Abstract
Surface-based ultrasound (SUS) systems have undergone substantial improvement over the years in image quality, ease-of-use, and reduction in size. Their ability to image organs non-invasively makes them a prime technology for the diagnosis and monitoring of various diseases and conditions. An example is the screening/risk- stratification of prostate cancer (PCa) using prostate-specific antigen density (PSAD). Current literature predominantly focuses on prostate volume (PV) estimation techniques that make use of magnetic resonance imaging (MRI) or transrectal ultrasound (TRUS) imaging, while SUS techniques are largely overlooked. If a reliable SUS PCa screening method can be introduced, patients may be able to forgo unnecessary MRI or TRUS scans. Such a screening procedure could be introduced into standard primary care settings with point-of-care ultrasound systems available at a fraction of the cost of their larger hospital counterparts. This review analyses whether literature suggests it is possible to use SUS-derived PV in the calculation of PSAD.
Keywords: Abdominal ultrasound, machine learning, prostate cancer, PSA-density, surface-based ultrasound
I. Introduction
Prostate cancer (PCa) is the second most diagnosed form of cancer in men, with a mortality rate second only to lung cancer [1]. It has a five-year survivability rate that drops from nearly 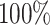 to
to 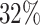 in those diagnosed with stage 3 and stage 4 cancer, respectively [2]. This dramatic decrease underscores the importance of earlier detection.
in those diagnosed with stage 3 and stage 4 cancer, respectively [2]. This dramatic decrease underscores the importance of earlier detection.
A variety of methods are available for detecting PCa, each with their own advantages and disadvantages, from the simple digital rectal exam (DRE), through to automatic segmentation of multi-parametric magnetic resonance images (mpMRI) leveraging deep learning. This review focuses on detecting PCa by combining the result of a prostate-specific antigen (PSA) blood test with prostate volume (PV) measurements, acquired using surface-based ultrasound (SUS), to give PSA-density (PSAD) [3].
The calculation of PSAD is shown in (1), where PSA is in 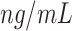 , PV is in
, PV is in 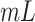 , and the resulting PSAD is in
, and the resulting PSAD is in 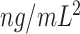 . The benefit of using PSAD over PSA is that it accounts for enlarged prostates naturally increasing PSA levels in the blood, which alone is not necessarily a sign of PCa.
. The benefit of using PSAD over PSA is that it accounts for enlarged prostates naturally increasing PSA levels in the blood, which alone is not necessarily a sign of PCa.
 |
It has been shown that PSAD is more reliable than PSA alone when attempting to detect PCa in patients with a Gleason Score of 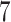 or higher [6] and can be used to safely avoid biopsies in patients with negative features on magnetic resonance images (MRI) [7], [8].
or higher [6] and can be used to safely avoid biopsies in patients with negative features on magnetic resonance images (MRI) [7], [8].
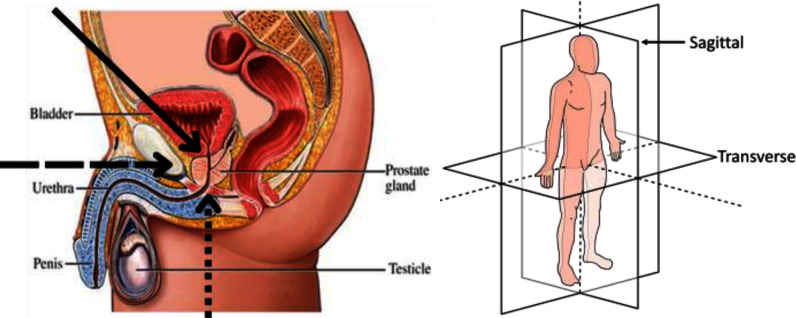
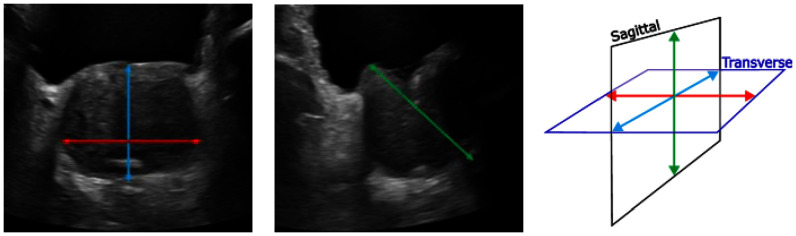
Acquiring PV for use in PSAD calculations can be tricky. The difficulty in acquiring the PV is in part due to there being no orthogonal line-of-site from outside the body to the prostate that is not at least partially obstructed by bone (see Fig. 1– Left, dashed and dotted arrows). While not a problem for MRI, it is a problem for SUS imaging.
Fig. 1.
Left – male abdominal diagram showing prostate gland location. Arrows indicate SUS viewing angles (solid and dotted – suitable; dashed – unsuitable) [4]. Right –anatomical planes [5].
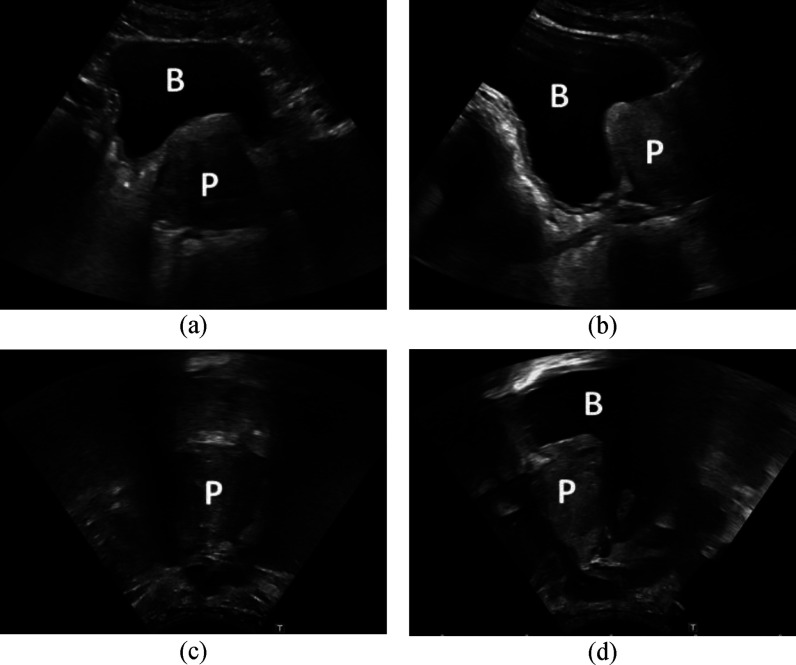
SUS scans of the prostate can be split into: Transabdominal ultrasound (AUS) scans and transperineal ultrasound (TPUS) scans. For AUS scans the probe is placed on the abdomen of the patient (see Fig. 1– Left solid arrow) whereas for TPUS scans the probe is placed on the perineum (see Fig. 1– Left dotted arrow). Each of these scanning positions are further subdivided into two orthogonal sets: transverse and sagittal. For each of these scans the ultrasound probe is aligned with the relevant anatomical plane in Fig. 1– Right. This results in four possible scanning planes: transverse-AUS (tAUS), sagittal-AUS (sAUS), transverse-TPUS (tTPUS), and sagittal-TPUS (sTPUS). Fig. 2 highlights the differences between AUS scans (top) and TPUS scans (bottom) of the prostate.
