Abstract
Viruses can be designed to be tools and carrier vehicles for intratumoural immunotherapy. Their nanometre-scale size and shape allow for functionalization with or encapsulation of medical cargoes and tissue-specific ligands. Importantly, immunotherapies may particularly benefit from the inherent immunomodulatory properties of viruses. For example, mammalian viruses have already been tested for oncolytic virotherapy, and bacteriophages and plant viruses can be engineered for immunotherapeutic treatment approaches. In this Review, we discuss how viruses — including oncolytic viruses, immunomodulatory plant viruses and bacteriophages — and virus-like particles can be designed for intratumoural immunotherapy to elicit anti-tumour immunity and induce systemic anti-tumour responses at distant non-injected sites. We further highlight the engineering of viruses and virus-like particles as drug-delivery systems, and outline key translational challenges and clinical opportunities.
Introduction
Immunotherapy is a new addition to long-established cancer treatment strategies, such as surgery, radiotherapy and chemotherapy1. Immunotherapy trains the patients’ immune system to recognize and eradicate cancer cells systemically2. Several immunotherapies have been approved by the FDA or are in clinical development, including immune checkpoint inhibitors (such as cytotoxic T-lymphocyte-associated antigen 4, CTLA-4 (ref. 3); programmed cell death 1 and its ligand, PD1–PDL1; and lymphocyte activation gene 3, Lag3), tumour vaccines (such as sipuleucel-T), cell-based immunotherapies (such as chimeric antigen receptor (CAR)-T-cell therapy4) and small-molecule-based immunotherapies (such as stimulator of interferon genes protein (STING) agonists)5. We note that the development of the FDA-approved drugs CTLA-4 and PD1–PDL1 was awarded the Nobel prize in 2018 (ref. 6). In addition, viruses are explored for immunotherapy owing to their intrinsic immunomodulatory features, because they can evade and/or generate immune responses in their hosts through structural recognition motifs and other molecular mechanisms7. Thereby, viruses can interface with and reprogram the immune system and thus be repurposed for immunotherapy8–10.
Our current understanding of the relation between the immune system and cancer is reflected by the cancer-immunity cycle11,12 (Fig. 1). Many factors contribute to tumour-mediated immune suppression and cancer progression and are targets for therapeutic intervention13. These factors include cancer-cell-surface receptors and secreted factors, matrix stiffness, interstitial pressure, hypoxia, low intratumoural pH, leaky vasculature, limited drainage to lymph nodes and prevention of infiltration by effector T cells and other immune cells into the tumour microenvironment (TME)13. Most tumours recruit and polarize immunosuppressive immune cells within the TME to generate local tumour-supporting immune suppression12. Activation of the cancer-immunity cycle, which is limited by this immunosuppressive environment, is the primary goal of immunotherapy. In particular, stimulation of the type I interferon (IFN) pathway is being investigated as a therapeutic strategy. Although type I IFN signalling can have anti-neoplastic effects, it is primarily (but not only) an antiviral response14,15. Upon pathogenic insult and recognition, local immune cells are alerted to secrete type I IFN16.
Fig. 1 |. Cancer-immunity cycle.
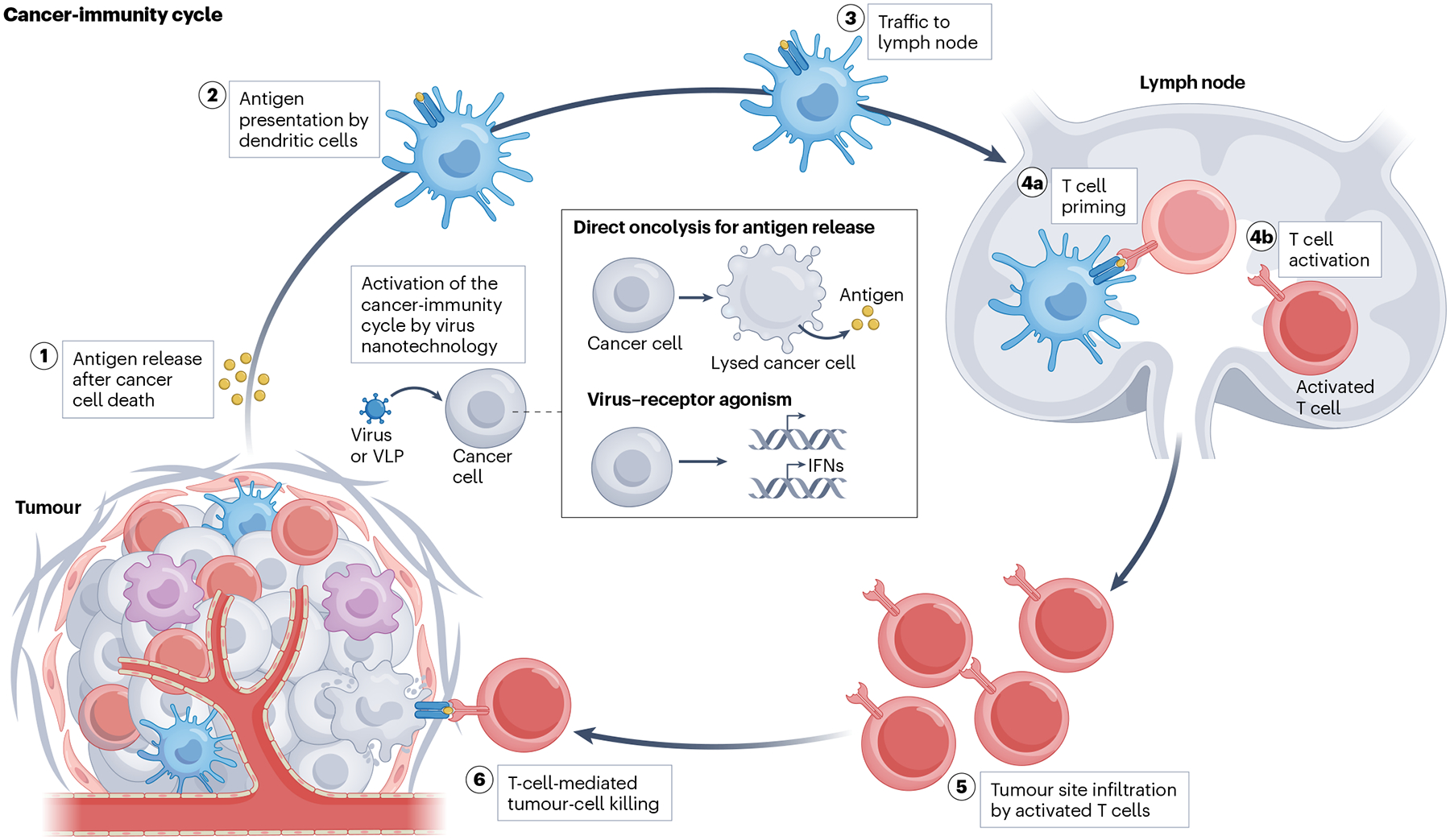
Antigens are released from dead or dying cancer cells. These antigens are then presented by dendritic cells and trafficked to lymph nodes, where T cells are primed and activated. Activated T cells infiltrate tumour sites to induce more cancer-cell death. The cancer-immunity cycle can be activated by oncolytic viruses that infect cancer cells to trigger their oncolysis and subsequent antigen release. Alternatively, viruses and virus-like particles (VLPs) can be designed to bind to specific receptors on cancer cells, promoting stimulation of the immune system by stimulating type I interferon (IFN) signalling.
Type I IFNs reprogram the TME through autocrine and paracrine circuits, which leads to the upregulation of IFN-stimulated genes and activation of the anti-pathogenic state of immune cells14. Specifically, type I IFN programs upregulate antigen presentation by dendritic cells, enhance the cytotoxicity of CD8+ T cells and natural killer cells, polarize macrophages toward inflammatory phenotypes and reduce the immunosuppressive state of regulatory T cells14,16. In addition, type I IFN promotes crosstalk that stimulates the adaptive immune system and establishes B- and T-cell-mediated antigen-specific memory16.
Stimulation of the type I IFN pathway also holds promise in cancer immunotherapy, and recombinant IFNα was one of the first approved cancer immunotherapy drugs17. However, recombinant IFNα has a short half-life in serum, and therefore treatment with recombinant IFNα requires high dosage through intravenous or subcutaneous injections of up to five times a week for extended periods18,19. Viruses can be repurposed as drugs to produce type I IFNs, enabling sustained IFNα levels in particular, because viruses and their nanoparticle formulations typically have good tissue residence and viral replication may further extend IFNα signalling. Thus, virus-based immunomodulation may allow the reduction of dosage, and hence the costs and infrastructural burden of IFNα immunotherapy. Several virus-based drug candidates that target the type I IFN signalling pathway are under development, including PVSRIPO (a modified poliovirus:rhinovirus chimera)20, vidutolimod (a bacteriophage virus-like particle (VLP) carrying TLR9 receptor agonists)21, and plant viruses, such as papaya mosaic virus22 and cowpea mosaic virus23.
In this Review, we discuss how virus nanotechnology can be designed to activate the cancer-immunity cycle. In particular, we examine the application of oncolytic viruses, which selectively replicate in and lyse tumour cells, non-infectious plant viruses, which can agonize the mammalian immune system, and virus-delivery systems, including plant virus- and bacteriophage-derived VLPs (which are not infectious to their hosts because they are devoid of genomic nucleic acid24), for intratumoural immunotherapy.
Intratumoural immunotherapy
The efficacy of intratumoural therapeutic delivery was first demonstrated by administering bacteria to tumours and surgical tumour sites, resulting in the local reduction in tumour growth in human patients25,26 (Fig. 2). Importantly, this therapeutic strategy can also reduce or eliminate distant untreated tumours. The idea that the immune system protects against cancer was proposed around 1909 (ref. 27), but the concept was only later developed in the late 1950s to early 1970s28,29 and is now known as cancer immunosurveillance. Various intratumoural immunotherapies have since been developed and approved, such as talimogene laherparepvec (TVEC)30. Intratumoural immunotherapy primarily acts on innate immune cells (such as dendritic cells, natural killer cells and macrophages) to rewire the TME and relieve local immunosuppression, which leads to crosstalk with adaptive immune cells (CD4+ and CD8+ T cells) to induce systemic immune-mediated tumour-cell death31. Compared to immunotherapy by systemic intravenous injections30, intratumoural immunotherapy achieves higher drug concentration at the tumour site, while considerably reducing systemic drug exposure, translating to increased safety and reduced costs32–34. Furthermore, host-immune responses can augment or compromise clinical drug efficacy after systemic administration30,35. For example, neutralizing anti-drug antibodies are a barrier for systemic delivery of oncolytic viriotherapies32, and non-neutralizing anti-drug antibodies can alter the biodistribution and pharmacokinetics of biotherapeutics36, which may be addressed by intratumoural immunotherapy.
Fig. 2 |. Milestones of virus nanotechnology and immunotherapy.
