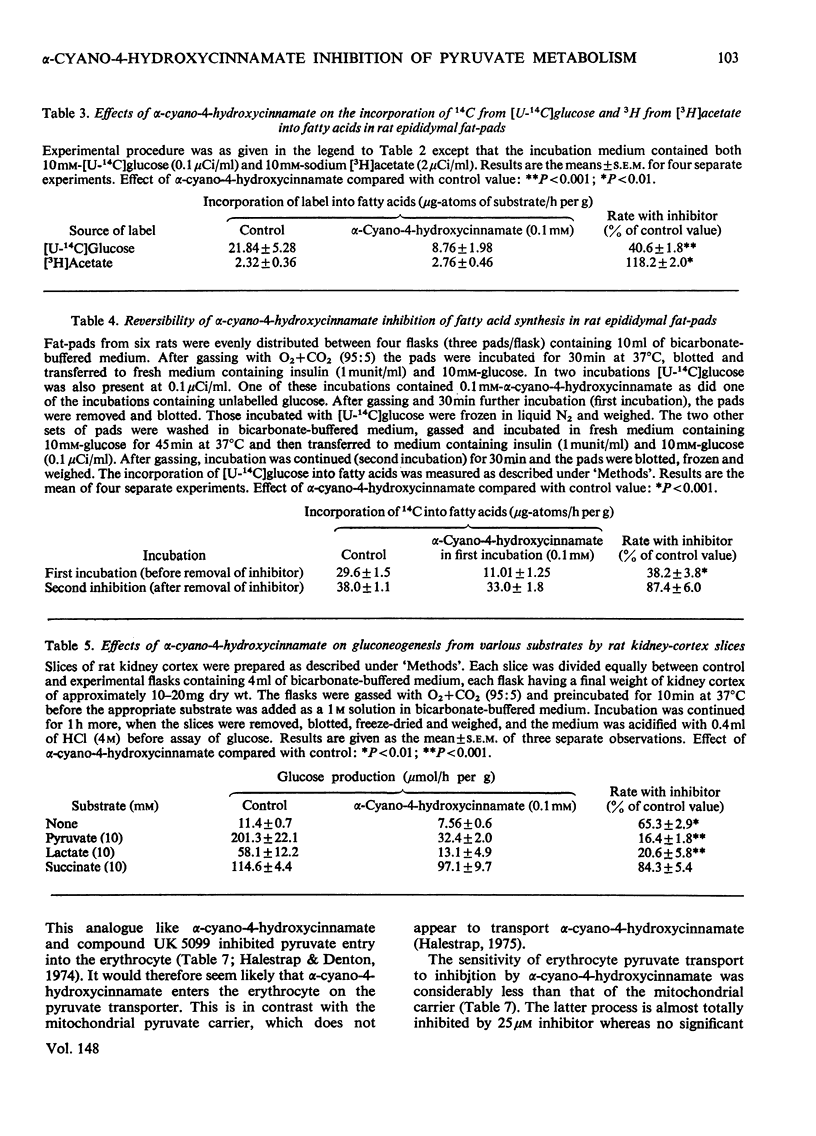
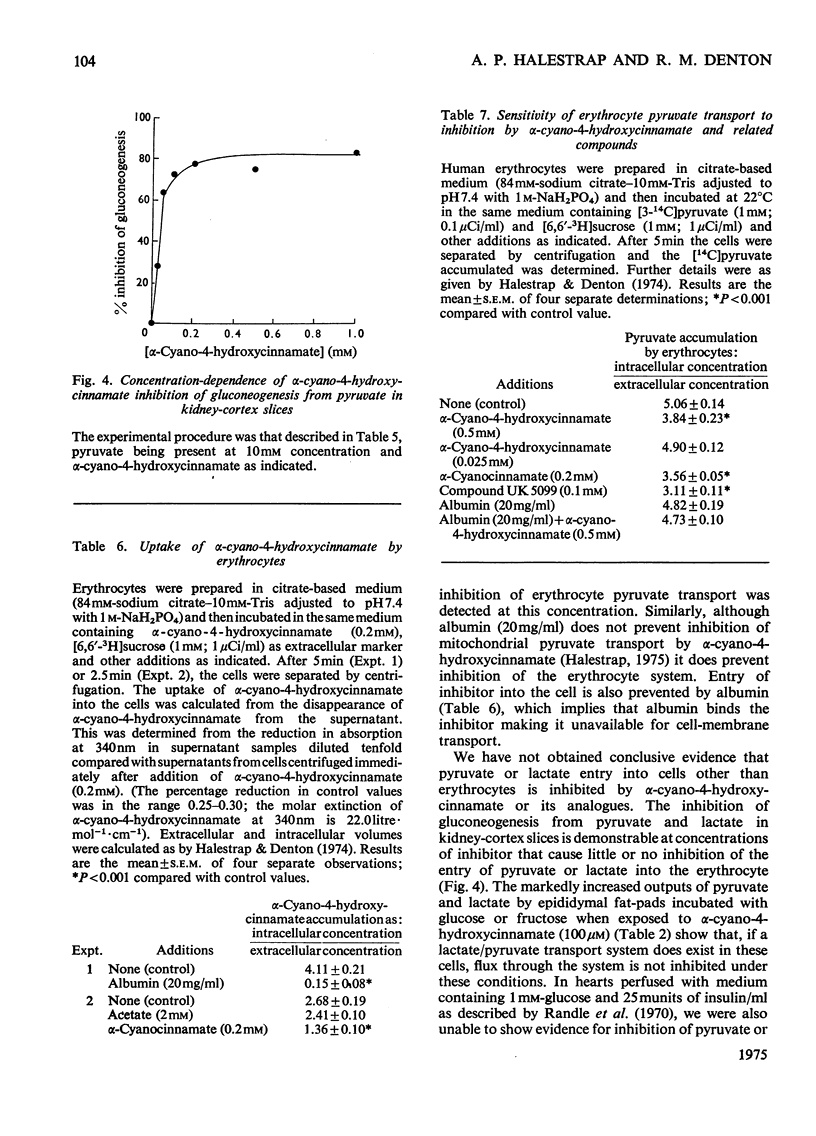
1. Effects of alpha-cyano-4-hydroxycinnamate and alpha-cyanocinnamate on a number of enzymes involved in pyruvate metabolism have been investigated. Little or no inhibition was observed of any enzyme at concentrations that inhibit completely mitochondrial pyruvate transport. At much higher concentrations (1 mM) some inhibition of pyruvate carboxylase was apparent. 2. Alpha-Cyano-4-hydroxycinnamate (1-100 muM) specifically inhibited pyruvate oxidation by mitochondria isolated from rat heart, brain, kidney and from blowfly flight muscle; oxidation of other substrates in the presence or absence of ADP was not affected. Similar concentrations of the compound also inhibited the carboxylation of pyruvate by rat liver mitochondria and the activation by pyruvate of pyruvate dehydrogenase in fat-cell mitochondria. These findings imply that pyruvate dehydrogenase, pyruvate dehydrogenase kinase and pyruvate carboxylase are exposed to mitochondrial matrix concentrations of pyruvate rather than to cytoplasmic concentrations. 3. Studies with whole-cell preparations incubated in vitro indicate that alpha-cyano-4-hydroxycinnamate or alpha-cyanocinnamate (at concentrations below 200 muM) can be used to specifically inhibit mitochondrial pyruvate transport within cells and thus alter the metabolic emphasis of the preparation. In epididymal fat-pads, fatty acid synthesis from glucose and fructose, but not from acetate, was markedly inhibited. No changes in tissue ATP concentrations were observed. The effects on fatty acid synthesis were reversible. In kidney-cortex slices, gluconeogenesis from pyruvate and lactate but not from succinate was inhibited. In the rat heart perfused with medium containing glucose and insulin, addition of alpha-cyanocinnamate (200 muM) greatly increased the output and tissue concentrations of lactate plus pyruvate but decreased the lactate/pyruvate ratio. 4. The inhibition by cyanocinnamate derivatives of pyruvate transport across the cell membrane of human erythrocytes requires much higher concentrations of the derivatives than the inhibition of transport across the mitochondrial membrane. Alpha-Cyano-4-hydroxycinnamate appears to enter erythrocytes on the cell-membrane pyruvate carrier. Entry is not observed in the presence of albumin, which may explain the small effects when these compounds are injected into whole animals.