Fig. 2.
A comparison between the different SUS images of the prostate. (a) – tAUS scan. (b) – sAUS scan. (c) – tTPUS scan. (d) – sTPUS scan. Each image was taken to be at the centre of the prostate. ‘B’ indicates the bladder and ‘P’ the prostate.
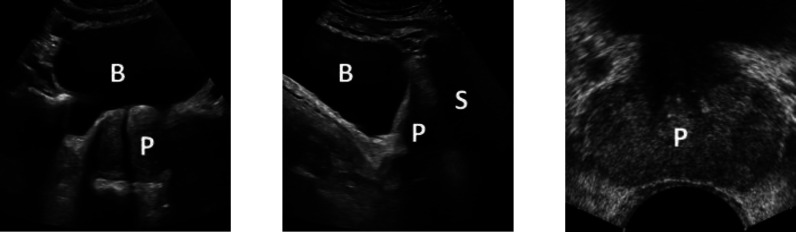
SUS scans are not subject to the patient discomfort of transrectal ultrasound (TRUS) scans or operating constraints of mpMRI scans. However, due to SUS having a lower signal-to-noise ratio compared to that of TRUS, SUS images containing multiple anatomical structures (see Fig. 3 – Left and Centre), and the shadowing caused by the pubic bone (Fig. 1– Left solid arrow and resultant shadowing in Fig. 3 – Centre), there is comparatively little research into the acquisition of PV using SUS images.
Fig. 3.
An example of a tAUS scan (Left), sAUS scan (Centre), and TRUS scan [9] (Right) of the prostate. ‘B’ indicates the bladder, ‘P’ the prostate, and ‘S’ shadowing caused by the pubic bone. The tAUS and sAUS images were acquired using a Canon Aplio i700 ultrasound system.
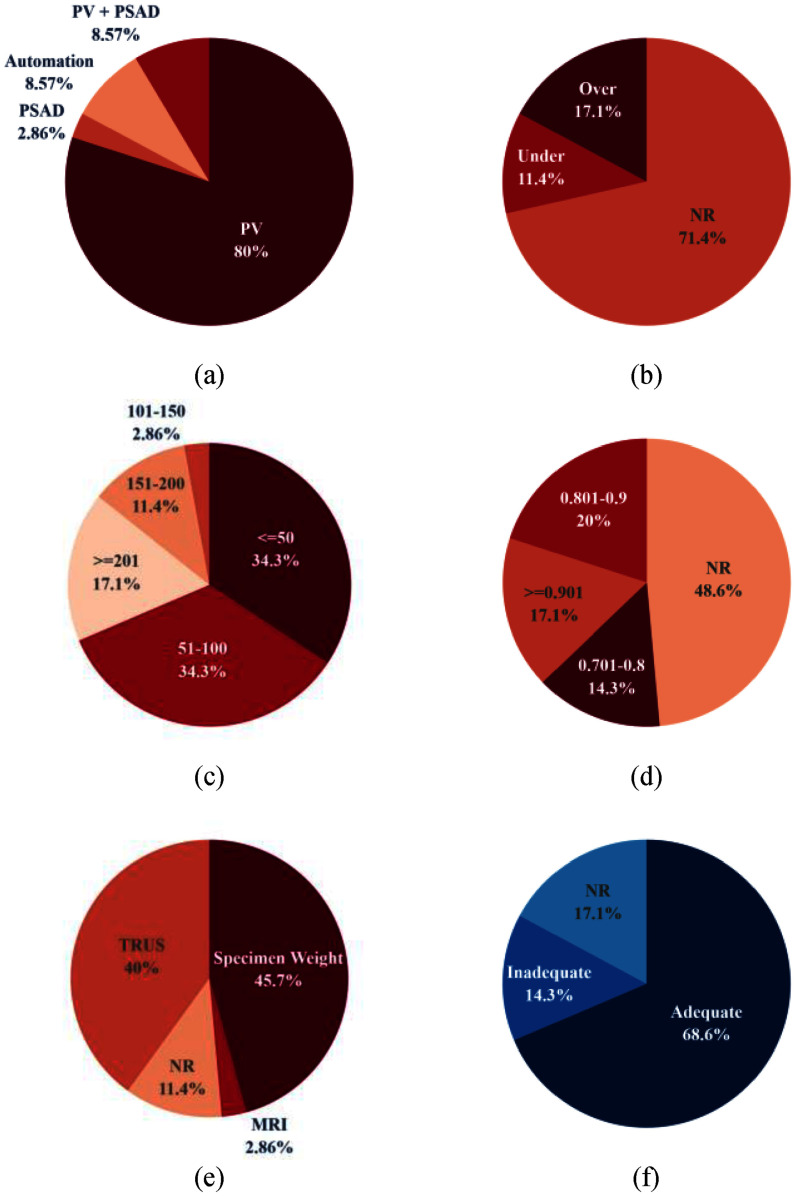
The current review covers the use of SUS scans in acquiring PV estimates, considering its suitability for use in PSAD calculations when testing for PCa. A summary of the studies reviewed is presented in Table 1, with some key metrics given in Fig. 5. The methodology followed can be found in the supplementary materials, as can the recognised limitations of this study.