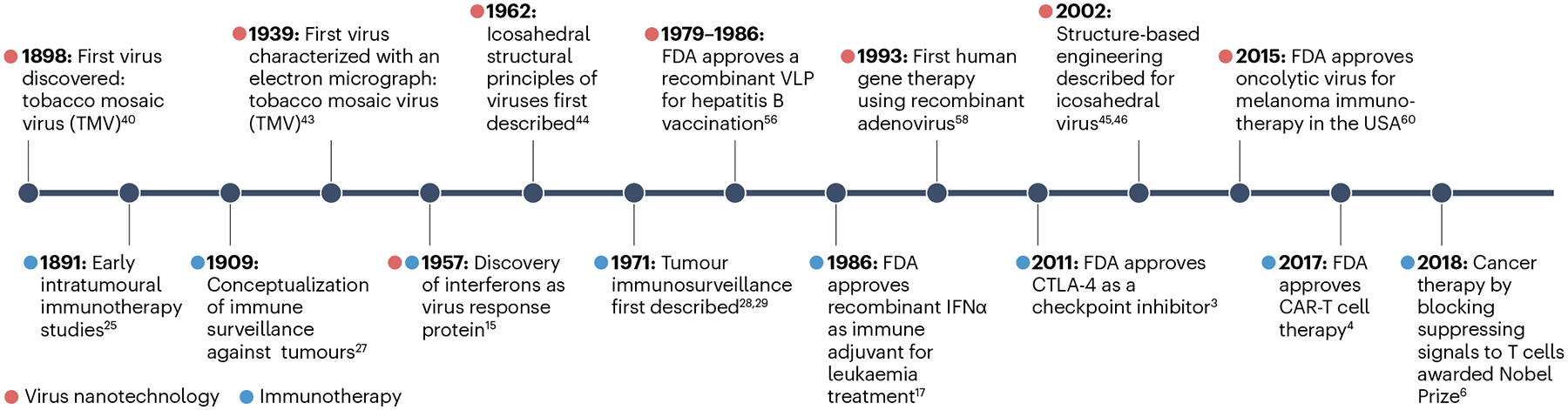
CAR-T cell, chimeric antigen receptor T cell; CTLA-4; cytotoxic T-lymphocyte-associated antigen 4. Data are taken from refs. 3,4,6,15,17,25,27–29,40,43–46,56,58,60.
However, intratumoural immunotherapy of large tumours requires multiple injections, leading to procedural complexities and efficacy variance. In addition, high intra-tumoural fluid pressure may prevent effective drug dispersion or cause the exit of drugs from the tumour37. Recurrent dosing may further affect patient compliance owing to discomfort38. Material and treatment designs, such as slow-release depots39, may be able to address these issues by stream-lining intratumoural administration, thereby alleviating the need for repeated treatment.
Virus nanotechnology
Virus nanotechnology refers to the repurposing of viruses and VLPs, that is, assembled virus particles without genomic content, for nanotechnology approaches. In particular, since its discovery in the 1890s as ‘contagium vivum fluidum’, the rod-shaped tobacco mosaic virus (TMV)40,41 has been used as a tool for virus nanotechnology42 (Fig. 2). In 1939, TMV was the first virus to be imaged using a electron microscope, greatly advancing the field of virology43. The structural principles and triangulation numbers of icosahedral-shaped viruses were described in the early 1960s44. In addition, structure-based engineering has been applied to create virus-based vaccines, and concepts such as genetic overcoat display (that is, display of proteins of interest on virus capsids through genetic engineering), encapsulation and bioconjugation have been developed for icosahedral-shaped viruses45,46. Viruses and VLPs are biological nanoscale materials that offer a design space for versatile applications, including drug and gene delivery47,48, light harvesting49,50, data and energy storage51,52 and nanobiocatalysis53,54. Viruses have also been engineered for medical applications, with the first DNA recombinant VLP vaccine approved by the FDA for hepatitis B in 1986 (refs. 55,56) (Fig. 2). The first human gene therapy using recombinant adenovirus was approved in 1993 (refs. 57,58), and replication-competent and -incompetent viruses are now being explored for cancer immunotherapy59,60.
Multiple pathways are involved in cancer progression61, and therefore, treatment approaches may benefit from viruses owing to their multi-mechanistic actions. In particular, the nanoscale size of viruses and VLPs enables tissue retention, delivery and protection of cargo, cell engagement and lymphatic drainage24,62. In addition, the highly ordered and repetitive arrangement of viral protein capsids serves as a pathogen-associated molecular pattern (PAMP) that can generate a response from immune cells63,64. Nucleoprotein assemblies may further contain multiple factors that activate the immune system; for example, nucleic acid sequences can target Toll-like receptors (TLRs), T helper (TH) cell epitopes or carbohydrates that stimulate T cells64. However, translating virus nanotechnology for cancer immunotherapy requires an understanding of how viral features, such as nucleic acids, capsids and ligand–receptor binding, can be intentionally harnessed and re-engineered to modulate the TME.
Oncolytic viruses
The increase in our knowledge of virus–host interactions and genetic engineering tools has enabled the development of oncolytic viruses as a cancer treatment modality65 (Fig. 3), with four therapies approved for intratumoural immunotherapy. These are ECHO-7 (echovirus, first approved in 2004 in Latvia, discontinued owing to lack of efficacy and manufacturing issues)66, H101 (adenovirus, approved in 2005 in China)67, TVEC (herpes simplex virus type 1 (HSV1), approved in 2015 in the USA)60, and Teserpaturev (HSV1, approved in 2021 in Japan)68. Oncolytic viruses are engineered to target, infect and lyse cancerous cells69, which causes the release of tumour-associated antigens and neoantigens (Fig. 4a). Viral replication and the expression of foreign, immunogenic viral proteins also lead to the release of proinflammatory cytokines and chemokines, which causes the recruitment and activation of innate and adaptive immune cells within the TME, resulting in antigen processing and presentation and thus systemic adaptive anti-tumour immunity70–73. To engineer an oncolytic-virus-based immunotherapy, four major parameters need to be considered: optimization of tropism for specific cancer cells; reduction of virulence toward healthy cells; improvement of the immune-stimulatory function of oncolytic viruses; and avoidance of drug neutralization by the host-immune response73,74.
Fig. 3 |. Research and investment in oncolytic viruses.
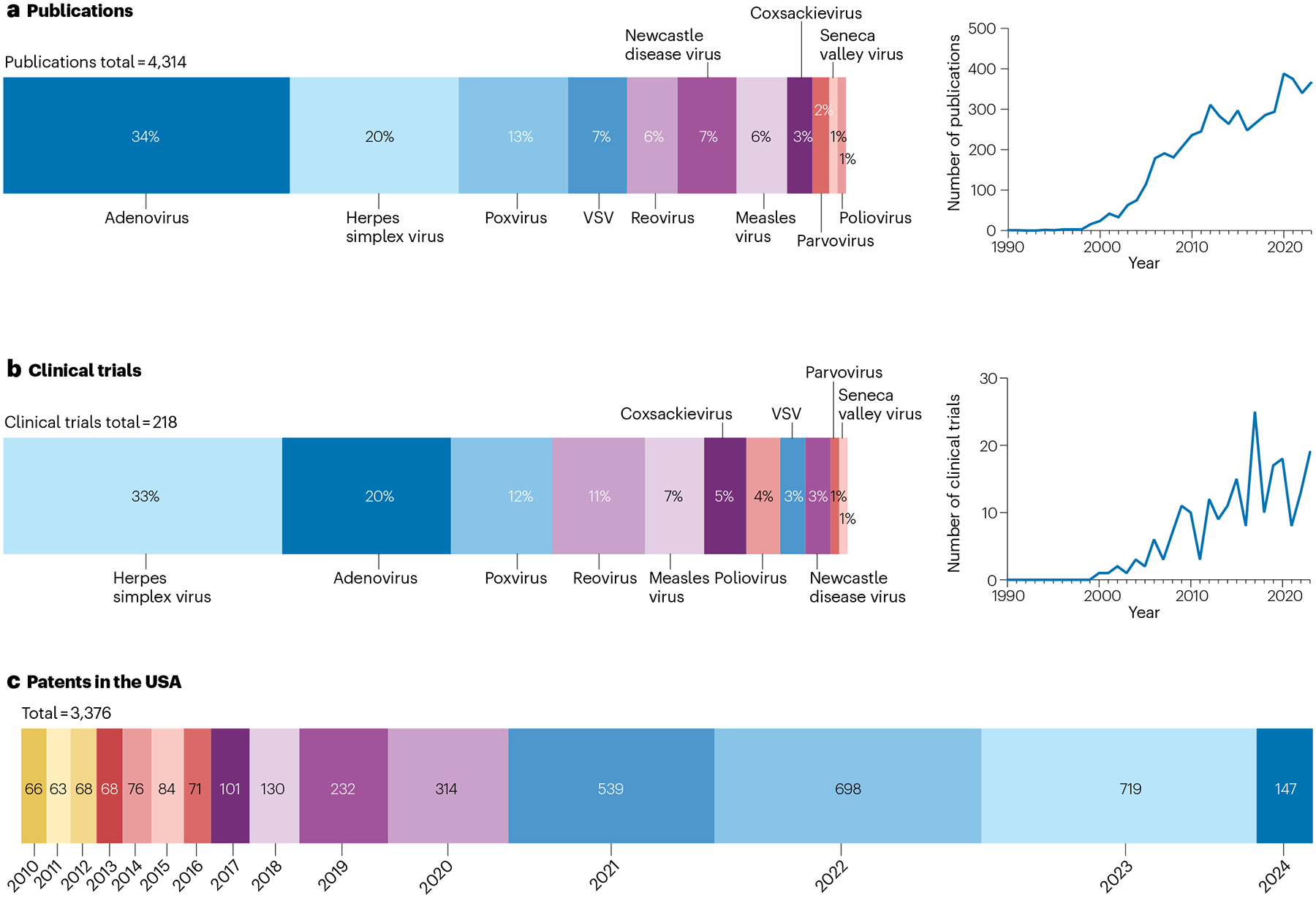
a, Peer-reviewed publications in 1990–2024 involving different types of oncolytic virus. Search terms in PubMed: [oncolytic virus] AND [cancer] AND [virus type name]. b, Clinical trials using oncolytic viruses in 1990–2024. Oncolytic viruses undergoing clinical trials were extracted from the Clinicaltrials.gov database using the following keywords in titles and abstracts: oncolytic virus, adenovirus, poxvirus, vaccinia, coxsackievirus, herpes simplex virus (HSV), measles virus, Newcastle disease virus, parvovirus, reovirus, Seneca Valley virus, vesicular stomatitis virus (VSV), poliovirus and cancer. c, Patents filed for oncolytic-virus platforms from 2010 to 2024 in the USA. Search terms in Google patents include [oncolytic virus] AND [cancer] + [country = USA].
Fig. 4 |. Mechanism of action of virus-based intratumoural immunotherapy.
