Abstract
Objectives
Cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) haploinsufficiency is a rare genetic condition characterized by development of immune cytopenia, hypogammaglobulinemia, and/or lymphoproliferative disorder, as well as multiple autoimmunity. Treatment with abatacept was shown to alleviate autoimmune conditions, yet its long-lasting impact on bone marrow function remains undetermined.
Methods
We here present the case of a now 39-year-old woman with CTLA-4 haploinsufficiency with predominant CNS affection, yet multiorgan autoimmunity and lymphopenia. We conducted single-cell RNA sequencing (scRNA-seq) of peripheral mononuclear blood cells before and after abatacept induction.
Results
After several high-efficacy immunosuppressive treatments with little-to-no response, she started abatacept in 2017 and experienced ongoing remission including resolution of pre-existing immune cytopenia and hypogammaglobulinemia. Using scRNA-seq, we were able to demonstrate reconstitution of peripheral B cells accompanied by reduction of CD8+ T cells. CD4+ and CD8+ T cells were characterized by downregulation of pathways involved in activation of innate immune cells.
Discussion
Our findings demonstrate long-lasting resolution of lymphopenia after abatacept treatment in CTLA-4 haploinsufficiency despite severity and duration of symptoms. Thus, abatacept should be considered throughout before stem cell transplantation also in CTLA-4 haploinsufficiency with severe symptoms.
Classification of Evidence
As a single report without controls, this report provides class IV evidence that abatacept might revert lymphopenia in patients with CTLA-4 haploinsufficiency.
Introduction
Cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) haploinsufficiency is a rare immune dysregulation syndrome characterized by regulatory T-cell dysfunction. Recently, several sequence variations have been described to cause this syndrome. Besides variations in the CTLA-4 gene itself, variations in the LRBA or DEF6 genes were also identified to subsequently induce CTLA-4 haploinsufficiency.1,2
Affected patients develop cytopenia, lymphoproliferative disorders, and hypogammaglobulinemia and are prone to a variety of autoimmune phenomena. Autoimmunity in patients with CTLA-4 haploinsufficiency regularly affects the CNS resulting in a relapsing, steroid-responsive disease with cell-rich infiltrates in the parenchyma accompanied by only minor demyelination or axonal loss, thus potentially mimicking relapsing multiple sclerosis (MS).3
Although only allogenic hematopoietic stem cell transplantation provides a causative approach to the condition,4 amelioration of the disease was described after administration of abatacept, a recombinant CTLA-4 fusion protein approved for treatment of rheumatoid arthritis.5 However, there remains a knowledge gap in respect to the influence of abatacept on lymphopenia. Thus, we here present the case of a now 39-year-old woman in whom abatacept induced permanent clinical remission including resolution of lymphopenia and repopulation of peripheral B cells.
Methods
A case report from a well-characterized patient with CTLA-4 haploinsufficiency is presented. To provide further in-depth analysis of her immunorepertoire, we performed single-cell RNA sequencing (scRNA-seq) of peripheral blood mononuclear cells at 2 time points spanning the period before and after abatacept treatment (2017 and 2019). In brief, scRNA-seq was performed using the GEXSCOPE Single Cell RNA Library Kit V2 as previously described.6 Both data sets were quality controlled, as well as harmonized and integrated using the Harmony algorithm.7 Cell populations and subpopulations were automatically annotated with ScType.8
Standard Protocol Approvals, Registrations, and Patient Consents
The patient gave written consent to publication of her medical history including publication of results from genetic analysis.
Data Availability
Anonymized data will be shared on reasonable request from qualified investigators.