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Parks R. E., Jr Purine nucleoside phosphorylase from human erythrocytes. IV. Crystallization and some properties. J Biol Chem. 1969 Feb 25;244(4):644–647. [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Halperin M. L. The control of fatty acid and triglyceride synthesis in rat epididymal adipose tissue. Roles of coenzyme A derivatives, citrate and L-glycerol 3-phosphate. Biochem J. 1968 Nov;110(1):27–38. doi: 10.1042/bj1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Brand M. D., Denton R. M. Inhibition of mitochondrial pyruvate transport by phenylpyruvate and alpha-ketoisocaproate. Biochim Biophys Acta. 1974 Oct 10;367(1):102–108. doi: 10.1016/0005-2736(74)90140-0. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974 Feb;138(2):313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J. 1975 Apr;148(1):85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M., Pask H. T., Randle P. J. Mechanisms regulating adipose-tissue pyruvate dehydrogenase. Biochem J. 1972 Sep;129(3):763–773. doi: 10.1042/bj1290763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure W. R., Lardy H. A., Wagner M., Cleland W. W. Rat liver pyruvate carboxylase. II. Kinetic studies of the forward reaction. J Biol Chem. 1971 Jun 10;246(11):3579–3583. [PubMed] [Google Scholar]
- Pogson C. I. Adipose-tissue pyruvate kinase. Properties and interconversion of two active forms. Biochem J. 1968 Nov;110(1):67–77. doi: 10.1042/bj1100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., England P. J., Denton R. M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970 May;117(4):677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970 Sep;119(2):193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of acetate, pyruvate, lactate, palmitate, electron-acceptors, uncoupling agents and oligomycin. Biochem J. 1972 Aug;128(5):1069–1078. doi: 10.1042/bj1281069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert D., Herlemann E. M., Albrecht E., Seubert W. On the mechanism of gluconeogenesis and its regulation. VII. Purification and properties of pyruvate carboxylase from rat liver. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):459–478. doi: 10.1515/bchm2.1971.352.1.459. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucki J. W., Brawand F., Walter P. Regulation of pyruvate metabolim in rat-liver mitochondria by adenine nucleotides and fatty acids. Eur J Biochem. 1972 May;27(1):181–191. doi: 10.1111/j.1432-1033.1972.tb01824.x. [DOI] [PubMed] [Google Scholar]
- Walter P., Stucki J. W. Regulation of pyruvate carboxylase in rat liver mitochondria by adenine nucleotides and short chain fatty acids. Eur J Biochem. 1970 Feb;12(3):508–519. doi: 10.1111/j.1432-1033.1970.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Wimhurst J. M., Manchester K. L. Some aspects of the kinetics of rat liver pyruvate carboxylase. Biochem J. 1970 Nov;120(1):79–93. doi: 10.1042/bj1200079. [DOI] [PMC free article] [PubMed] [Google Scholar]