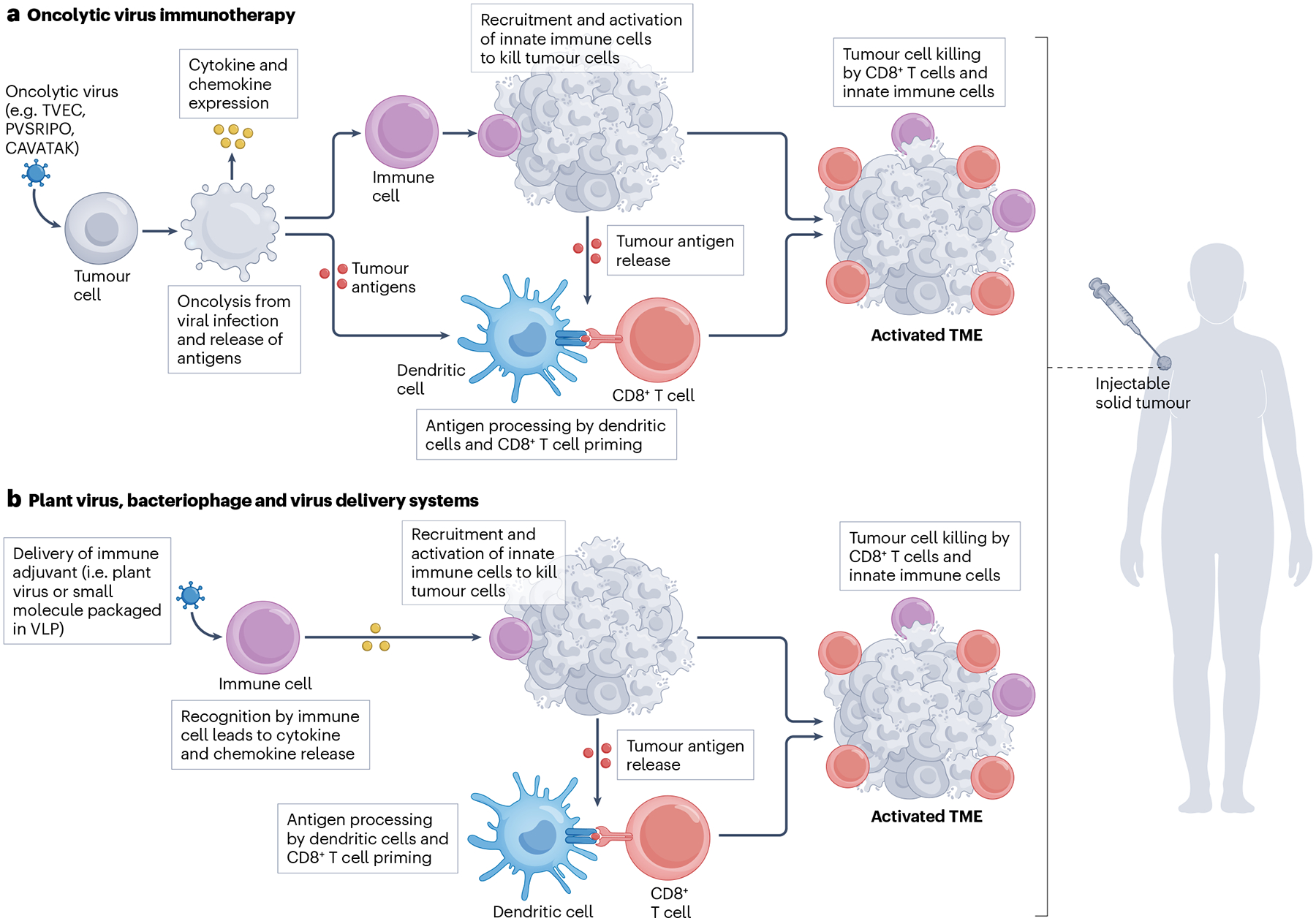
a, Oncolytic viruses selectively infect and lyse tumour cells to release antigens. Antigens are used by dendritic cells to prime CD8+ T cells, which induce tumour killing. b, Plant viruses and virus-like particles (VLPs) are recognized by the immune system, which, in response, launches an activation programme. Recognized antigens prime CD8+ T cells for tumour killing. TME, tumour microenvironment; TVEC, herpes simplex virus 1; PVSRIPO, poliovirus; CAVATAK, coxsackievirus.
Cancer-cell targeting
Cancer cells can be targeted by exploiting upregulated or aberrant expression of viral receptors, such as CD46 (a membrane cofactor protein)75, CD155 (a poliovirus receptor), herpes virus entry mediator (HVEM)76, nectin-1 or -2 (herpesvirus entry mediator C or B)77,78 and integrins (transmembrane receptors)79. Oncolytic viruses can then use these receptors for viral entry; for example, HSV1 enters host cells through interaction with HVEM or nectin-1/-2, a mechanism exploited in TVEC80; measles virus binds to CD46 for cell entry81; and poliovirus enters via CD155 (ref. 82). Moreover, viruses without natural tropism for cancer cells can be genetically engineered to acquire tumour-cell-targeting properties; for example, in the chimeric oncolytic adenovirus 5/3, the Ad5 fibre knob is replaced by the CD46-specific and desmoglein-2-specific Ad3 fibre knob83. In addition, an RGD peptide can be integrated in oncolytic viruses to allow binding to integrin receptors overexpressed on cancer cells, thereby increasing tumour-cell penetration and engagement84,85. An alternative targeting strategy can take advantage of proteases found within the TME, such as matrix metalloproteinase 9 (MMP9). For example, a tuneable adeno-associated virus (AAV) has been developed to be selectively activated only in the presence of MMP9 (ref. 86). These targeting strategies, which can be applied to viruses, biologics or synthetic nanoparticles, have been widely explored in nanomedicine, albeit with limited translational success87,88.
Cancer cells often possess irregular transcriptional and signalling pathways owing to mutations89. This not only contributes to their uncontrolled cell replication but may also lead to a compromised antiviral response, such as suppressed type I IFN responses and inhibited cell apoptosis for viral clearance90,91. Therefore, oncolytic viruses can be engineered to preferentially replicate in tumour cells rather than in healthy cells. For example, overexpression of anti-apoptotic B-cell leukaemia/lymphoma 2 (Bcl-2) family proteins can inhibit cancer-cell apoptosis92,93. This can be exploited by engineering a Newcastle disease virus-based oncolytic virus that can replicate in human B-cell lymphoma extra-large (Bcl-xL) over-expressing non-small-cell lung cancer cell line (A549), allowing the spread of infection and thus oncolytic effects94. Oncolytic viruses can also be designed to replicate under cancer-specific or tissue-specific control. For example, an oncolytic adenovirus can be engineered to express the adenoviral E1A protein (which enhances viral replication) under control of the prostate specific antigen (PSA) promoter, thereby achieving selective E1A expression in PSA-expressing human prostate cancer cells and its xenografts in mice95. Here, adenoviral E1A protein expression inhibits cell cycle arrest, enabling sufficient virus replication to achieve oncolysis96. Although these strategies can be applied to target tumour cells, such engineered oncolytic viruses may also infect healthy cells that express the same tissue-specific promoters. Therefore, tumour and healthy cells should be profiled by proteomics or gene sequencing to delineate signatures that can be specifically targeted.
Immunostimulation
As pathogens, oncolytic viruses are recognized by pathogen recognition receptors (PRRs), which, together with viral replication and protein expression, prime antiviral immune responses, causing the release of pro-inflammatory cytokines, such as type I IFNs. Nevertheless, tumours can have impaired antiviral functions, and thus, transgenes can be incorporated into viruses73 to augment this immune response and reprogramme the TME65,97. For example, genes can be implemented that encode immunostimulatory cytokines and chemokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), the interleukins IL-2, IL-12 and IL-15, and the CXC motif chemokine ligands CXCL9 and CXCL10 (ref. 73). Furthermore, preclinical and clinical studies have shown that treatment with oncolytic viruses can increase the expression of programmed cell death-ligand 1 (PD-L1) in cancer cells98. Accordingly, oncolytic viruses can be engineered to express anti-PD-L199,100. Other immune-activating ligands, such as cluster of differentiation 40 ligand (CD40L), OX40 ligand (OX40L) and the 4–1BB ligand (4–1BBL)101–103, as well as suicide genes (for example, thymidine kinase to activate the prodrug ganciclovir) can be introduced into oncolytic viruses, an approach that has been tested in preclinical and clinical studies104–106. Transgenes can also be introduced in non-oncolytic viruses, including in viral vectors and VLPs. For example, transgenes for human IFNα2b have been introduced in the FDA-approved therapy nadofaragene firadenovec, a non-replicating adenoviral vector encoding IFNα2b for bacillus Calmette–Guerin (BCG)-unresponsive, non-muscle-invasive bladder cancer (NMIBC) treatment36,107.
Safety
The clinical translation of oncolytic viruses remains limited by pathogenicity-related safety concerns36 as well as efficacy issues, partly owing to neutralization by pre-existing antiviral immunity (that is, anti-drug antibodies)108. However, the safety of oncolytic viruses can be improved by deleting or replacing virulence genes. For example, TVEC has a deletion of the γ34.5 gene encoding the neurovirulence factor ICP34.5 within HSV1109,110, which diminishes its pathogenicity; in the poliovirus-based oncolytic virus PVSRIPO, the original viral internal ribosome entry site sequence is replaced with a sequence from human rhinovirus type 2 to avoid neuron infection111.
Anti-drug antibodies
Oncolytic viruses are typically administered through intratumoural injection112 to enable the treatment of recognized tumours and to limit systemic virus exposure and organ damage113. However, both intravenous and intratumoural virus administration are challenged by pre-existing or newly established anti-drug antibodies, that is, antibodies developed owing to prior exposure to the virus or in response to repetitive intravenous administration, respectively. This is particularly problematic for Ad5 and HSV1, to which many people have previously been exposed114,115. The presence of anti-drug antibodies leads to clearance of intravenously injected oncolytic viruses, thus limiting their accumulation in tumour sites. Importantly, anti-drug antibodies can also prevent oncolytic viruses from infecting tumour cells following intratumoural injection. To escape from anti-drug antibody-mediated clearance, oncolytic viruses can be coated with polymers116, liposomes117 or graphene sheets118. Alternatively, albumin-binding protein can be genetically inserted on the drug surface, leading to the binding of albumin to viruses instead of antiviral antibodies to prolong circulation and proliferation119. In addition, patient-derived mesenchymal stem cells, neural stem cells or other immune cells can be used as carriers for oncolytic-virus delivery, because viruses loaded in such immune cells can be protected from antiviral antibody recognition and clearance, thereby achieving longer circulation and delivery to tumour sites120–122.
Abscopal effect
The clinical impact of intratumoural immunotherapy depends on its systemic efficacy, that is, the elimination or inhibition of distant, non-injected tumours. This effect, which is termed the ‘abscopal effect’, results from the activation of systemic anti-tumour immunity. The abscopal effect, or a reduction in tumour volume at non-injected distant metastases, has been clinically observed for TVEC10, V937 (oncolytic coxsackievirus A21)123 and Pexa-Vec (JX-594, oncolytic vaccinia virus)124. However, the abscopal effect remains a rare and unpredictable phenomenon, and the underlying mechanisms and how these can be harnessed for cancer treatment remain to be investigated.
Translational challenges
Despite preclinical and clinical efforts36,112,125 (Fig. 3; Table 1), challenges remain to be overcome for the wider clinical translation of oncolytic-virus-based intratumoural immunotherapy. In particular, tumour heterogeneity may impede oncolytic virus infection; the presence of anti-drug antibodies may cause virus clearance; and solo treatment with an oncolytic virus may be insufficient to launch an anti-tumoural immune response. Therefore, most clinical trials on oncolytic-virus-based treatment are investigating combination approaches with chemotherapy, radiotherapy, CAR-T-cell and immune checkpoint blockade therapy to identify combination therapies that improve treatment outcomes.
Table 1 |.
Selected completed clinical trials of oncolytic viruses
| Virus type | Drug | Cancer type | Clinical trial number | Phase | Refs. |
|---|---|---|---|---|---|
| Adenovirus | CG0070 | Bladder cancer | NCT02365818 | Phase 2, single group, open label | 170 |
| DNX-2401 | Glioblastoma, gliosarcoma | NCT02798406 | Phase 2, single group, open label | 171 | |
| Brainstem glioma | NCT03178032 | Phase 1, single group, open label | 172 | ||
| NSC-CRAd-S-p7 | Glioma | NCT03072134 | Phase 1, open label | 173 | |
| Picornavirus | PVSRIPO | Melanoma | NCT03712358 | Phase 1, non-randomized, open label | 174 |
| SVV-001 | Solid tumours with neuroendocrine features | NCT00314925 | Phase 1, non-randomized, open label | 175 | |
| CVA21 (CAVATAK) | Malignant melanoma | NCT01227551 | Phase 2, single group, open label | 123 | |
| Uveal melanoma | NCT03408587 | Phase 1b, randomized, open label | 176 | ||
| Herpes simplex virus | TVEC | Melanoma | NCT00289016 | Phase 2, single group, open label | 177 |
| Melanoma | NCT00769704 | Phase 3, randomized, open label | 10,178 | ||
| Melanoma | NCT01740297 | Phase 1/2, randomized, open label | 179 | ||
| HSV1716 | Non-central nervous system solid tumours | NCT00931931 | Phase 1, single group, open label | 180,181 | |
| Parvovirus | H-1PV | Glioblastoma | NCT01301430 | Phase 1/2a, single group, open label | 182 |
| Poxvirus | JX-594 | Melanoma | NCT00429312 | Phase 1/2, single group, open label | 183 |
| Hepatocellular carcinoma | NCT00554372 | Phase 2a, randomized, open label | 184 | ||
| GL-ONC1 (Olvi-Vec) | Ovarian cancer, peritoneal carcinomatosis | NCT02759588 | Phase 1b/2, single group, open label | 185 |
Data extracted from the Clinicaltrials.gov database using the following keywords: oncolytic virus, adenovirus, poxvirus, coxsackievirus, herpes simplex virus, parvovirus, reovirus, Seneca Valley virus, vesicular stomatitis virus, poliovirus and cancer.