Results
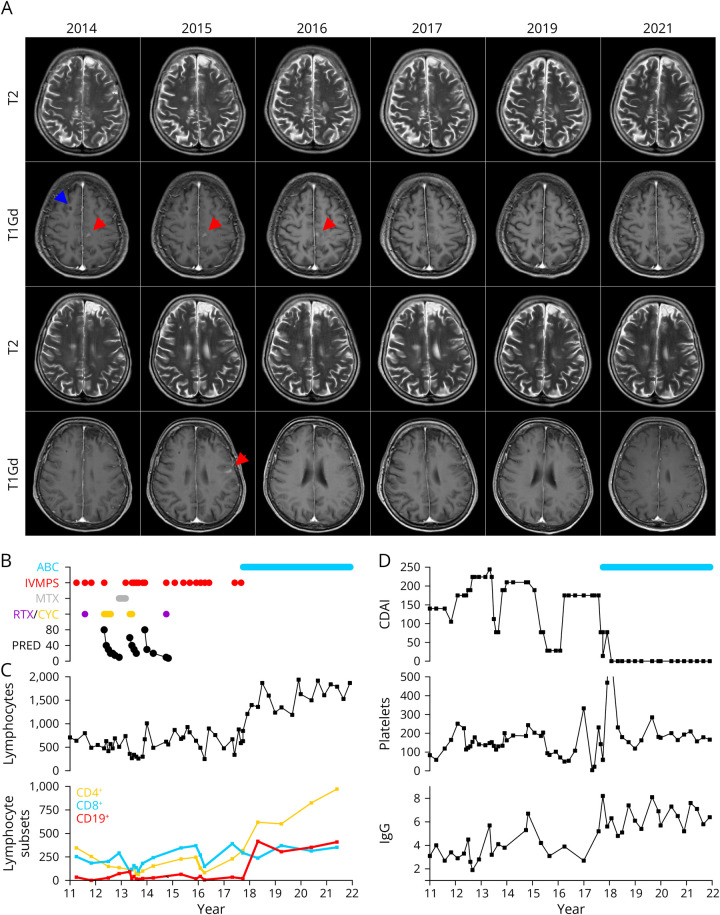
A 14-year-old adolescent girl first developed a relapsing-remitting inflammatory CNS disorder and thrombocytopenia in 1999. Her relapses presented with various symptoms yet always responded to IV methylprednisolone (IVMPS). On first admission to our University Hospital in 2004, she underwent extensive differential diagnosis. CSF analysis showed intrathecal synthesis of immunoglobulins G and M. MRI displayed disseminated T2-hyperintense lesions (spinal MRI was unremarkable) with some lesions indicating long-lasting gadolinium enhancement (Figure 1A). Laboratory testing was negative for infectious or rheumatologic conditions, and brain vessel angiography was normal as well. Full blood count indicated lymphopenia, anemia, and thrombopenia. Furthermore, hypogammaglobulinemia was diagnosed. The patient was treated with romiplostim for thrombopenia since 2008 and received IV immunoglobulins since 2004. The patient also reported chronic diarrhea and presented with signs of malabsorption including hair loss and chronic bowel inflammation diagnosed by colon biopsies.
Figure 1. Clinical Course of the Presented Patient.
(A) T1-weighted axial MRI scans after gadolinium administration before (2014–2016) and after (2017–2021) abatacept treatment. Red arrows indicate lesions with (persistent) gadolinium enhancement. The blue arrow indicates the site of brain biopsy in 2011. (B) Immunomodulatory treatment of the patient since 2011. ABC = abatacept; CYC = cyclophosphamide; IVMPS = IV methylprednisolone; PRED = prednisolone (daily dosage as indicated); RTX = rituximab (2011: 2 × 1000 mg iv; 2014: 2 × 10 mg ith). (C) Development of the blood lymphocyte count and lymphocyte subsets over time (cells/mm3). (D) Crohn disease activity index (CDAI) indicating severity of symptoms of the patient's autoimmune enteropathy (upper graph), blood platelet count (platelets/mm3; middle graph), and serum immunoglobulin G (IgG) levels (g/L).
Given the repeated symptoms and persistent contrast enhancement suggestive of a neoplastic condition, the patient underwent matrix-metalloproteinase PET scan–guided brain biopsy. It showed sustained myelin integrity as well as massive infiltration of T cells and presence of few perivascular B cells as well as plasma cells. Infectious or neoplastic conditions were ruled out.