TABLE 1. Paper Search Results, With Key SUS Conclusions, Organised by Year Published.
| Ref. | Focus | Cohort Size | SUS Position | Equation | Reference | Over/Under/Accurate (SUS w.r.t. reference PV) | Correlation (SUS w.r.t. reference) | Stratification | SUS Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| [16] | PV | 15 | AUS | PE | Specimen | - | - | - | Useful applications where approximate assessments are required. |
| [17] | PV | 8 | AUS | Sphere | Specimen | - | • | - | AUS is more accurate than CT and clinical estimates in PV estimation. |
| [29] | PV | 29 | AUS | Sphere | Specimen | - | Highly significant correlation. • 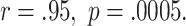
|
Better correlation for larger prostates. | AUS should receive more attention as it is an accurate predictor of PV. |
| [38] | PV | 50 | AUS | PE | Specimen | - | Correlation. • 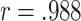
|
- | AUS is an accurate method for estimation of PV. |
| [44] | PV | 33 | AUS | PE | Specimen | - | • Insignificant difference. | - | AUS is reasonably accurate with good correlation to specimen weight. |
| [45] | PV | 85 | AUS | - | Specimen | - | - | - | Close correlation between AUS and sum of resected and postoperative PV. |
| [27] | PV | 26 | AUS | PE | Specimen | Underestimated. | Pearson's correlation. • 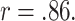
Spearman's correlation. 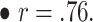
|
- | AUS is quite accurate in PV estimation has definite value in the assessment of prostatic size. |
| [46] | PV | 88 | AUS | - | TRUS | - | • Correlated well with good agreement. | - | AUS correlated well with TRUS. |
| [39] | PV | 107 | AUS | Sphere | Specimen | - | Pearson's correlation.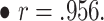
|
- | AUS was well adapted for screening of prostatic diseases. |
| [30] | PV | 50 | TPUS | PE1 | TRUS | - | Student's two-tailed t-test. • 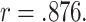
|
- | TPUS measurements are comparable to TRUS in dimensions and volume. |
| [40] | PV | 80 | TPUS | PE | Specimen | - | Pearson's correlation to TPUS. • Specimen: 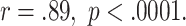
• AUS: 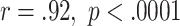 . . |
- | TPUS is accurate in evaluating PV. |
| [41] | PV | 44 | AUS | PE | TRUS | - | Pearson's correlation. • 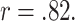
|
- | No statistically significant difference between AUS and TRUS. |
| [42] | PV | 95 | AUS | PE | TRUS | - | Pearson's correlation. • 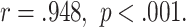
|
- | AUS could replace TRUS for PV determination. |
| [23] | PV | 196 | AUS | PE | TRUS | Overestimated: 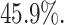
Underestimated: 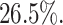
Accurate: 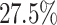 . . |
- | - | AUS less accurate than TRUS |
| [47] | PSAD | 420 | AUS | PE5 | Biopsy | - | PSAD ROC curve areas. • TRUS: 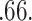
• AUS: 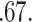
Sensitivity and specificity. • TRUS: 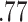 and and 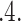
• AUS: 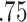 and and 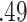 . . |
- | AUS derived PSAD is as useful as TRUS derived PSAD. |
| [31] | PV | 22 | AUS | PE | TRUS | - | Spearman's correlation4. • 100mL: 
• 200mL: 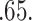
• 300mL: 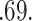
• 400mL: 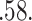
• 500mL: 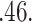
|
- | AUS PV correlates well with TRUS PV when bladder volume 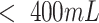 . . |
| [32] | PV | 200 | AUS | PE | TRUS | - | Pearson's correlation.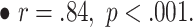
|
- | No statistical difference between AUS PV and TRUS PV. |
| [48] | ML | 11 | AUS | - | - | - | • - | - | - |
| [22] | PV + PSAD | 238 | AUS | PE2 | TRUS | Overestimated: 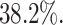
Underestimated: 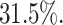
Accurate: 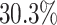 . . |
Pearson's correlation (PV). • All: 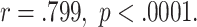
• >50mL: 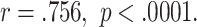
• <50mL: 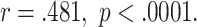
|
Better correlation for larger prostates. | AUS PSAD is worthwhile as it significantly improves on PSA alone and can reduce unnecessary biopsies. |
| [43] | PV | 287 | TPUS | PE | TRUS | - | Interclass correlation (PV). • 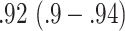
|
- | TPUS provides an accurate alternative to TRUS. |
| [25] | PV | 94 | AUS | PE | TRUS | Overestimated: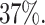
Underestimated 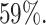
Accurate: 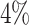 . . |
Pearson's correlation. • All: 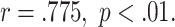
• Beginner: 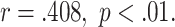
• Trained: 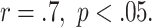
• Expert: 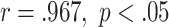 . . |
- | |
| [19] | PV | 100 | AUS | PE | TRUS | Overestimated by 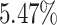 . . |
Pearson's correlation. • All: 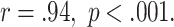
• >50mL: 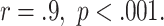
• <=50mL: 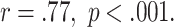
|
Better correlation for larger prostates. | Strong correlation between AUS and TRUS for volume and dimensions. AUS can be an alternative to TRUS. |
| [20] | PV | 71 | AUS | PE | Specimen | Overestimated by 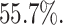
|
Pearson's correlation. • 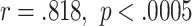 . . |
- | AUS shows significant overestimation of specimen weight. |
| [21] | PV + PSAD | 60 | AUS | PE | Specimen | Overestimated. | Pearson's correlation. • PV: 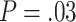 . .• PSAD: 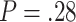 . . |
- | AUS PSAD showed no statistically significant difference to specimen PSAD. AUS overestimation of specimen volume statically significant. |
| [24] | PV | 163 | AUS | PE | Specimen | Underestimated by 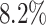 . . |
95% confidence interval.