Plant viruses and bacteriophages
Cowpea mosaic virus
In contrast to oncolytic viruses, non-mammalian viruses, such as plant viruses, do not infect mammalian cells; however, they can also be designed for intratumoural immunotherapy (Fig. 4b). In particular, non-cytolytic plant viruses, such as the cowpea mosaic virus (CPMV), can be repurposed for intratumoural immunotherapy. Plant viruses contain PAMPs that are recognized by PRRs and stimulate innate immune cells, thereby reprogramming the TME to launch systemic and durable anti-tumour immunity upon intratumoural administration9. For example, in tumour mouse models and canine cancer patients126, systemic efficacy (the abscopal effect) can be achieved by intratumourally administered CPMV (or VLPs thereof); here, both CPMV-injected and distant non-injected tumours shrink upon treatment with CPMV126,127 owing to durable CD8+ T-cell-mediated systemic anti-tumour responses that also prevent recurrence after re-challenge in mice9. Long-lasting protection has also been achieved in canine cancer patients (pets) with advanced mammary cancer, who received CPMV VLP intratumoural immunotherapy as neoadjuvant therapy prior to surgery126.
CPMV interacts with the immune system in a multivalent manner, resulting in a cascade of events. Although CPMV is a plant virus, it resembles animal picornaviruses (Box 1) and is recognized by PRRs. Upon intratumoural delivery, the capsid proteins of CPMV interact with and stimulate TLR2 and TLR4; its positive strand single-stranded RNA (ssRNA) agonizes TLR7 and activates antiviral IFN signalling through the MyD88 pathway23. Thus, intratumourally delivered CPMV polarizes the TME to an immune-activated phenotype, thereby transforming ‘cold’ (immune-suppressed) tumours with poor prognosis into ‘hot’ (immune-activated) tumours. Accordingly, CPMV treatment results in the infiltration and activation of innate immune cells, such as natural killer cells, anti-tumour neutrophils (N1), macrophages (which switch from an immune suppressive to a proinflammatory phenotype) and dendritic cells9. This immune reprogramming is generated in response to a suspected viral threat. The mammalian immune system does not discriminate between viruses from different kingdoms and reacts with antiviral responses if pathogen-recognizing receptors, such as TLRs, are activated, no matter the type of virus. Importantly, the mechanism of action of CPMV and other plant viruses is distinct from that of oncolytic viruses. Plant viruses are not pathogenic and do not lyse cancer cells directly, and so immune stimulation does not stem from viral replication or foreign protein synthesis. Although plant viruses, such as CPMV, act on innate immune cells, they also trigger adaptive and durable immunity (that is, activation of CD4+ and CD8+ effector and memory T cells). Therefore, plant virus immunotherapy interfaces with the immune system and restores normal function (that is, immunosurveillance), which kickstarts the cancer-immunity cycle. Moreover, anti-CPMV antibodies cannot neutralize the anti-tumour efficacy of CPMV (shown in an ovarian tumour model as well as canine cancer patients)128,129. By contrast, anti-CPMV antibodies increase opsonization of CPMV and uptake by antigen-presenting cells, which increases, rather than reduces, efficacy, and is likely to be responsible for building the T-cell memory compartment128.
Box 1 |. Virus taxonomy informing nanomedicine design: a case for Picornavirales.
Several Picornavirales-based intratumoural immunotherapy strategies have been developed, including PVSRIPO, CAVATAK and GD7-KS1. These viruses contain pico-RNA (a small RNA genome) and are positive-sense RNA viruses with an icosahedral morphology186, with a diverse range of hosts, including insects, vertebrates and plants. However, their structural morphology and genetic arrangement remain homologous across species187 (see Box 1 figure panels a and b). The structural recognition of virus motifs by the host-immune system contributes to their potent anti-tumour immunity. For example, in addition to the oncolytic activity of PVSRIPO, its RNAs are recognized by the cytoplasmic pattern recognition receptors (PRRs) mitochondrial antiviral signalling protein (MAVS), melanoma differentiation associated gene 5 (MDA5) and retinoic acid-inducible gene I (RIG-I), resulting in the generation of type I interferon (IFN)188,189. CPMV RNAs are recognized by Toll-like receptor (TLR)-7, which activates myeloid differentiation primary response 88 (MyD88) signalling to generate type I IFN23. Different types of PRR recognize the different viruses, probably owing to differences in intracellular processing; upon cell entry, PVSRIPO uncoats and introduces its RNA into the cytoplasm for translation188, whereas CPMV localizes in the endolysosome for an extended period, where its RNAs agonize TLR7 (ref. 23).
Cowpea mosaic virus (CPMV) is from the Secoviridae family (within the Picornavirales order) that naturally infects beans and legumes190. Other plant viruses that are not in the picorna family, such as cowpea chlorotic mottle virus (CCMV), sesbania mosaic virus (SeMV) and physalis mottle virus (PhMV), show no efficacy as intratumoural immunotherapy agents191. However, cowpea severe mosaic virus (CPSMV) and tobacco ringspot virus (TRSV) — also members of the plant picornaviruses — show potency against tumours in mouse models, albeit with reduced efficacy, reflected in reduced type I IFNs and TLR7 stimulation, compared to CPMV192. The potency of CPMV may be related to the conserved structure and genetic organization between plant and animal picornaviruses, such as the polio virus (see Box 1 figure panels a and b)193,194. In addition, antigens are shared between plant and mammalian picornaviruses195, which may suggest a common ancestor.
Box Fig. 1 |.
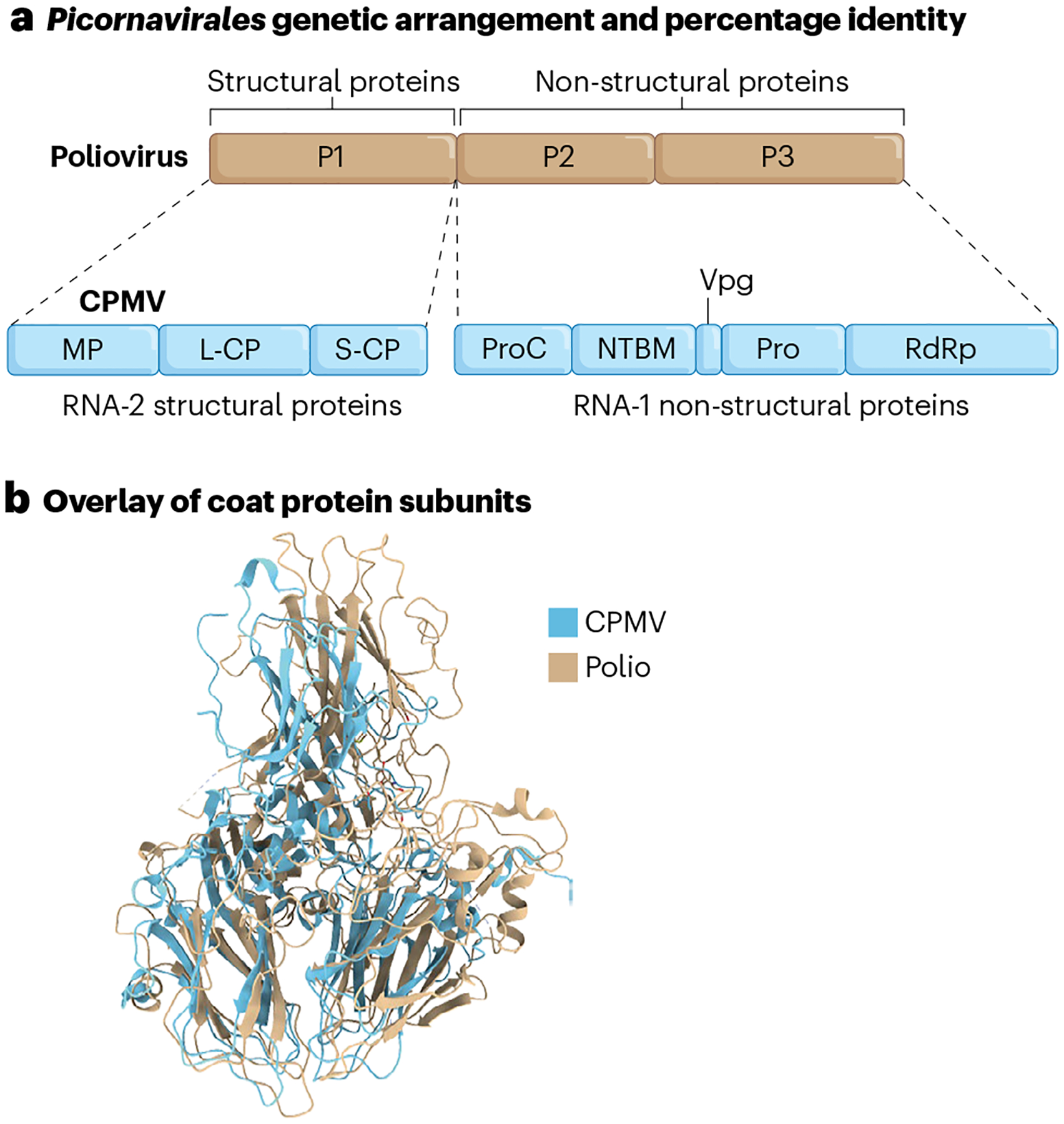
a, Genetic arrangement and homology between CPMV and poliovirus. P1 is the region encoding poliovirus structural proteins (the capsid). P2 and P3 are the regions encoding poliovirus nonstructural proteins (viral genome protein, protease and RNA-dependent RNA polymerase). L-CP, S-CP, large- and small-coat proteins, respectively; MP, movement protein; NTBM, NTP binding motif; Pro, protease; ProC, protease cofactor; RdRp, RNA-dependent RNA polymerase; Vpg, viral genome protein. b, The Protein Data Bank ID (PDB) entries for coat proteins are 1NY7 (CPMV) and 1POV (polio).
Filamentous plant viruses and bacteriophages
Filamentous plant viruses are also being investigated for intratumoural immunotherapy. For example, the papaya mosaic virus (PapMV) of the Alphaflexiridae family is a 530-nm flexuous virus that naturally infects papaya plants130,131. PapMV VLPs were first developed as a vaccine platform against bacterial and viral infections132,133, and are now also being explored for intratumoural immunotherapy. In PapMV VLPs, a packaged non-coding ssRNA functions as a TLR7 agonist22,134, leading to the induction of type I IFN133. In B16-OVA melanoma tumour mouse models, PapMV intratumoural immunotherapy substantially reduced tumour burden, decreasing tumour proliferation markers and increasing major histocompatibility complex MHC-I surface expression on B16-OVA tumour cells22. In addition, the treatment led to an increase in chemokines (such as interferon-γ-induced protein 10, IP-10 and monocyte chemoattractant protein 1, MCP-1) and pro-inflammatory cytokine (such as IL-1α and IL-5) concentrations in the TME, which can convert the TME immunotype from suppressed to activated22. The filamentous plant virus potato virus X (PVX) also shows anti-tumour efficacy in mouse models135, triggering the upregulation of proinflammatory cytokines and chemokines, such as IL-1α, IL1β, IP-10 and MCP-1, thereby delaying tumour progression135.
In addition to plant viruses, M13 bacteriophage (Fig. 5), a filamentous positive-sense ssDNA bacteria-infecting virus of the Inoviridae family, showed anti-tumour efficacy in mouse models8,136. Within the TME, M13 stimulates a MyD88-dependent anti-tumour pathway136, thereby promoting macrophage and neutrophil infiltration as well as the upregulation of antigen presentation and co-stimulatory receptors8. M13 is endocytosed and localizes to the endolysosome137, where it functions as a TLR9 agonist based on its ssDNA cargo, highlighting the role of virus nucleic acid recognition in inducing tumour immunity136.