Afterward, the patient received 2 courses of IV rituximab in 2011 (Figure 1B). After further relapses, immunomodulatory treatment was escalated to oral prednisolone and 4 courses of IV cyclophosphamide (750 mg/m2 every 4 weeks) from May 2012 to August 2012, followed by methotrexate. However, within 2 months, the patient developed atypical pneumonia and had a clinical relapse with hemiparesis and was again treated with 2 courses of cyclophosphamide.
She then continued oral prednisolone but experienced at least 6 further relapses including one relapse with brainstem involvement and cranial nerve palsy, which only resolved after repeated IVMPS. Apart from 2 intrathecal injections of rituximab (10 mg), she rejected any further treatment until 2017 but received at least 8 further courses of IVMPS.
In 2017, we thoroughly re-evaluated the patient and performed sequencing of the LRBA and CTLA-4 genes. Although LRBA sequencing was normal, we found a heterozygous insertion variant in the CTLA-4 gene ((NM_005214.5): c.322_323insT; p.(Ser108MetfsTer46)).
The variant is classified as likely pathogenic according to ACMG/AMP criteria, fulfilling PVS1 (pathogenic very strong) as a null frameshift variation predicted to cause nonsense-mediated decay in the Ig-like V-type domain and PM2 (pathogenic moderate) due to its absence in gnomAD.
Given the severity of the clinical course (severe thrombocytopenia (nadir: 4/nL) with oral and gastrointestinal bleeding), we immediately commenced IV treatment with abatacept (500 mg every 2 weeks). After abatacept treatment, lymphopenia resolved and CD4+ T cells and CD19+ B cells normalized in the peripheral blood (Figure 1C). The patient experienced rapid resolution of her autoimmune conditions, and within few months, immunoglobulin substitution and romiplostim treatment were stopped (Figure 1D). She now continues treatment with abatacept every 4 weeks and experiences ongoing remission.
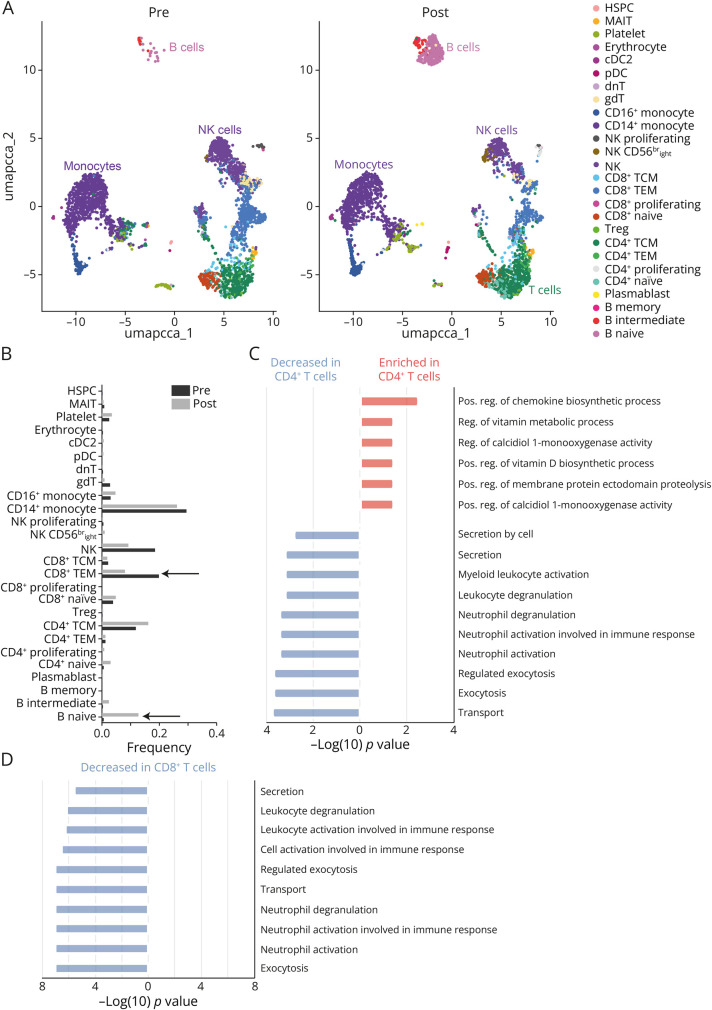
The immune landscape of this patient was characterized by a distinct loss of the B-cell repertoire and, to a lesser extent, of the T effector memory (TEM) population. After abatacept treatment, we observed repopulation of the B-cell and TEM compartments eventually associated with the resolution of hypogammaglobulinemia (Figure 2, A and B).