|
Most accurate estimation between 41g-60g.. | AUS PV correlated well with specimen. |
| [33] | PV | 40 | AUS | PE | TRUS | - | Pearson's correlation. • 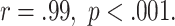
|
- | AUS does not agree sufficiently with TRUS according to LOA. |
| [34] | PV | 60 | AUS | NR | Specimen | - | Pearson's correlation. • r=.77, p<.001. Coefficient of linear regression. 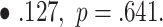
|
- | Significant correlation between AUS and specimen. |
| [35] | PV | 49 | AUS | PE | TRUS | - | Spearman's correlation. • 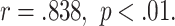
|
- | TRUS and AUS are not different in PV estimation. |
| [49] | ML | 210 | AUS | - | - | - | - | - | - |
| [28] | PV | 236 | AUS | PE | TRUS | Mix of overestimation, underestimation, and accurate estimation. | Pearson's correlation. • 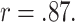
Intraclass correlation. • 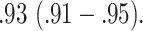
|
Better agreement5 for smaller prostates. | AUS excellent surrogate for TRUS in most cases, but not for larger prostates. |
| [18] | PV | 170 | AUS | PE | Specimen | Overestimated. | Spearman's correlation. • 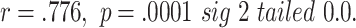
|
- | Positive correlation between AUS and specimen. |
| [36] | PV | 92 | AUS | PE | Specimen | - | Pearson's correlation. • 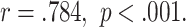
|
- | AUS showed statistically significant difference to specimen. |
| [26] | PV | 98 | AUS | PE | Specimen | Overestimated: 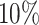 . .Underestimated: 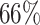 . .Accurate: 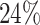
|
Pearson's correlation. • All: 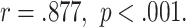
• <30g: 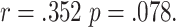
• 30g-60g: 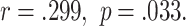
• >60g: 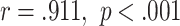
|
Better correlation for larger prostates. | AUS comparable, and acceptable alternative, to TRUS. |
| [50] | ML | 305 | AUS | PE | - | - | - | - | - |
| [37] | PV + PSAD | 64 | AUS / TPUS | PE | mpMRI | - | Interclass correlation (PV). • TAUS to MRI: 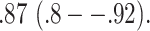
• TPUS to MRI: . 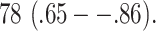
|
- | AUS and TPUS PV has good agreement with MRI PV. AUS and TPUS PSAD has good agreement with MRI PSAD. |
Bolded Rows Attempted to Automate All/Part of the PV Estimation Process. PE – Prolate Ellipsoid From (2). PE1 – Modification of (2): 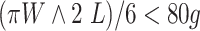 or
or 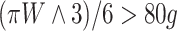 . PE2 – Modification of (2):
. PE2 – Modification of (2): 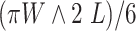 . PE3 – Modification of (2):
. PE3 – Modification of (2): 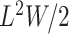 . Spearman's Correlation4: With Respect to Bladder Volume. Agreement5: As Determined Using Bland Altman Plots and Limits-of-Agreement
. Spearman's Correlation4: With Respect to Bladder Volume. Agreement5: As Determined Using Bland Altman Plots and Limits-of-Agreement
Fig. 5.
Graphical summary of key metrics from Table 1. NR – Not report or not applicable. (a) Focus of paper. (b) Overestimation or underestimation of SUS-derived PV w.r.t. reference method. (c) Study cohort size, grouped by 50. (d) Reported PCC. (e) PV estimation reference method. (f) Qualitative conclusion regarding suitability of AUS in PV estimation.
II. Review Search Results
The results of the literature search can be categorised into four groups: How PV is estimated using SUS scans; The accuracy of PV estimates calculated using SUS images; How SUS-derived PSAD values fare in the clinical decision process in comparison to more contemporary methods; Automating the process of PV estimation using SUS images.
A. SUS-Based PV Estimation
In a clinical setting, there are generally two approaches used to estimate the PV from images: stepwise planimetry and geometric models. To the best of the authors’ knowledge, there are no studies done to date that use SUS images of the prostate for stepwise planimetry, and as such, only the geometric model is considered.
The geometric model assumes that the prostate is shaped like an ellipse. This is not entirely true, and as patients age the prostate can become more irregularly shaped. Under this assumption (2) is used to estimate PV, where 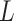 ,
, 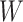 , and
, and 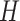 are the length, width, and height of the gland, respectively, and C is a constant. For the sake of this review and the equations presented in Table 1,
are the length, width, and height of the gland, respectively, and C is a constant. For the sake of this review and the equations presented in Table 1, 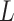 is the anterior-posterior dimension,
is the anterior-posterior dimension, 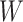 is the left-right dimension, and
is the left-right dimension, and 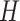 is the superior-inferior dimension. The value of the constant C varies based on the assumed shape of the prostate [10], [11], [12], [13].
is the superior-inferior dimension. The value of the constant C varies based on the assumed shape of the prostate [10], [11], [12], [13].
 |
Two orthogonal images of the prostate are acquired, and a clinician measures the three required dimensions. See Fig. 4 showing sample measurements on both a tAUS (Left) and sAUS (Middle) image. Studies tend to conclude that the geometric model is accurate enough when compared to the more accurate stepwise planimetry [14], [15].
Fig. 4.
AUS measurement locations used to estimate PV. Left - transverse plane showing axial 1 and axial 2 measurements. Middle - sagittal plane showing cranio-caudal measurement. Right – anatomical scan planes with corresponding dimensions superimposed.
B. SUS-Based PV Accuracy
The use of AUS scans to estimate PV was mentioned as far back as 1973 [16]. It was concluded that the SUS-derived PV estimates were useful in applications that required approximate assessment of the prostate. In 1977, [17] concluded that SUS-derived PVs were more accurate than CT-derived and clinically derived values when compared to specimen weights, and that SUS could be used when estimating PV.
Since then, multiple studies have tested the accuracy of SUS-derived PV estimates in comparison to one or more reference values. Some found that SUS-derived PVs tended to overestimate the reference method [18], [19], [20], [21], [22], [23], others showed a tendency to underestimate [24], [25], [26], [27], while some found a mixture of underestimation, overestimation, and accurate estimation [22], [23], [25], [26], [28]. In attempts to determine if measurements of smaller or larger prostates using SUS scans correlated more with the reference method some studies stratified their results based on volume estimates. References [19], [22], [26], [29] showed better correlation with larger prostates, [28] showed more agreement for smaller prostates, and [24] showed the best correlation for medium sized prostates. Of the 31 studies that compared SUS-derived PV with a reference value, quantitatively 24 of them found that SUS-derived PV estimates correlated well with the chosen reference method [18], [19], [20], [22], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], with six studies not reporting a quantitative correlation value [16], [17], [23], [44], [45], [46]. One study did not report a correlation coefficient, only that the SUS-derived PV significantly overestimated the reference PV [21].
Of these 24 studies, 5 concluded that even with the reported correlation values SUS-derived PV is not suitable when compared to the chosen reference method [20], [21], [23], [33], [36]: Three studies concluded that SUS-derived PVs overestimated specimen weight [20], [21], [23]; One study explicitly claimed that SUS could not be used in place of TRUS, stating that SUS-derived PV estimates did not agree with TRUS-derived PV estimates using Bland Altman plots and limits-of-agreement [33]; One study concluded that there was a statistically significant difference between SUS-derived PV and specimen weight [36].
C. Clinical Decisions
A 2002 study compared SUS-derived and TRUS-derived PSAD values with the results of a biopsy [47], with the conclusion being that SUS-derived PSAD is as useful as TRUS-derived PSAD.