Fig. 5 |. Structure and scale of viruses and virus-like particles used for intratumoural immunotherapy.
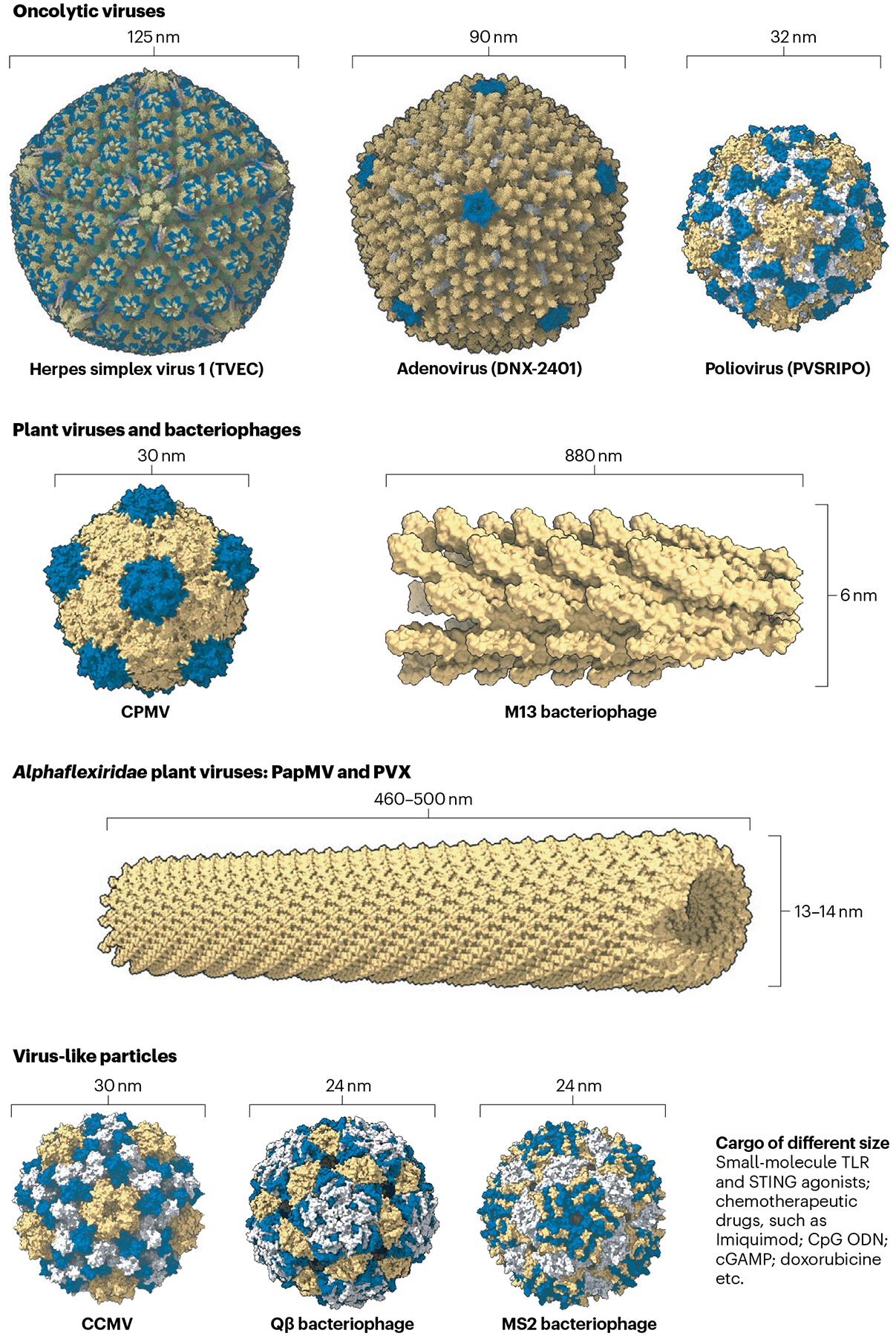
Virus and virus-like particle (VLP) structures are diverse. This allows them to be engineered for intratumoural immunotherapy. Mammalian viruses include the herpes simplex virus 1 (TVEC; Protein Data Bank ID (PDB): 6CGR), adenovirus (DNX-2401; PDB: 6CGV) and poliovirus (PVSRIPO; PDB: 1POV). TVEC is currently approved for melanoma, and DNX-2401 and PVSRIPO are currently being tested in clinical trials. Cowpea mosaic virus (CPMV; PDB: 1NY7), M13 bacteriophage (PDB: 2MJZ), the Alphaflexiridae plant viruses papaya mosaic virus (PapMV) and potato virus X (PVX; PDB: 4DOX), cowpea chlorotic mottle virus (CCMV; PDB: 1ZA7), Qβ bacteriophage (PDB: 1QBE) and MS2 bacteriophage (PDB: 2MS2) are currently in the preclinical development pipeline. Viruses and VLPs can deliver Toll-like receptors (TLRs) and stimulator of interferon gene (STING) agonists.
Viruses and VLPs as delivery systems
The properties of both mammalian and plant viruses can be harnessed and engineered to achieve new functionality. However, in contrast to mammalian viruses that require controlled environmental conditions to function (that is, physiological pH and temperature), plant viruses and bacteriophages (and their derived VLPs) are more robust and can withstand a range of environmental conditions throughout their life cycle138,139. Importantly, viruses can inherently serve as delivery vehicles, because they can encapsulate, protect and deliver nucleic acid cargo into their host cells for propagation. Therefore, viruses and VLPs can be repurposed as drug and gene carriers through genetic programming, bioconjugation or encapsulation140, for example, for the delivery of genes encoding cytokines (for example, TVEC’s genome encodes GM-CSF) and small-molecule drugs, such as STING and TLR agonists30,141–145. Packaging of small-molecule agents, such as TLR agonists, into nanoparticles or VLPs overcomes their rapid leaching from tumours, protects them from enzymatic degradation and improves immune cell uptake, thereby boosting efficacy.
Vidutolimod as a Qβ bacteriophage drug carrier
Also known as CMP-001, vidutolimod is a VLP derived from the Qβ bacteriophage146 of the Leviviridae family (Fig. 5). This bacteriophage has been engineered to carry unmethylated CpG-dense DNA (a TLR9 agonist). CpG molecules can activate immune cells and generate type I IFNs147, but are limited by low tissue retention, rapid clearance and degradation by nucleases upon administration148. In vidutolimod formulation, CpG is encapsulated in a Qβ bacteriophage nanoparticle to circumvent these problems and enhance its multi-mechanistic action143, which has been tested in several clinical trials (NCT04698187, NCT05445609 and NCT04633278). Upon intratumoural administration, vidutolimod remodels the TME by activating plasmacytoid dendritic cells to generate type I IFNs and other innate immune cells (such as natural killer cells143), causing downstream cross-talk with the adaptive immune system and priming of CD8+ T cells for anti-tumour activity149.
Interestingly, in mouse models, the anti-tumour efficacy of vidutolimod depends on antibody-mediated immune-cell targeting to plasmacytoid dendritic cells and monocytes. Furthermore, the presence of antibodies in vitro increases immune-cell uptake of vidutolimod, and pre-immunization of mice before treatment also enhances its efficacy150, because antibody opsonization of vidutolimod promotes immune-cell uptake through Fc-receptor engagement150. Therefore, the clinical protocol requires induction of anti-drug antibodies through immunization against the Qβ carrier VLP prior to treatment143,149,150.
Other virus and VLP drug carriers
VLPs from CCMV can also be engineered to encapsulate or covalently display small-molecule agonists to target TLR7 (ref. 145). Agonists that target the STING pathway have been delivered by VLPs made of HIV-1 structural proteins combined with the envelope glycoprotein from vesicular stomatitis virus (VSV)151. Another VLP drug carrier example is Ad5D24–CpG, which is an oncolytic adenovirus with unmethylated CpG DNA synthetically engineered into its genome to enable delivery and targeting of TLR9 (ref. 152).
Outlook
Various virus nanotechnologies have been tested for intratumoural immunotherapy, but only TVEC has been approved for clinical use by the FDA thus far. The efficacy of virus-nanotechnology-based intratumoural immunotherapy might be limited by the presence of pre-existing neutralizing antibodies. In addition, achieving the translation of local to systemic efficacy, that is, the abscopal effect, remains challenging. Intratumoural immunotherapy can reverse immunosuppression within the injected tumour; however, immune-cell recruitment to distant non-injected tumours remains difficult to achieve, thereby limiting treatment success. Although the abscopal effect has been reported in patients10,123,124, it is considered a rare and unpredictable event.
In addition, although targeting nucleic-acid-recognition receptors (such as TLRs and the STING pathway) can promote the anti-tumour immunity of virus-based therapies by launching antineoplastic type I IFN responses, it also triggers antiviral programs that may reduce the ability of oncolytic viruses to replicate and lyse tumour cells. Indeed, retinoic-acid-inducible gene I (RIG-I) detection of viral RNA can negatively regulate oncolytic efficacy, and STING signalling activated through viral double-stranded DNA recognition can interfere with the efficacy of oncolytic viruses153,154. Therefore, the right balance between an anti-tumour and an antiviral response must be considered when developing virus-based therapies — a balance that is an inherent characteristic of the immune system and the cancer-immunity cycle.
Furthermore, intratumour mutational heterogeneity and related T-cell priming in mismatch-repair-related tumour models may be a limiting or promoting factor in immunotherapy155,156. That is, levels of mutational burden in tumours as well as the diversity of mutations can have a role in the immunotherapy response, and may have to be considered in the design of virus-based immunotherapy. However, the mechanism of virus nanotechnology is considered to be tumour-agnostic: the intention is to overcome these limitations by releasing tumour antigens into the TME through oncolysis (either promoted directly by an oncolytic virus or indirectly through recruitment of natural killer cells or neutrophils by a VLP or virus) and by serving as an adjuvant for the immunological processing of the released antigens. Therefore, a particular mutation of an antigen may not necessarily interfere with the mechanisms of virus platforms. Mutations may even create a favourable environment for oncolytic viral activity89. However, specialized infrastructure and training may be required to produce replication-competent oncolytic viruses157. Similarly, although they are safe, the manufacture of plant viruses and VLPs may involve specialized plant molecular farming platforms or multi-step assembly approaches for packaging therapeutic cargo (Box 2).
Box 2 |. Translational considerations for plant viruses and virus-like particles.
The clinical translation of plant viruses and plant virus-like particles (VLPs) faces several challenges. In particular, plants are typically not used for biomanufacturing196, knowledge about manufacturing of biologics in plants is limited (for example, compared to Chinese hamster ovary cells, which are often used for biomanufacturing), and only a few contract development and manufacturing organizations have been established to facilitate process development and current good manufacturing practice (cGMP) manufacturing. In addition, many reagents and assays for the production and quality control of plant-virus-based products differ from those used for mammalian systems and are thus not readily commercially available.
Plant-based biomanufacturing also requires custom-designed and contained growth facilities, whereas upstream production equipment, such as bioreactors, is available off the shelf. Typically, host plants are manually infected with viral stocks or transfected by recombinant transfer DNAs, delivered by Agrobacterium tumefaciens, which can be difficult to scale up. To obtain the clarified extract, each combination of plant host and virus requires process optimization197, and laboratory processes need to be adapted to robust, high-yield and scalable industrial unit operations. In addition, although the large size difference between plant viruses and plant host proteins (for example, plant viruses typically have a size of 3,000 kDa, which is ten times bigger than the plant host cell protein RuBisCo, of ~500 kDa) is an advantage for ultrafiltration, the high mass transport and size exclusion are disadvantages for column chromatography. Importantly, scalable systems are being developed for plant-virus-based vaccine production; for example, the Coalition for Epidemic Preparedness Innovations (CEPI) has funded LenioBio’s plant-cell-lysate-based technology for vaccine production198.