Figure 2. Single-Cell RNA Sequencing of Peripheral Blood Mononuclear Cells (PBMCs).
(A) Dimensional reduction with Uniform Manifold Approximation and Projection (UMAP) for cells before (pre) and after (post) abatacept treatment. Both data sets were integrated and harmonized using canonical correlation analysis. Cells were annotated based on canonical markers. (B) Frequencies of specific cell populations and subpopulations comparing pretreatment and post-treatment status. (C) Gene set enrichment analysis (GSEA) of differentially expressed genes (DEGs) between pretreatment and post-treatment status for the CD4+ T-cell compartment. Enrichment indicates a higher GSEA score in the pretreatment status and a decrease in the post-treatment status. (D) GSEA for the CD8+ T-cell compartment. All gene sets were reduced in the post-treatment status.
We performed gene set enrichment analysis (GSEA) on the differentially expressed genes (DEGs) of CD4+ and CD8+ T cells. After abatacept treatment, CD4+ T cells demonstrated a downregulation of pathways related to recruitment of innate immune cells, while pathways related to vitamin metabolic processes and chemokine biosynthesis were upregulated (compared with baseline; Figure 2C). Concurrently, CD8+ T cells were characterized by a marked downregulation of pathways associated with recruitment of innate immune cells, such as activation or degranulation of granulocytes (compared with baseline; Figure 2D). These observations suggest that abatacept affects the regulation of innate immunity by restoring T-cell regulatory properties.
Discussion
CTLA-4 haploinsufficiency has become an increasingly recognized condition comprising immune dysregulation with subsequent multiorgan autoimmunity on the one hand and immune cytopenia on the other hand.1 CNS involvement seems common in the disease and is characterized by mixed cellular infiltrates and only minor structural damage as was evident in our patient as well.9 Although some patients present with secondary lymphoproliferative disorders, our patient belonged to the subtype with persistent lymphopenia.5,10
In healthy T cells, CTLA-4 acts as an immune checkpoint in effector T cells and a costimulatory receptor on regulatory T (Treg) cells. Absence of CTLA-4 thus leads to Treg insufficiency and subsequent expansion of effector T cells such as follicular T helper cells.11 Among those dysregulated T cells, a subset of CD57+CD4+ T cells has attracted attention because these cells exhibit cytotoxic properties and are capable of inhibiting B-cell responses.12
Of interest, abatacept seemed to predominantly affect regulatory functions exerted by CD4+ and CD8+ T cells on innate immune cells, such as neutrophils, as was shown in our pathway analyses. Although we did not perform bone marrow biopsy, these findings might explain the underlying mechanisms by which T cells suppress bone marrow function or maintain autoantibody production. Previous studies have shown that abatacept interferes with production of effector cytokines by innate immune cells such as macrophages and thus reduces autoantibody production and autoimmunity in vitro besides induction of regulatory T cells.13,14
Clinically, our patient responded dramatically to treatment. Of note, she also recovered from hypogammaglobulinemia, which was not described in recent cohorts after abatacept treatment but only after stem cell transplantation.4 However, the absence of a lymphoproliferative disorder may have correlated with a less profound impairment of B-cell precursors despite a long duration of hypogammaglobulinemia. Alternatively, B-cell depletion might have been caused by peripheral exhaustion, which was sensitive to treatment.15
Taken together, our findings support the use of abatacept in patients with CTLA-4 haploinsufficiency before stem cell transplantation, even in patients with persistent lymphopenia.