In a 2005 study PSAD values calculated using SUS-derived and TRUS-derived PV estimates were compared [22]. Although it was found that SUS-derived and TRUS-derived PVs correlated well, the SUS-derived PSAD values did not perform as well as the TRUS-derived values in detecting PCa. However, it was noted that SUS-derived PSAD values significantly outperformed PSA alone.
A 2013 study showed that due to SUS-derived PV estimates tending towards overestimation of the specimen volume, 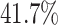 of cancers would have been missed, in their cohort, if SUS-derived PSAD values were used for PCa prediction [21]. This was in comparison to the
of cancers would have been missed, in their cohort, if SUS-derived PSAD values were used for PCa prediction [21]. This was in comparison to the 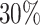 that would have been missed if specimen volumes were used in the calculations of PSAD values. The study concluded that SUS-derived PSAD values performed similarly to TRUS-derived values for active surveillance.
that would have been missed if specimen volumes were used in the calculations of PSAD values. The study concluded that SUS-derived PSAD values performed similarly to TRUS-derived values for active surveillance.
In a more recent study (2022) PSAD values derived from SUS scans were compared with MRI-derived PSAD values [37]. The aim of the study was to ascertain whether SUS-derived PSAD values could be used in triage as a risk-stratification tool. A more conservative PSAD threshold was used with sensitivities and specificities of up to 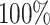 achieved. It was concluded that SUS-derived PSAD has a good agreement with MRI-derived values. They also suggested that unnecessary MRIs can be avoided by using the PSAD values obtained from SUS scans.
achieved. It was concluded that SUS-derived PSAD has a good agreement with MRI-derived values. They also suggested that unnecessary MRIs can be avoided by using the PSAD values obtained from SUS scans.
D. Automating SUS-Based PV Estimation
A 2004/5 study attempted to automatically segment AUS images of the prostate using a combination of a contour enhancing filter and a heuristic optimisation algorithm [48]. They found that their system produced contours that were very similar to those created through manual segmentation.
In 2017 [49] and 2022 [50] a multi-task quadruplet deep convolutional neural network (QDCNN) was developed to infer four points on tAUS images of the prostate, and two points on sAUS images. These six points were then used to estimate the PV using (2) (see Fig. 2 of the Supplementary Material for sample results). It was found that the QDCNN system's estimated PVs fell within experts’ estimations, and that the system could be used by experts as an aid to increase the reliability of their own PV estimates.
III. Discussion
While SUS PV estimation is not a new idea its limitations have resulted in the technique largely being ignored in favour of other imaging modalities, which are considered superior when imaging the prostate. However, they are subject to higher operating costs, increased time requirements, and patient discomfort. SUS scans are not subject to any of these limitations, with point-of-care ultrasound (POCUS) devices allowing for routine scanning in a primary care setting. SUS scans exhibit almost no patient discomfort and are non-invasive, however, the images are affected by shadowing from the pubic bone. This shadowing can lead to further inaccuracies in the PV estimates as the prolate ellipsoid dimensions can be difficult to estimate. To minimise the effects of this pubic bone shadowing, patients are asked to present with a full bladder (see Fig. 3 of the Supplementary Material). If SUS systems can be shown to be good enough for PCa detection purposes, they could be used as a pre-MRI/TRUS scan, or for use during active surveillance, and potentially for the screening of PCa. For this to be realised, SUS-derived PV estimates need to be shown to correlate well with either mpMRI, TRUS, or specimen volumes, but more importantly the resulting PSAD values need to be shown as effective in detecting PCa.
When manually estimating the PV simpler methods/ techniques are favoured by clinicians due to time constraints and ease of use. Generally, the higher the required accuracy the more complicated/time-consuming the estimation method will be (manual stepwise planimetry versus geometric models). Manual stepwise planimetry of mpMRI has been suggested by some to be used as the gold-standard, whereas prolate ellipsoid PV estimates are just considered good enough or reasonably accurate [14], [51]. The drop in accuracy between manual planimetry and the prolate ellipsoid formula can be attributed to the limitations associated with using a simple geometric model to represent the prostate, which can have a variety of shapes that tend to change as the patient ages [12], [13]. This variability in prostate shape suggests that the constant value of the geometric model may not be the most accurate formulation, and that instead a variable constant (possibly a function of the prostate size) should be used. Such a formula has been reported previously, where the “adjusted PV” is a function of patient age and the calculated prolate ellipsoid volume of the prostate [36].
While the absolute accuracy of SUS-derived PV estimates may vary between studies the general consensus is that they are fairly accurate when compared to either mpMRI, TRUS, or specimen weight (Fig. 5(f)). Of the five studies that found AUS-derived PV estimates were significantly different from their chosen reference method, only one explicitly claimed that AUS-derived PV estimates could not be used in place of TRUS-derived estimates [33]. However, in a later study, it was noted that the cohort of the 2015 study was relatively small and homogeneous [28]. Fig. 5(d) highlights the strong correlation between SUS-derived PV values and the chosen reference method for the papers included in this review. When reported whether SUS-derived PV was overestimated, underestimated, or accurately estimated, most studies found overestimation to be more common (Fig. 5(b)).
Studies that have used SUS-derived PSAD values to detect PCa show that even though the absolute accuracy of geometric SUS-derived PV estimates may not always be very reliable, the resulting PSAD values are accurate enough to be used in the process of detecting, and possibly screening for, PCa [21], [22], [37], [47]. The results of [37] are particularly promising for SUS PCa screening. By simply lowering the threshold PSAD value they were able to reach a sensitivity of 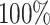 for SUS-derived PSAD values. Although PVs estimated from SUS scans have been shown to not be entirely accurate, they are consistent enough that merely changing other parameters (not related to the acquisition of the PV) to accommodate this lack of accuracy is sufficient.
for SUS-derived PSAD values. Although PVs estimated from SUS scans have been shown to not be entirely accurate, they are consistent enough that merely changing other parameters (not related to the acquisition of the PV) to accommodate this lack of accuracy is sufficient.
In comparison to MRI- and TRUS-based automated PV estimation studies, there are not many that use SUS scans. The three studies presented are the only studies that work towards automatically estimating PV from AUS-based scans, with [50] a continuation of [49]. The first of these two studies did not attempt to estimate PV as only the first two dimensions of (2) were inferred from tAUS images. The second study improved on this limitation by incorporating inference of the third dimension from sAUS images. [48] only attempted to segment one image of the prostate, and no volume calculations were attempted. The presented results of [50] are encouraging as the level of accuracy their system was capable of fell within those of expert values. They also made their dataset available for public use, which helps address a major bottleneck researchers face when creating new machine learning models: data availability. When attempting to create systems that can automate the task of estimating PV from SUS scans acquiring datasets for a single study can be an expensive and protracted process. Public datasets alleviate this and can grant researchers access to a more diverse pool than when using their own datasets. Fig. 5(c) shows the limited cohort sizes in the studies presented. Only six studies had more than 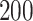 patients participate, with most studies making use of data from less than
patients participate, with most studies making use of data from less than 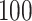 patients.
patients.