For the scale-up translation of plant viruses and VLPs, regulatory guidelines for cGMP-compliant biomanufacturing can be adapted from existing plant-based biologics199. In addition, turnkey vertical farming solutions with low footprint, high yield, automation and energy efficiency are being marketed. Such scalable systems cover all scale requirements, from initial clinical development to marketing. Automation of the manual infection process can be achieved with the aid of robotics, camera systems and artificial intelligence. Large-scale extraction and clarification are routinely done in the food industry; however, developing suitable down-scale models remains difficult. Filtration technology has high scalability and is available off the shelf, and further downstream processing could be designed as in approaches used for non-enveloped oncolytic viruses. Cost models for large-scale plant-based cGMP manufacturing facilities have demonstrated economic viability for several products that have higher dosage than those required for viral nanotechnology for intratumoural immunotherapy200–202.
Various combination strategies are currently tested in clinical trials to improve immunotherapy outcomes. In particular, immune checkpoint therapy has shown clinical responses158, thereby driving its integration into first- and second-line therapies. However, only a minority of patients respond to immune checkpoint therapy, largely because the immunosuppressive TME contains physical and/or chemical barriers to effective T-cell anti-tumour immunity158. Preclinical studies have shown that intratumoural immunotherapy with VLPs encapsulating small-molecule agonists, plant viruses or oncolytic viruses synergize with immune checkpoint therapy (for example, treatment with anti-PD-1 antibodies) by increasing the expression of checkpoint markers within the TME and by expanding the pool of tumour-specific CD8+ effector T cells159–162. Of note, vidutolimod is undergoing clinical testing as both a solo therapy and with an immune checkpoint therapy combination arm163–165. Moreover, for virus and VLP drug carriers that are not directly cytotoxic, combination with treatment regimens that lyse tumours and release tumour antigens (chemotherapy, cryoablation, photothermal therapy and radiation) hold promise for holistic immunotherapy. Combination approaches could be implemented with virus-based intratumoural immunotherapy as an adjuvant or neoadjuvant therapy. For example, neoadjuvant treatment with TVEC prior to surgery improves recurrence-free survival in human patients166.
Accessibility is a key requirement for intratumoural therapy, which may be challenging to achieve for disseminated peritoneal cancers (ovarian and colon cancers), metastatic disease or haematological cancers30. However, interventional radiology and image-guided procedures may improve accessibility in such cancers. In addition, delivery techniques (for example, multiside hole needles) and slow-release devices167,168 that can overcome barriers, such as high interstitial fluid pressure in tumours limiting drug penetration37, may be applied. Delivery devices and slow-release depots may also be designed to avoid repeated intratumoural dosing, which adds to treatment costs and may cause discomfort, thereby affecting patient compliance38,169. Given their robust nature, plant viruses and bacteriophages are particularly well suited to be integrated into medical devices39.
A better understanding of the underlying mechanisms of virus–host cell interactions, the clinical application of cancer immunotherapy and the safety and affordability of intratumoural immunotherapy as well as its rapid systemic anti-tumour response (weeks between diagnosis and surgery) suggest that virus-nanotechnologybased intratumoural immunotherapy may well become integrated into standard-of-care cancer treatments. However, how best to generate an abscopal effect and how to technically combine various immunotherapies remains to be identified.
Key points.
Viruses are immunomodulatory biologics that can be repurposed for intratumoural immunotherapy to kickstart the cancer immunity cycle.
Mammalian viruses, non-mammalian viruses and virus-like particles can be engineered to trigger immune responses or deliver therapeutic cargo for immunotherapy.
Virus-associated pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) recognition (through replication, nucleic acid payload, protein expression or structure) can induce type I interferon (IFN) responses and promote anti-tumour immunity.
Intratumoural immunotherapy using virus-based nanomaterials and gene-delivery vectors benefit from low costs and dose requirements as well as minimal side-effects and systemic toxicity.
Acknowledgements
This work was supported by the NIH (R01-CA224605, R01-CA274640, R01-CA253615), the American Cancer Society and F. M. Kirby Foundation Inc. Mission Boost Grant (MBGI-23-1030244-01-MBG) and the Shaughnessy Family Fund for Nano-ImmunoEngineering at the University of California, San Diego. A.O.O. acknowledges support from San Diego Fellowship and the Alfred P. Sloan Foundation’s Minority PhD Program (G-2020-14067).
Footnotes
Competing interests
The authors declare the following competing financial interest(s): N.F.S. and S.N.F. are co-founders of, have equity in, and have a financial interest with Mosaic ImmunoEngineering Inc. N.F.S. is a co-founder of, and serves as manager of, Pokometz Scientific LLC, under which she is a paid consultant to Flagship Labs 95 Inc. and Arana Biosciences Inc. M.S. is a consultant of and has equity in Mosaic ImmunoEngineering Inc. The other authors declare no potential conflicts of interest.
References
- 1.Murciano-Goroff YR, Warner AB & Wolchok JD The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 30, 507–519 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mellman I, Coukos G & Dranoff G Cancer immunotherapy comes of age. Nature 480, 480–489 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron F, Whiteside G & Perry C Ipilimumab: first global approval. Drugs 71, 1093–1104 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Mullard A FDA approves first CAR T therapy. Nat. Rev. Drug. Discov 16, 669–669 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Riley RS, June CH, Langer R & Mitchell MJ Delivery technologies for cancer immunotherapy. Nat. Rev. Drug. Discov 18, 175–196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledford H, Else H & Warren M Cancer immunologists scoop medicine Nobel prize. Nature 562, 20–21 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Katze MG, He Y & Gale M Jr. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol 2, 675–687 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Murgas P et al. A filamentous bacteriophage targeted to carcinoembryonic antigen induces tumor regression in mouse models of colorectal cancer. Cancer Immunol. Immunother 67, 183–193 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lizotte PH et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol 11, 295–303 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andtbacka RH et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol 33, 2780–2788 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Chen DS & Mellman I Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Mellman I, Chen DS, Powles T & Turley SJ The cancer-immunity cycle: indication, genotype, and immunotype. Immunity 56, 2188–2205 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Chao Y & Liu Z Biomaterials tools to modulate the tumour microenvironment in immunotherapy. Nat. Rev. Bioeng 1, 125–138 (2023). [Google Scholar]
- 14.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ & Kroemer G Type I interferons in anticancer immunity. Nat. Rev. Immunol 15, 405–414 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Isaacs A & Lindenmann J Virus interference. I. The interferon. Proc. R. Soc. Lond. B 147, 258–267 (1957). [PubMed] [Google Scholar]
- 16.Ivashkiv LB & Donlin LT Regulation of type I interferon responses. Nat. Rev. Immunol 14, 36–49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quesada JR et al. Treatment of hairy cell leukemia with recombinant alpha-interferon. Blood 68, 493–497 (1986). [PubMed] [Google Scholar]
- 18.Hauschild A et al. Practical guidelines for the management of interferon-α-2b side effects in patients receiving adjuvant treatment for melanoma: expert opinion. Cancer 112, 982–994 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Wills RJ Clinical pharmacokinetics of interferons. Clin. Pharmacokinet 19, 390–399 (1990). [DOI] [PubMed] [Google Scholar]
- 20.Brown MC et al. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci. Transl. Med 9, eaan4220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabree SA et al. Monocytes exposed to immune complexes reduce pDC type 1 interferon response to vidutolimod. Vaccines 9, 982 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebel M-È et al. Potentiating cancer immunotherapy using papaya mosaic virus-derived nanoparticles. Nano Lett. 16, 1826–1832 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Mao C, Beiss V, Fields J, Steinmetz NF & Fiering S Cowpea mosaic virus stimulates antitumor immunity through recognition by multiple MYD88-dependent Toll-like receptors. Biomaterials 275, 120914 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeltins A Construction and characterization of virus-like particles: a review. Mol. Biotechnol 53, 92–107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coley WB II. Contribution to the knowledge of sarcoma. Ann. Surg 14, 199–220 (1891). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starnes CO Coley’s toxins. Nature 360, 23 (1992). [DOI] [PubMed] [Google Scholar]
- 27.Ehrlich P Ueber den jetzigen Stand Der Karzinomforchung. Nederl. Tijdschr. Geneeskd 53, 273–290 (1909). [Google Scholar]
- 28.Burnet FM Immunological surveillance in neoplasia. Transpl. Rev 7, 3–25 (1971). [DOI] [PubMed] [Google Scholar]
- 29.Burnet M Cancer — a biological approach: III. Viruses associated with neoplastic conditions. IV. Practical applications. Br. Med. J 1, 841 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melero I, Castanon E, Alvarez M, Champiat S & Marabelle A Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat. Rev. Clin. Oncol 18, 558–576 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheen MR & Fiering S In situ vaccination: harvesting low hanging fruit on the cancer immunotherapy tree. WIREs Nanomed. Nanobiotechnol 11, e1524 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Russell SJ, Peng K-W & Bell JC Oncolytic virotherapy. Nat. Biotechnol 30, 658–670 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breitbach CJ et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477, 99–102 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Maroun J et al. Designing and building oncolytic viruses. Future Virol. 12, 193–213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YL, Peng HH, Su SY & Lin CT Combined immunotherapy (OK-432, IL-2) with chemotherapy decrease the recurrence rate in advanced ovarian cancer. Reprod. Sci 26, 244–249 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Shalhout SZ, Miller DM, Emerick KS & Kaufman HL Therapy with oncolytic viruses: progress and challenges. Nat. Rev. Clin. Oncol 20, 160–177 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Heldin CH, Rubin K, Pietras K & Ostman A High interstitial fluid pressure — an obstacle in cancer therapy. Nat. Rev. Cancer 4, 806–813 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Puts MTE et al. Factors influencing adherence to cancer treatment in older adults with cancer: a systematic review. Ann. Oncol 25, 564–577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung YH et al. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater 7, 372–388 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beijerinck MW Ueber ein contagium vivum fluidum als Ursache der Fleckenkrankheit der Tabaksblatter [transl. Concerning a contagium vivum fluidum as cause of the spot disease of tobacco leaves.] Verhandelingen der Koninklyke akademie van Wettenschappen te Amsterdam [transl. American Phytopathological Society] https://dwc.knaw.nl/DL/publications/PU00011860.pdf (1898). [Google Scholar]
- 41.Scholthof KB Tobacco mosaic virus: a model system for plant biology. Annu. Rev. Phytopathol 42, 13–34 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Lomonossoff GP & Wege C TMV particles: the journey from fundamental studies to bionanotechnology applications. Adv. Virus Res 102, 149–176 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kausche GA, Pfankuch E & Ruska H Die Sichtbarmachung von pflanzlichem virus im Übermikroskop. Naturwissenschaften 27, 292–299 (1939). [Google Scholar]
- 44.Caspar DL & Klug A Physical principles in the construction of regular viruses. Cold Spring Harb. Symp. Quant. Biol 27, 1–24 (1962). [DOI] [PubMed] [Google Scholar]
- 45.Strable E & Finn MG Chemical modification of viruses and virus-like particles. Curr. Top. Microbiol. Immunol 327, 1–21 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Lin T, Tang L, Johnson JE & Finn MG Icosahedral virus particles as addressable nanoscale building blocks. Angew. Chem. Int. Edn Engl 41, 459–462 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Maheshri N, Koerber JT, Kaspar BK & Schaffer DV Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol 24, 198–204 (2006). [DOI] [PubMed] [Google Scholar]
- 48.McNeale D, Dashti N, Cheah LC & Sainsbury F Protein cargo encapsulation by virus-like particles: strategies and applications. Wiley Interdisc. Rev. Nanomed. Nanobiotechnol 15, e1869 (2023). [DOI] [PubMed] [Google Scholar]