Author Contributions
S. Pfeuffer: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. C. Nelke: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. M. Pawlitzki: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. T. Ruck: drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data. C.B. Schroeter: drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data. C. Thomas: drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data. G. Kobbe: drafting/revision of the manuscript for content, including medical writing for content. S. Dietrich: drafting/revision of the manuscript for content, including medical writing for content. A.A. Zimprich: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. H. Wiendl: drafting/revision of the manuscript for content, including medical writing for content. S.G. Meuth: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data.
Study Funding
The authors report no targeted funding.
Disclosure
S. Pfeuffer received travel grants from Roche, Biogen, Sanofi, and Merck; lecturing and consulting honoraria from Alexion, Hexal, Sanofi, Mylan, Merck, Biogen, Novartis, and Roche; and research support from Diamed, Merck, Biogen, Novartis, and the German Multiple Sclerosis Society North-Rhine-Westphalia. C. Nelke declares no conflicts of interest. M. Pawlitzki received honoraria for lecturing and travel expenses for attending meetings from Alexion, ArgenX, Bayer Health Care, Biogen, Hexal, Merck Serono, Novartis, Roche, Sanofi-Aventis, Takeda, and Teva. His research is funded by ArgenX, Biogen, Hexal and Novartis, all outside the scope of this study. C.B. Schroeter and C. Thomas declare no conflicts of interest. G. Kobbe had an advisory role or received speaker honoraria from MSD, Pfizer, Amgen, Novartis, Gilead, BMS-Celgene, Abbvie, Biotest, Takeda, Eurocept, JAZZ, and Medac; and received research funding from BMS-Celgene, Amgen, Abbvie, Eurocept, and Medac. S. Dietrich had an advisory role or received speaker honoraria from Roche and Gilead, and received research funding from Roche. A.A. Zimprich declares no conflicts of interest. H. Wiendl is a member of scientific advisory boards/steering committees for Bayer, Biogen, the health care business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva; received speaker honoraria and travel support from Bayer, Biogen, CSL Behring, EMD Serono, Fresenius Medical Care, Genzyme, the health care business of Merck KGaA (Darmstadt, Germany), Omniamed, Novartis, Sanofi, and Teva; received compensation as a consultant from Biogen, the health care business of Merck KGaA (Darmstadt, Germany), Novartis, Omniamed, Roche, and Sanofi; and has received research support from Bayer, Biogen, the health care business of Merck KGaA (Darmstadt, Germany), Novartis, Sanofi, Teva, as the German Ministry for Education and Research (BMBF), the German Research Foundation (DFG), the Else Kröner Fresenius Foundation, the Fresenius Foundation, the Hertie Foundation, the NRW Ministry of Education and Research, the Interdisciplinary Center for Clinical Studies (IZKF) Muenster, and the RE Children's Foundation. S.G. Meuth received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen Idec, Celgene, Diamed, Sanofi-Aventis, MedDay, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Bundesinstitut fuer Risikobewertung (BfR), Deutsche Forschungsgemeinschaft (DFG), the Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), the German Academic Exchange Service, the Hertie Foundation, the Interdisciplinary Center for Clinical Studies (IZKF) Muenster, the German Foundation Neurology, Alexion, Almirall, Amicus Therapeutics Germany, Biogen Idec, Diamed, Fresenius Medical Care, Sanofi-Aventis, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. Go to Neurology.org/NN for full disclosures.