IV. Conclusion
When it comes to testing for PCa using PV and PSAD the most trusted imaging modality is mpMRI. This is followed by TRUS which is considered invasive. SUS comes in last, due to its lower signal-to-noise ratio, and the presence of multiple confounding anatomical structures in its images. However, the results of this review show that even though SUS-based PV estimates are not the most accurate, they are accurate and consistent enough that they can be used for the calculation of PSAD in a clinically appropriate setting.
Due to the known limitations of SUS scans of the prostate there is comparatively little research into using machine learning to either aid in, or fully-automate, the process of calculating the PV. However, the few studies that have attempted to automate the process of calculating PSAD (by first automating the process of estimating PV) using machine learning have shown very promising results.
Therefore, given that SUS scans of the prostate return “good enough” PV estimates with significant correlation to more accurate methods, and machine learning has been shown to be capable of returning results within the range of experts, further investigation into the use of SUS scans of the prostate for the screening of PCa is definitely warranted.
Supplementary Materials
Supplementary materials have been supplied as a separate file. The methodology followed during the literature search, some sample images, and a limitations section can be found there.
Acknowledgment
The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Funding Statement
The work of Tristan Barrett was supported by the NIHR Cambridge Biomedical Research Centre under Grant NIHR203312, in part by Cancer Research U.K., Cambridge Imaging Centre under Grant C197/A16465, in part by the Engineering and Physical Sciences Research Council Imaging Centre in Cambridge and Manchester, and in part by the Cambridge Experimental Cancer Medicine Centre. This work was supported in part by the Academy of Medical Sciences Professorship (APR6\1011), in part by Royal Society Wolfson Fellowship, Cancer Research UK under Grant EDDPMA-Nov21\100026, in part by the National Institutes of Health (NIH) Bench-to-Bedside Award, in part by the NIH Center for Interventional Oncology under Grant ZID BC011242 and Grant CL040015, and in part by the Intramural Research Program of the National Institutes of Health.
References
- [1].“Key statistics for prostate cancer | prostate cancer facts,” Mar. 10, 2023. Accessed: Mar. 10, 2023. [Online]. Available: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
- [2].“Prostate cancer: Statistics | Cancer.Net,” Mar. 10, 2023. Accessed: Oct. 05, 2023. [Online]. Available: https://www.cancer.net/cancer-types/prostate-cancer/statistics
- [3].Benson M. C. et al. , “Prostate specific antigen density: A means of distinguishing benign prostatic hypertrophy and prostate cancer,” J. Urol., vol. 147, no. 3, pp. 815–816, 1992, doi: 10.1016/s0022-5347(17)37393-7. [DOI] [PubMed] [Google Scholar]
- [4].“Prostate cancer — Singapore Urological Association,” Accessed: Oct. 12, 2023. [Online]. Available: https://sua.sg/prostate-cancer/
- [5].“File:anatomical Planes.svg - wikimedia commons,” Mar. 19, 2023. Accessed: Mar. 19, 2023. [Online]. Available: https://commons.wikimedia.org/wiki/File:Anatomical_Planes.svg
- [6].Nordström T., Akre O., Aly M., Grönberg H., and Eklund M., “Prostate-specific antigen (PSA) density in the diagnostic algorithm of prostate cancer,” Prostate Cancer Prostatic Dis., vol. 21, no. 1, pp. 57–63, Apr. 2018, doi: 10.1038/s41391-017-0024-7. [DOI] [PubMed] [Google Scholar]
- [7].Boesen L. et al. , “Prebiopsy biparametric magnetic resonance imaging combined with prostate-specific antigen density in detecting and ruling out Gleason 7–10 prostate cancer in biopsy-naïve men,” Eur. Urol. Oncol., vol. 2, no. 3, pp. 311–319, May 2019, doi: 10.1016/j.euo.2018.09.001. [DOI] [PubMed] [Google Scholar]
- [8].Hansen N. L. et al. , “The influence of prostate-specific antigen density on positive and negative predictive values of multiparametric magnetic resonance imaging to detect Gleason score 7–10 prostate cancer in a repeat biopsy setting,” BJU Int., vol. 119, no. 5, pp. 724–730, May 2017, doi: 10.1111/bju.13619. [DOI] [PubMed] [Google Scholar]
- [9].Mitterberger M. J. et al. , “Comparative efficiency of contrast-enhanced colour Doppler ultrasound targeted versus systematic biopsy for prostate cancer detection,” Eur. Radiol., vol. 20, no. 12, pp. 2791–2796, Dec. 2010, doi: 10.1007/s00330-010-1860-1. [DOI] [PubMed] [Google Scholar]
- [10].MacMahon P. J., Kennedy A. M., Murphy D. T., Maher M., and McNicholas M. M., “Modified prostate volume algorithm improves transrectal US volume estimation in men presenting for prostate brachytherapy,” Radiology, vol. 250, no. 1, pp. 273–280, 2009, doi: 10.1148/radiol.2501080290. [DOI] [PubMed] [Google Scholar]
- [11].Lee J. S. and Chung B. H., “Transrectal ultrasound versus magnetic resonance imaging in the estimation of prostate volume as compared with radical prostatectomy specimens,” Urologia Internationalis, vol. 78, no. 4, pp. 323–327, May 2007, doi: 10.1159/000100836. [DOI] [PubMed] [Google Scholar]
- [12].Rodriguez E., Skarecky D., Narula N., and Ahlering T. E., “Prostate volume estimation using the ellipsoid formula consistently underestimates actual gland size,” J. Urol., vol. 179, no. 2, pp. 501–503, 2008, doi: 10.1016/j.juro.2007.09.083. [DOI] [PubMed] [Google Scholar]