- 49.Bischoff AJ et al. Protein-based model for energy transfer between photosynthetic light-harvesting complexes is constructed using a direct protein–protein conjugation strategy. J. Am. Chem. Soc 145, 15827–15837 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai J et al. A membrane-associated light-harvesting model is enabled by functionalized assemblies of gene-doubled TMV proteins. Small 19, e2207805 (2023). [DOI] [PubMed] [Google Scholar]
- 51.Oh D et al. Biologically enhanced cathode design for improved capacity and cycle life for lithium–oxygen batteries. Nat. Commun 4, 2756 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tseng RJ et al. Digital memory device based on tobacco mosaic virus conjugated with nanoparticles. Nat. Nanotechnol 1, 72–77 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Carette N et al. A virus-based biocatalyst. Nat. Nanotechnol 2, 226–229 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Patterson DP, Schwarz B, Waters RS, Gedeon T & Douglas T Encapsulation of an enzyme cascade within the bacteriophage P22 virus-like particle. ACS Chem. Biol 9, 359–365 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Bucci M Milestones: First recombinant DNA vaccine for HBV. Nature https://www.nature.com/articles/d42859-020-00016-5 (2020). [Google Scholar]
- 56.Valenzuela P, Medina A, Rutter WJ, Ammerer G & Hall BD Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 298, 347–350 (1982). [DOI] [PubMed] [Google Scholar]
- 57.Crystal RG Adenovirus: the first effective in vivo gene delivery vector. Hum. Gene Ther 25, 3–11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zabner J et al. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 75, 207–216 (1993). [DOI] [PubMed] [Google Scholar]
- 59.Garber K China approves world’s first oncolytic virus therapy for cancer treatment. J. Natl Cancer Inst 98, 298–300 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Greig SL Talimogene laherparepvec: first global approval. Drugs 76, 147–154 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Hanahan D Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022). [DOI] [PubMed] [Google Scholar]
- 62.Shahrivarkevishahi A et al. Virus-like particles: a self-assembled toolbox for cancer therapy. Mater. Today Chem 24, 100808 (2022). [Google Scholar]
- 63.Bachmann MF et al. The influence of antigen organization on B cell responsiveness. Science 262, 1448–1451 (1993). [DOI] [PubMed] [Google Scholar]
- 64.Bachmann MF & Jennings GT Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol 10, 787–796 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Kaufman HL, Kohlhapp FJ & Zloza A Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug. Discov 14, 642–662 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alberts P, Tilgase A, Rasa A, Bandere K & Venskus D The advent of oncolytic virotherapy in oncology: the Rigvir® story. Eur. J. Pharmacol 837, 117–126 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Liang M Oncorine, the world first oncolytic virus medicine and its update in China. Curr. Cancer Drug. Targets 18, 171–176 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Fukuhara H, Ino Y & Todo T Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci 107, 1373–1379 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaurasiya S, Chen NG & Fong Y Oncolytic viruses and immunity. Curr. Opin. immunology 51, 83–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prestwich RJ et al. Oncolytic viruses: a novel form of immunotherapy. Expert. Rev. Anticancer. Ther 8, 1581–1588 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiocca EA & Rabkin SD Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol. Res 2, 295–300 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aurelian L Oncolytic viruses as immunotherapy: progress and remaining challenges. Onco Targets Ther. 9, 2627–2637 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian Y, Xie D & Yang L Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal. Transduct. Target. Ther 7, 117 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jhawar SR et al. Oncolytic viruses — natural and genetically engineered cancer immunotherapies. Front. Oncol 7, 202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderson BD, Nakamura T, Russell SJ & Peng KW High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 64, 4919–4926 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Malissen N et al. HVEM has a broader expression than PD-L1 and constitutes a negative prognostic marker and potential treatment target for melanoma. Oncoimmunology 8, e1665976 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oshima T et al. Nectin-2 is a potential target for antibody therapy of breast and ovarian cancers. Mol. Cancer 12, 60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamada M et al. Nectin-1 expression in cancer-associated fibroblasts is a predictor of poor prognosis for pancreatic ductal adenocarcinoma. Surg. Today 48, 510–516 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Davidson B et al. αV- and β1-integrin subunits are commonly expressed in malignant effusions from ovarian carcinoma patients. Gynecol. Oncol 90, 248–257 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Kohlhapp F, Zloza A & Kaufman H Talimogene laherparepvec (T-VEC) as cancer immunotherapy. Drugs Today 51, 549–558 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Dörig RE, Marcil A, Chopra A & Richardson CD The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75, 295–305 (1993). [DOI] [PubMed] [Google Scholar]
- 82.Carlsten M et al. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J. Immunol 183, 4921–4930 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Yang M et al. A novel fiber chimeric conditionally replicative adenovirus-Ad5/F35 for tumor therapy. Cancer Biol. Ther 18, 833–840 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puig-Saus C et al. iRGD tumor-penetrating peptide-modified oncolytic adenovirus shows enhanced tumor transduction, intratumoral dissemination and antitumor efficacy. Gene Ther. 21, 767–774 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Martinez-Velez N et al. The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models. Nat. Commun 10, 2235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Judd J et al. Tunable protease-activatable virus nanonodes. ACS Nano 8, 4740–4746 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van der Meel R et al. Smart cancer nanomedicine. Nat. Nanotechnol 14, 1007–1017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Y & Lammers T Combining nanomedicine and immunotherapy. Acc. Chem. Res 52, 1543–1554 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhagwat AS & Vakoc CR Targeting transcription factors in cancer. Trends Cancer 1, 53–65 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Critchley-Thorne RJ et al. Impaired interferon signaling is a common immune defect in human cancer. Proc. Natl Acad. Sci. USA 106, 9010–9015 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matveeva OV & Chumakov PM Defects in interferon pathways as potential biomarkers of sensitivity to oncolytic viruses. Rev. Med. Virol 28, e2008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaloni D, Diepstraten ST, Strasser A & Kelly GL BCL-2 protein family: attractive targets for cancer therapy. Apoptosis 28, 20–38 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trisciuoglio D et al. BCL-X overexpression promotes tumor progression-associated properties. Cell Death Dis. 8, 3216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mansour M, Palese P & Zamarin D Oncolytic specificity of newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J. Virol 85, 6015–6023 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez R et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 57, 2559–2563 (1997). [PubMed] [Google Scholar]
- 96.DeWeese TL et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 61, 7464–7472 (2001). [PubMed] [Google Scholar]
- 97.Gujar S, Pol JG, Kim Y, Lee PW & Kroemer G Antitumor benefits of antiviral immunity: an underappreciated aspect of oncolytic virotherapies. Trends Immunol. 39, 209–221 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Kelly KR et al. Oncolytic reovirus sensitizes multiple myeloma cells to anti-PD-L1 therapy. Leukemia 32, 230–233 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feist M et al. Oncolytic virus promotes tumor-reactive infiltrating lymphocytes for adoptive cell therapy. Cancer Gene Ther. 28, 98–111 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chouljenko DV et al. Induction of durable antitumor response by a novel oncolytic herpesvirus expressing multiple immunomodulatory transgenes. Biomedicines 8, 484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wenthe J et al. Immunostimulatory oncolytic virotherapy for multiple myeloma targeting 4–1BB and/or CD40. Cancer Gene Ther. 27, 948–959 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ylösmäki E et al. Characterization of a novel OX40 ligand and CD40 ligand-expressing oncolytic adenovirus used in the PeptiCRAd cancer vaccine platform. Mol. Ther. Oncolyt 20, 459–469 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eriksson E et al. Activation of myeloid and endothelial cells by CD40L gene therapy supports T-cell expansion and migration into the tumor microenvironment. Gene Ther. 24, 92–103 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J-C et al. Tolerability and safety of EUS-injected adenovirus-mediated double-suicide gene therapy with chemotherapy in locally advanced pancreatic cancer: a phase 1 trial. Gastrointest. Endosc 92, 1044–1052.e1041 (2020). [DOI] [PubMed] [Google Scholar]
- 105.Doronin K et al. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J. Virol 74, 6147–6155 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barton KN et al. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol. Ther 13, 347–356 (2006). [DOI] [PubMed] [Google Scholar]
- 107.Boorjian SA et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 22, 107–117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shin DH et al. Current strategies to circumvent the antiviral immunity to optimize cancer virotherapy. J. Immunother. Cancer 9, e002086 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu B et al. ICP34. 5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 10, 292–303 (2003). [DOI] [PubMed] [Google Scholar]
- 110.Chou J & Roizman B The gamma 1 (34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc. Natl Acad. Sci. USA 89, 3266–3270 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH & Wimmer E Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl Acad. Sci. USA 97, 6803–6808 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yun C-O, Hong J & Yoon A Current clinical landscape of oncolytic viruses as novel cancer immunotherapeutic and recent preclinical advancements. Front. Immunol 13, 953410 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith T et al. In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus–adenovirus receptor. Mol. Ther 5, 770–779 (2002). [DOI] [PubMed] [Google Scholar]
- 114.Groeneveldt C, van den Ende J & van Montfoort N Preexisting immunity: barrier or bridge to effective oncolytic virus therapy? Cytokine Growth Factor. Rev 70, 1–12 (2023). [DOI] [PubMed] [Google Scholar]
- 115.Wakimoto H et al. The complement response against an oncolytic virus is species-specific in its activation pathways. Mol. Ther 5, 275–282 (2002). [DOI] [PubMed] [Google Scholar]
- 116.Nosaki K et al. A novel, polymer-coated oncolytic measles virus overcomes immune suppression and induces robust antitumor activity. Mol. Ther. Oncolytics 3, 16022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang HW et al. Full encapsulation of oncolytic virus using hybrid erythroctye-liposome membranes for augmented anti-refractory tumor effectiveness. Nano Today 47, 101671 (2022). [Google Scholar]
- 118.Xia M et al. Graphene oxide arms oncolytic measles virus for improved effectiveness of cancer therapy. J. Exp. Clin. Cancer Res 38, 408 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rojas LA et al. Albumin-binding adenoviruses circumvent pre-existing neutralizing antibodies upon systemic delivery. J. Control. Rel 237, 78–88 (2016). [DOI] [PubMed] [Google Scholar]
- 120.Martinez-Quintanilla J, He D, Wakimoto H, Alemany R & Shah K Encapsulated stem cells loaded with hyaluronidase-expressing oncolytic virus for brain tumor therapy. Mol. Ther 23, 108–118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hammad M et al. Neural stem cells improve the delivery of oncolytic chimeric orthopoxvirus in a metastatic ovarian cancer model. Mol. Ther. Oncolyt 18, 326–334 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cornejo Y et al. NSCs are permissive to oncolytic Myxoma virus and provide a delivery method for targeted ovarian cancer therapy. Oncotarget 11, 4693 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Andtbacka RHI et al. Clinical responses of oncolytic coxsackievirus A21 (V937) in patients with unresectable melanoma. J. Clin. Oncol 39, 3829–3838 (2021). [DOI] [PubMed] [Google Scholar]
- 124.Breitbach CJ, Moon A, Burke J, Hwang TH & Kirn DH A phase 2, open-label, randomized study of pexa-vec (JX-594) administered by intratumoral injection in patients with unresectable primary hepatocellular carcinoma. Methods Mol. Biol 1317, 343–357 (2015). [DOI] [PubMed] [Google Scholar]
- 125.Lin D, Shen Y & Liang T Oncolytic virotherapy: basic principles, recent advances and future directions. Signal. Transduct. Target. Ther 8, 156 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alonso-Miguel D et al. Neoadjuvant in situ vaccination with cowpea mosaic virus as a novel therapy against canine inflammatory mammary cancer. J. Immunother. Cancer 10, e004044 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mao C et al. In situ vaccination with cowpea mosaic virus elicits systemic antitumor immunity and potentiates immune checkpoint blockade. J. Immunother. Cancer 10, e005834 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shukla S, Wang C, Beiss V & Steinmetz NF Antibody response against cowpea mosaic viral nanoparticles improves in situ vaccine efficacy in ovarian cancer. ACS Nano 14, 2994–3003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Valdivia G et al. Neoadjuvant intratumoral immunotherapy with cowpea mosaic virus induces local and systemic antitumor efficacy in canine mammary cancer patients. Cells 12, 2241 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davies JW Molecular Plant Virology (CRC Press, 1985). [Google Scholar]
