Abstract
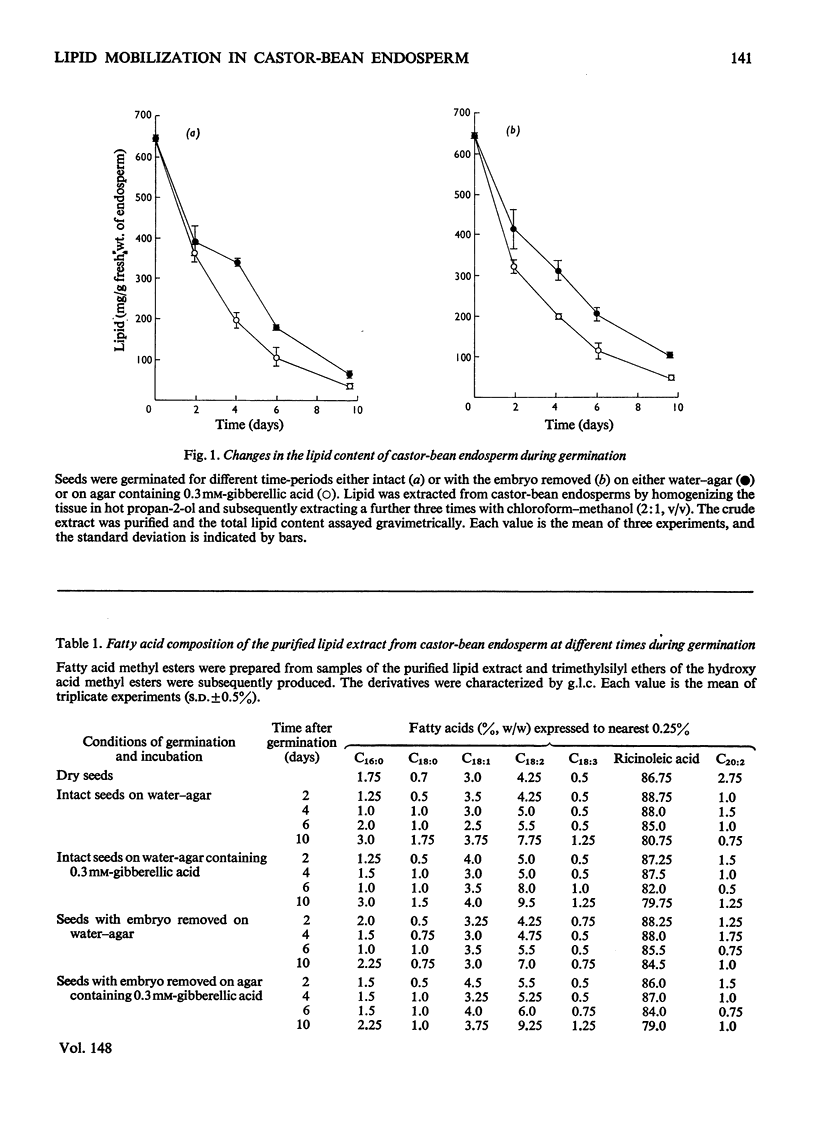
1. Lipid extracts were obtained from castor-bean endosperm tissue at various times during germination and, after purification, the total lipid content was determined. Quantitative measurements of the triglyceride and phospholipid content together with the fatty acid composition were made. 2. The total lipid content of the endosperm rapidly decreased during germination; after 10 days less than 20% of the original weight of lipid remained. In contrast, the phospholipid content (initially less than 0.5% of the total lipid) increased slightly during this time. The fatty acid composition and the relative proportions of the triglyceride species of the total lipid extract remained constant during 10 days of germination. 3. Gibberellic acid (0.3 mM) markedly stimulated the rate of lipid breakdown but did not alter either the fatty acid composition or the relative proportion of triglyceride species. 4. The embryo had little effect on lipid metabolism in the endosperm tissue; only after 6 days of germination were differences observed in the rate of fat utilization in the presence and absence of the embryo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES A. T., MARTIN A. J. P. Gas-liquid partition chromatography; the separation and micro-estimation of volatile fatty acids from formic acid to dodecanoic acid. Biochem J. 1952 Mar;50(5):679–690. doi: 10.1042/bj0500679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado P., Schwendimann M., Marrè E. Repression of isocitrate lyase synthesis in seeds germinated in the presence of glucose. Biochim Biophys Acta. 1968 Mar 18;157(1):140–148. doi: 10.1016/0005-2787(68)90272-4. [DOI] [PubMed] [Google Scholar]
- MARCUS A., FEELEY J. ISOCITRIC LYASE FORMATION IN THE DISSECTED PEANUT COTYLEDON. Biochim Biophys Acta. 1964 Jul 8;89:170–171. doi: 10.1016/0926-6569(64)90116-6. [DOI] [PubMed] [Google Scholar]
- Muto S., Beevers H. Lipase Activities in Castor Bean Endosperm during Germination. Plant Physiol. 1974 Jul;54(1):23–28. doi: 10.1104/pp.54.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. W., Moorhouse R. The separation, structure and metabolism of monogalactosyl diglyceride species in Chlorella vulgaris. Lipids. 1969 Sep;4(5):311–316. doi: 10.1007/BF02530998. [DOI] [PubMed] [Google Scholar]
- Noma A., Borgström B. The acid lipase of castor beans. Positional specificity and reaction mechanism. Biochim Biophys Acta. 1971 Jan 13;227(1):106–115. doi: 10.1016/0005-2744(71)90172-0. [DOI] [PubMed] [Google Scholar]
- ORY R. L., ST ANGELO A. J., ALTSCHUL A. M. Castor bean lipase: action on its endogenous substrate. J Lipid Res. 1960 Apr;1:208–213. [PubMed] [Google Scholar]
- Ory R. L., Kiser J., Pradel P. A. Studies on positional specificity of the castor bean acid lipase. Lipids. 1969 Jul;4(4):261–264. doi: 10.1007/BF02533183. [DOI] [PubMed] [Google Scholar]
- Penner D., Ashton F. M. Hormonal control of isocitrate lyase synthesis. Biochim Biophys Acta. 1967 Nov 28;148(2):481–485. doi: 10.1016/0304-4165(67)90145-6. [DOI] [PubMed] [Google Scholar]
- SAVARY P., FLANZY J., DESNUELLE P. Sur l'hydrolyse des triglycérides par la lipase du ricin. Bull Soc Chim Biol (Paris) 1958 Jun 9;40(4):637–645. [PubMed] [Google Scholar]
- VAN HANDEL E., ZILVERSMIT D. B. Micromethod for the direct determination of serum triglycerides. J Lab Clin Med. 1957 Jul;50(1):152–157. [PubMed] [Google Scholar]
- Vincenzini M. T., Vincieri F., Vanni P. The Effects of Octanoate and Oleate on Isocitrate Lyase Activity during the Germination of Pinus pinea Seeds. Plant Physiol. 1973 Dec;52(6):549–553. doi: 10.1104/pp.52.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]