- [13].Aprikian S. et al. , “Improving ultrasound-based prostate volume estimation,” BMC Urol., vol. 19, no. 1, Jul. 2019, Art. no. 68, doi: 10.1186/s12894-019-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bezinque A., Moriarity A., Farrell C., Peabody H., Noyes S. L., and Lane B. R., “Determination of prostate volume: A comparison of contemporary methods,” Academic Radiol., vol. 25, no. 12, pp. 1582–1587, Dec. 2018, doi: 10.1016/j.acra.2018.03.014. [DOI] [PubMed] [Google Scholar]
- [15].Christie D. R. H. and Sharpley C. F., “How accurately can prostate gland imaging measure the prostate gland volume? Results of a systematic review,” Prostate Cancer, vol. 2019, 2019, Art. no. 6932572, doi: 10.1155/2019/6932572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Whittingham T. A. and Bishop R., “Ultrasonic estimation of the volume of the enlarged prostate,” Brit. J. Radiol., vol. 46, no. 541, pp. 68–70, Jan. 1973, doi: 10.1259/0007-1285-46-541-68-B. [DOI] [PubMed] [Google Scholar]
- [17].Sukov R. J., Scardino P. T., Sample F. W., Winter J., and Confer D. J., “Computed tomography and transabdominal ultrasound in the evaluation of the Prostate,” J. Comput. Assist. Tomography (Computed Tomography), vol. 1, no. 3, pp. 281–289, 1977. [DOI] [PubMed] [Google Scholar]
- [18].Gok B. et al. , “Which is the best radiological imaging method for predicting actual prostate weight?,” Andrologia, vol. 52, no. 10, Nov. 2020, Art. no. e13770, doi: 10.1111/and.13770. [DOI] [PubMed] [Google Scholar]
- [19].Özden E. et al. , “Analysis of suprapubic and transrectal measurements in assessment of prostate dimensions and volume is transrectal ultrasonography really necessary for prostate measurements?” 2009. [Online]. Available: www.uj.unrc.ir [PubMed]
- [20].Stravodimos K. G. et al. , “TRUS versus transabdominal ultrasound as a predictor of enucleated adenoma weight in patients with BPH: A tool for standard preoperative work-up?,” Int. Urol. Nephrol., vol. 41, no. 4, pp. 767–771, 2009, doi: 10.1007/s11255-009-9554-9. [DOI] [PubMed] [Google Scholar]
- [21].Varkarakis I., Zarkadoulias A., Bourdoumis A., Chatzidarellis E., Antoniou N., and Deliveliotis C., “Measurement of PSA density by 3 imaging modalities and its correlation with the PSA density of radical prostatectomy specimen,” Urologic Oncol., Seminars Original Investigations, vol. 31, no. 7, pp. 1038–1042, Oct. 2013, doi: 10.1016/j.urolonc.2011.11.033. [DOI] [PubMed] [Google Scholar]
- [22].Kobayashi T., Kawahara T., Nishizawa K., Ogura K., Mitsumori K., and Ide Y., “Value of prostate volume measurement using transabdominal ultrasonography for the improvement of prostate-specific antigen-based cancer detection,” Int. J. Urol., vol. 12, no. 10, pp. 881–885, 2005, doi: 10.1111/j.1442-2042.2005.01162.x. [DOI] [PubMed] [Google Scholar]
- [23].Blanc M. et al. , “Prostatic volume: Suprapubic versus transrectal ultrasonography in the control of benign prostatic hyperplasia,” La Radiologia Medica, vol. 95, no. 3, pp. 182–187, Mar. 1998. [PubMed] [Google Scholar]
- [24].Kiliç M., Özdemir A. T., Altinova S., Atmaca A. F., Canda A. E., and Balbay M. D., “What is the best radiological method to predict the actual weight of the prostate?,” Turkish J. Med. Sci., vol. 44, no. 1, pp. 31–35, 2014, doi: 10.3906/sag-1205-33. [DOI] [PubMed] [Google Scholar]
- [25].Sun H. K. and Seung H. K., “Correlations between the various methods of estimating prostate volume: Transabdominal, transrectal, and three-dimensional US,” Korean J. Radiol., vol. 9, no. 2, pp. 134–139, Apr. 2008, doi: 10.3348/kjr.2008.9.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moussaoui G. et al. , “Accuracy of clarius, handheld wireless point-of-care ultrasound, in evaluating prostate morphology and volume compared to radical prostatectomy specimen weight: Is there a difference between transabdominal vs transrectal approach?,” J. Endourol., vol. 35, no. 9, pp. 1300–1306, Sep. 2021, doi: 10.1089/end.2020.0874. [DOI] [PubMed] [Google Scholar]
- [27].Vilmann P., Hancke S., Strange-Vognsen H. H., Nielsen K., and Sørensen S. M., “The reliability of transabdominal ultrasound scanning in the determination of prostatic volume,” Scand. J. Urol. Nephrol., vol. 21, no. 1, pp. 5–7, 1987, doi: 10.3109/00365598709180281. [DOI] [PubMed] [Google Scholar]
- [28].Pate W. R., Garg N., Wang L. B., Wason S. E., and Barbosa P. V., “Comparison of transabdominal and transrectal ultrasound for sizing of the prostate,” Urology, vol. 141, pp. 125–129, Jul. 2020, doi: 10.1016/j.urology.2020.04.054. [DOI] [PubMed] [Google Scholar]
- [29].Henneberry M., Carter M. F., and Neiman H. L., “Estimation of prostatic size by suprapubic ultrasonography,” J. Urol., vol. 121, no. 5, pp. 615–616, 1979, doi: 10.1016/S0022-5347(17)56904-9. [DOI] [PubMed] [Google Scholar]
- [30].Terris M. K., Hammerer P. G., and Nickas M. E., “Comparison of ultrasound imaging in patients undergoing transperineal and transrectal prostate ultrasound,” Urology, vol. 52, no. 6, pp. 1070–1072, 1998, doi: 10.1016/s0090-4295(98)00409-9. [DOI] [PubMed] [Google Scholar]
- [31].Shyi J. et al. , “Effects of bladder volume on transabdominal ultrasound measurements of intravesical prostatic protrusion and volume,” Int. J. Urol., vol. 9, pp. 225–229, 2002, doi: 10.1046/j.0919-8172.2002.00453.x. [DOI] [PubMed] [Google Scholar]
- [32].Chung J. W. N. C. H. F., De Vries S. H., Raaijmakers R., Postma R., Bosch J. L. H. R., and Van Mastrigt R., “Prostate volume ultrasonography: The influence of transabdominal versus transrectal approach, device type and operator,” Eur. Urol., vol. 46, no. 3, pp. 352–356, Sep. 2004, doi: 10.1016/j.eururo.2004.05.002. [DOI] [PubMed] [Google Scholar]