- 131.Yang S et al. Crystal structure of the coat protein of the flexible filamentous papaya mosaic virus. J. Mol. Biol 422, 263–273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Denis J et al. Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform. Vaccine 26, 3395–3403 (2008). [DOI] [PubMed] [Google Scholar]
- 133.Lebel ME et al. Nanoparticle adjuvant sensing by TLR7 enhances CD8+ T cell-mediated protection from Listeria monocytogenes infection. J. Immunol 192, 1071–1078 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Mathieu C, Rioux G, Dumas MC & Leclerc D Induction of innate immunity in lungs with virus-like nanoparticles leads to protection against influenza and Streptococcus pneumoniae challenge. Nanomedicine 9, 839–848 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Lee KL et al. Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano Lett. 17, 4019–4028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eriksson F et al. Tumor-specific bacteriophages induce tumor destruction through activation of tumor-associated macrophages. J. Immunol 182, 3105–3111 (2009). [DOI] [PubMed] [Google Scholar]
- 137.Tian Y et al. Probing the endocytic pathways of the filamentous bacteriophage in live cells using ratiometric pH fluorescent indicator. Adv. Healthc. Mater 4, 413–419 (2015). [DOI] [PubMed] [Google Scholar]
- 138.Prangishvili D, Forterre P & Garrett RA Viruses of the Archaea: a unifying view. Nat. Rev. Microbiol 4, 837–848 (2006). [DOI] [PubMed] [Google Scholar]
- 139.Krupovic M, Cvirkaite-Krupovic V, Iranzo J, Prangishvili D & Koonin EV Viruses of Archaea: structural, functional, environmental and evolutionary genomics. Virus Res. 244, 181–193 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Young TDAM Viruses: making friends with old foes. Science 312, 873–875 (2006). [DOI] [PubMed] [Google Scholar]
- 141.De Lombaerde E, De Wever O & De Geest BG Delivery routes matter: safety and efficacy of intratumoral immunotherapy. Biochim. Biophys. Acta Rev. Cancer 1875, 188526 (2021). [DOI] [PubMed] [Google Scholar]
- 142.Tariq H, Batool S, Asif S, Ali M & Abbasi BH Virus-like particles: revolutionary platforms for developing vaccines against emerging infectious diseases. Front. Microbiol 12, 790121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sabree SA et al. Direct and indirect immune effects of CMP-001, a virus-like particle containing a TLR9 agonist. J. Immunother. Cancer 9, e002484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cai H, Shukla S & Steinmetz NF The antitumor efficacy of CpG oligonucleotides is improved by encapsulation in plant virus-like particles. Adv. Funct. Mater 30, 1908743 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jung E, Chung YH & Steinmetz NF TLR agonists delivered by plant virus and bacteriophage nanoparticles for cancer immunotherapy. Bioconjug. Chem 34, 1596–1605 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Storni T et al. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J. Immunol 172, 1777–1785 (2004). [DOI] [PubMed] [Google Scholar]
- 147.Krug A et al. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur. J. Immunol 31, 2154–2163 (2001). [DOI] [PubMed] [Google Scholar]
- 148.Mutwiri GK, Nichani AK, Babiuk S & Babiuk LA Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides. J. Control. Rel 97, 1–17 (2004). [DOI] [PubMed] [Google Scholar]
- 149.Cheng Y et al. In situ immunization of a TLR9 agonist virus-like particle enhances anti-PD1 therapy. J. Immunother. Cancer 8, e000940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lemke-Miltner CD et al. Antibody opsonization of a TLR9 agonist-containing virus-like particle enhances in situ immunization. J. Immunol 204, 1386–1394 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bakhos Jneid AB et al. Selective STING stimulation in dendritic cells primes antitumor T cell responses. Sci. Immunol 8, 1–17 (2023). [DOI] [PubMed] [Google Scholar]
- 152.Cerullo V et al. An oncolytic adenovirus enhanced for toll-like receptor 9 stimulation increases antitumor immune responses and tumor clearance. Mol. Ther 20, 2076–2086 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang P, Han X, Tan W, Chen D & Sun Q RIG-I-mediated innate immune signaling in tumors reduces the therapeutic effect of oncolytic vesicular stomatitis virus. Thorac. Cancer 14, 246–253 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thorne SH Adding STING to the tale of oncolytic virotherapy. Trends Cancer 2, 67–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Westcott PMK et al. Mismatch repair deficiency is not sufficient to elicit tumor immunogenicity. Nat. Genet 55, 1686–1695 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Germano G et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 552, 116–120 (2017). [DOI] [PubMed] [Google Scholar]
- 157.Hom V, Karonis E, Sigidi T & Cawley K Development of a nursing policy for the administration of an oncolytic virus in the outpatient setting. Semin. Oncol. Nurs 35, 150928 (2019). [DOI] [PubMed] [Google Scholar]
- 158.Galon J & Bruni D Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug. Discov 18, 197–218 (2019). [DOI] [PubMed] [Google Scholar]
- 159.Bommareddy PA-O, Aspromonte S, Zloza A, Rabkin SA-O & Kaufman HL MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Sci. Transl. Med 10, eaau0417 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang C & Steinmetz NF A combination of cowpea mosaic virus and immune checkpoint therapy synergistically improves therapeutic efficacy in three tumor models. Adv. Funct. Mater 30, 2002299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Passaro C et al. Arming an oncolytic herpes simplex virus type 1 with a single-chain fragment variable antibody against PD-1 for experimental glioblastoma therapy. Clin. Cancer Res 25, 290–299 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zamarin D et al. PD-L1 in tumor microenvironment mediates resistance to oncolytic immunotherapy. J. Clin. Invest 128, 1413–1428 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shakoora AS et al. Direct and indirect immune effects of CMP-001, a virus-like particle containing a TLR9 agonist. J. Immunother. Cancer 9, e002484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yinwen C et al. in situ immunization of a TLR9 agonist virus-like particle enhances anti-PD1 therapy. J. Immunother. Cancer 8, e000940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ribas A et al. Overcoming PD-1 blockade resistance with CpG — a Toll-like receptor 9 agonist vidutolimod in patients with metastatic melanoma. Cancer Discov. 11, 2998–3007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dummer R et al. Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage IIIB-IVM1a melanoma: a randomized, open-label, phase 2 trial. Nat. Med 27, 1789–1796 (2021). [DOI] [PubMed] [Google Scholar]
- 167.Hong WX et al. Intratumoral immunotherapy for early-stage solid tumors. Clin. Cancer Res 26, 3091–3099 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Munoz NM et al. Influence of injection technique, drug formulation and tumor microenvironment on intratumoral immunotherapy delivery and efficacy. J. Immunother. Cancer 9, e001800 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mohsen MO et al. In situ delivery of nanoparticles formulated with micron-sized crystals protects from murine melanoma. J. Immunother. Cancer 10, e004643 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Packiam VT et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: interim results. Urol. Oncol 36, 440–447 (2018). [DOI] [PubMed] [Google Scholar]
- 171.Nassiri F et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial. Nat. Med 29, 1370–1378 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gallego Perez-Larraya J et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N. Engl. J. Med 386, 2471–2481 (2022). [DOI] [PubMed] [Google Scholar]
- 173.Fares J et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 22, 1103–1114 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Beasley GM et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J. Immunother. Cancer 9, e002203 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rudin CM et al. Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features. Clin. Cancer Res 17, 888–895 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lutzky J et al. Phase 1b study of intravenous coxsackievirus A21 (V937) and ipilimumab for patients with metastatic uveal melanoma. J. Cancer Res. Clin 1449, 6059–6066 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Senzer NN et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol 27, 5763–5771 (2009). [DOI] [PubMed] [Google Scholar]
- 178.Andtbacka RH et al. Cutaneous head and neck melanoma in OPTiM, a randomized phase 3 trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor for the treatment of unresected stage IIIB/IIIC/IV melanoma. Head Neck 38, 1752–1758 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chesney JA et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. J. Immunother. Cancer 11, e006270 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Streby KA et al. First-in-human intravenous seprehvir in young cancer patients: a phase 1 clinical trial. Mol. Ther 27, 1930–1938 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Streby KA et al. Intratumoral injection of HSV1716, an oncolytic herpes virus, is safe and shows evidence of immune response and viral replication in young cancer patients. Clin. Cancer Res 23, 3566–3574 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Geletneky K et al. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: parvOryx01 protocol. BMC Cancer 12, 99 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hwang TH et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol. Ther 19, 1913–1922 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Heo J et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med 19, 329–336 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Holloway RW et al. Clinical activity of olvimulogene nanivacirepvec-primed immunochemotherapy in heavily pretreated patients with platinum-resistant or platinum-refractory ovarian cancer: the nonrandomized phase 2 VIRO-15 clinical trial. JAMA Oncol. 9, 903–908 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Le Gall O et al. Picornavirales, a proposed order of positive-sense single-stranded RNA viruses with a pseudo-T=3 virion architecture. Arch. Virol 153, 715–727 (2008). [DOI] [PubMed] [Google Scholar]
- 187.Lin T & Johnson JE Structures of picorna-like plant viruses: implications and applications. Adv. Virus Res 62, 167–239 (2003). [DOI] [PubMed] [Google Scholar]
- 188.Brown MC et al. Oncolytic polio virotherapy of cancer. Cancer 120, 3277–3286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Walton RW, Brown MC, Sacco MT & Gromeier M Engineered oncolytic poliovirus PVSRIPO subverts MDA5-dependent innate immune responses in cancer cells. J. Virol 92, e00879–e00918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lomonossoff GP in Encyclopedia of Virology 569–574 (Elsevier, 2008). [Google Scholar]
- 191.Shukla S et al. The unique potency of cowpea mosaic virus (CPMV) in situ cancer vaccine. Biomater. Sci 8, 5489–5503 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Beiss V, Mao C, Fiering SN & Steinmetz NF Cowpea mosaic virus outperforms other members of the secoviridae as in situ vaccine for cancer immunotherapy. Mol. Pharm 19, 1573–1585 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Argos P, Kamer G, Nicklin MJH & Wimmer E Similarity in gene organization and homology between proteins of animal picomaviruses and a plant comovirus suggest common ancestry of these virus families. Nucl. Acids Res 12, 7251–7267 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Franssen H, Leunissen J, Goldbach R, Lomonossoff G & Zimmern D Homologous sequences in non-structural proteins from cowpea mosaic virus and picornaviruses. EMBO J. 3, 855–861 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Beck MA et al. Comoviruses and enteroviruses share a T cell epitope. Virology 186, 238–246 (1992). [DOI] [PubMed] [Google Scholar]
- 196.Tripathi NK & Shrivastava A Recent developments in bioprocessing of recombinant proteins: expression hosts and process development. Front. Bioeng. Biotechnol 7, 420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wellink J Comovirus isolation and RNA extraction. Methods Mol. Biol 81, 205–209 (1998). [DOI] [PubMed] [Google Scholar]
- 198.Coalition for Epidemic Preparedness Innovations (CEPI). Plant-based ALiCE® technology could shave weeks off vaccine production. CEPI https://cepi.net/plant-based-alicer-technology-could-shave-weeks-vaccine-production (2024). [Google Scholar]
- 199.Lee J, Lee SK, Park JS & Lee KR Plant-made pharmaceuticals: exploring studies for the production of recombinant protein in plants and assessing challenges ahead. Plant. Biotechnol. Rep 17, 53–65 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Alam A et al. Technoeconomic modeling of plant-based griffithsin manufacturing. Front. Bioeng. Biotechnol 6, 102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nandi S et al. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. MAbs 8, 1456–1466 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Holtz BR et al. Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant. Biotechnol. J 13, 1180–1190 (2015). [DOI] [PubMed] [Google Scholar]