References
- 1.Jamee M, Hosseinzadeh S, Sharifinejad N, et al. Comprehensive comparison between 222 CTLA-4 haploinsufficiency and 212 LRBA deficiency patients: a systematic review. Clin Exp Immunol. 2021;205(1):28-43. doi: 10.1111/cei.13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serwas NK, Hoeger B, Ardy RC, et al. Human DEF6 deficiency underlies an immunodeficiency syndrome with systemic autoimmunity and aberrant CTLA-4 homeostasis. Nat Commun. 2019;10(1):3106. doi: 10.1038/s41467-019-10812-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coustal C, Goulabchand R, Labauge P, et al. Clinical, radiologic, and immunologic features of patients with CTLA4 deficiency with neurologic involvement. Neurology. 2023;101(15):e1560-e1566. doi: 10.1212/WNL.0000000000207609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slatter MA, Engelhardt KR, Burroughs LM, et al. Hematopoietic stem cell transplantation for CTLA4 deficiency. J Allergy Clin Immunol. 2016;138(2):615-619.e1. doi: 10.1016/j.jaci.2016.01.045 [DOI] [PubMed] [Google Scholar]
- 5.Egg D, Rump IC, Mitsuiki N, et al. Therapeutic options for CTLA-4 insufficiency. J Allergy Clin Immunol. 2022;149(2):736-746. doi: 10.1016/j.jaci.2021.04.039 [DOI] [PubMed] [Google Scholar]
- 6.Nelke C, Schroeter CB, Theissen L, et al. Senescent fibro-adipogenic progenitors are potential drivers of pathology in inclusion body myositis. Acta Neuropathol. 2023;146(5):725-745. doi: 10.1007/s00401-023-02637-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korsunsky I, Millard N, Fan J, et al. Fast, sensitive and accurate integration of single-cell data with harmony. Nat Methods. 2019;16(12):1289-1296. doi: 10.1038/s41592-019-0619-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ianevski A, Giri AK, Aittokallio T. Fully-automated and ultra-fast cell-type identification using specific marker combinations from single-cell transcriptomic data. Nat Commun. 2022;13(1):1246. doi: 10.1038/s41467-022-28803-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schindler MK, Pittaluga S, Enose-Akahata Y, et al. Haploinsufficiency of immune checkpoint receptor CTLA4 induces a distinct neuroinflammatory disorder. J Clin Invest. 2020;130(10):5551-5561. doi: 10.1172/JCI135947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taghizade N, Babayeva R, Kara A, et al. Therapeutic modalities and clinical outcomes in a large cohort with LRBA deficiency and CTLA4 insufficiency. J Allergy Clin Immunol. 2023;152(6):1634-1645. doi: 10.1016/j.jaci.2023.08.004 [DOI] [PubMed] [Google Scholar]
- 11.Alroqi FJ, Charbonnier LM, Baris S, et al. Exaggerated follicular helper T-cell responses in patients with LRBA deficiency caused by failure of CTLA4-mediated regulation. J Allergy Clin Immunol. 2018;141(3):1050-1059.e10. doi: 10.1016/j.jaci.2017.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao Y, Miraghazadeh B, Chand R, et al. CTLA4 protects against maladaptive cytotoxicity during the differentiation of effector and follicular CD4(+) T cells. Cell Mol Immunol. 2023;20(7):777-793. doi: 10.1038/s41423-023-01027-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozec A, Luo Y, Engdahl C, Figueiredo C, Bang H, Schett G. Abatacept blocks anti-citrullinated protein antibody and rheumatoid factor mediated cytokine production in human macrophages in Ido-dependent manner. Arthritis Res Ther. 2018;20(1):24. doi: 10.1186/s13075-018-1527-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallikourdis M, Martini E, Carullo P, et al. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat Commun. 2017;8:14680. doi: 10.1038/ncomms14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uzel G, Keller B, Warnatz K. Hypogammaglobulinemia and immune dysregulation-not just 2 sides of a coin. J Allergy Clin Immunol. 2024;153(1):90-92. doi: 10.1016/j.jaci.2023.11.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on reasonable request from qualified investigators.