- [33].Jandaghi A. B. et al. , “Application of bland-altman method in comparing transrectal and transabdominal ultrasonography for estimating prostate volume,” IJMS, vol. 40, no. 1, pp. 34–39, 2015. [PMC free article] [PubMed] [Google Scholar]
- [34].Demir A., Karadağ M. A., Çeçen K., and Türkeri L., “Abdominal or transrectal ultrasonographic prostate volume and cystoscopic prostatic urethral length measurements to determine the surgical technique for prostatectomy in patients with benign prostate hyperplasia,” J. Urological Surg., vol. 3, no. 4, pp. 119–122, Sep. 2016, doi: 10.4274/jus.954. [DOI] [Google Scholar]
- [35].Kalkanli A. et al. , “Intravesical prostatic protrusion: A potential marker of alpha-blocker treatment success in patients with benign prostatic enlargement,” Urology, vol. 88, pp. 161–165, Feb. 2016, doi: 10.1016/j.urology.2015.11.029. [DOI] [PubMed] [Google Scholar]
- [36].Dekalo S. et al. , “Novel ultrasound-based volume estimation of prostatic benign enlargement to improve decision-making on surgical approach,” Therapeutic Adv. Urol., vol. 13, 2021, Art. no. 1756287221993301, doi: 10.1177/1756287221993301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pantelidou M., Caglic I., George A., Blyuss O., Gnanapragasam V. J., and Barrett T., “Evaluation of transabdominal and transperineal ultrasound-derived prostate specific antigen (PSA) density and clinical utility compared to MRI prostate volumes: A feasibility study,” PLoS One, vol. 17, no. 9, Sep. 2022, Art. no. e0274014, doi: 10.1371/journal.pone.0274014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Smith H.-J. and Haveland H., “Pre-operative and post-operative volumetry of the prostate by transabdominal ultrasonography,” Brit. J. Urol., vol. 54, no. 5, pp. 531–535, Oct. 1982, doi: 10.1111/J.1464-410X.1982.TB13583.X. [DOI] [PubMed] [Google Scholar]
- [39].Ishida N. et al. , “The evaluation of simple estimation method of prostate size by transabdominal ultrasound,” Nihon Hinyokika Gakkai Zasshi, vol. 80, no. 6, pp. 832–837, 1989, doi: 10.5980/JPNJUROL1989.80.832. [DOI] [PubMed] [Google Scholar]
- [40].Rathaus V., Richter S., Nissenkorn I., and Goldberg E., “Transperineal ultrasound examination in the evaluation of prostatic size,” Clin. Radiol., vol. 44, no. 6, pp. 383–385, Dec. 1991, doi: 10.1016/S0009-9260(05)80654-3. [DOI] [PubMed] [Google Scholar]
- [41].Jabaloyas J. M. M., Cerdá J. L. R., García J. M. O., Chinesta S. S., Tormo F. B., and Cruz J. F. J., “Vesicoprostatic echography versus transrectal planimetry in the determination of prostatic volume,” Actas Urologicas Espanolas, vol. 17, no. 5, pp. 310–314, May 1993. [PubMed] [Google Scholar]
- [42].Prassopoulos P., Charoulakis N., Anezinis P., Daskalopoulos G., Cranidis A., and Gourtsoyiannis N., “Suprapubic versus transrectal ultrasonography in assessing the volume of the prostate and the transition zone in patients with benign prostatic hyperplasia,” Abdom Imag., vol. 21, pp. 75–77, 1996, doi: 10.1007/s002619900017. [DOI] [PubMed] [Google Scholar]
- [43].Griffiths K. A., Ly L. P., Jin B., Chan L., and Handelsman D. J., “Transperineal ultrasound for measurement of prostate volume: Validation against transrectal ultrasound,” J. Urol., vol. 178, no. 4, pp. 1375–1380, Oct. 2007, doi: 10.1016/j.juro.2007.05.163. [DOI] [PubMed] [Google Scholar]
- [44].Abu-Yousef M. M. and Narayana A. S., “Transabdominal ultrasound in the evaluation of prostate size,” J. Clin. Ultrasound, vol. 10, no. 6, pp. 275–278, Jul. 1982, doi: 10.1002/JCU.1870100606. [DOI] [PubMed] [Google Scholar]
- [45].Walz P. H., Wenderoth U., and Jacobi G. H., “Suprapubic transvesical sonography of the prostate:determination of prostate size,” Eur. Urol., vol. 9, no. 3, pp. 148–152, Mar. 1983, doi: 10.1159/000474070. [DOI] [PubMed] [Google Scholar]
- [46].Styles R. A., Neal D. E., and Powell P. H., “Reproducibility of measurement of prostatic volume by ultrasound,” Eur. Urol., vol. 14, no. 4, pp. 266–269, Apr. 1988, doi: 10.1159/000472957. [DOI] [PubMed] [Google Scholar]
- [47].Rodriguez R. P., Dehesa M., Zucharino L., and Galan G., “Comparison of prostate volume measurements by transrectal or abdominal ultrasound and its involvement in PSA density assessment for prostate cancer diagnosis AIM,” 2002. [Online]. Available: https://pesquisa.bvsalud.org/portal/resource/pt/ibc-13292
- [48].Betrouni N., Vermandel M., Pasquier D., Maouche S., and Rousseau J., “Segmentation of abdominal ultrasound images of the prostate using a priori information and an adapted noise filter,” Computerized Med. Imag. Graph., vol. 29, no. 1, pp. 43–51, Jan. 2005, doi: 10.1016/j.compmedimag.2004.07.007. [DOI] [PubMed] [Google Scholar]
- [49].Albayrak N. B., Yildirim E., and Akgul Y. S., “Prostate size inference from abdominal ultrasound images with patch based prior information,” in Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics), Berlin, Germany: Springer, 2017, pp. 249–259. doi: 10.1007/978-3-319-70353-4_22. [DOI] [Google Scholar]
- [50].Albayrak N. B. and Akgul Y. S., “Estimation of the prostate volume from abdominal ultrasound images by image-patch voting,” Appl. Sci., vol. 12, no. 3, Feb. 2022, Art. no. 1390, doi: 10.3390/app12031390. [DOI] [Google Scholar]
- [51].Wasserman N. F., Niendorf E., and Spilseth B., “Measurement of prostate volume with MRI (a guide for the perplexed): Biproximate method with analysis of precision and accuracy,” Sci. Rep., vol. 10, no. 1, Dec. 2020, Art. no. 575, doi: 10.1038/s41598-019-57046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]