Abstract
The cytokine production patterns of human peripheral blood mononuclear cells (PBMC) in response to Salmonella typhi flagella (STF) were examined in culture supernatants of PBMC stimulated with STF. Consistent with previous findings in volunteers vaccinated with aroC aroD deletion mutants of S. typhi, PBMC from volunteers immunized with the licensed live Ty21a S. typhi vaccine secreted gamma interferon following exposure to STF. Stimulation with STF induced rapid de novo synthesis of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), followed by IL-6 and IL-10. Trypsin treatment of STF abrogated their effects, while polymyxin B had no effect. Intracellular cytokine measurements of STF-stimulated PBMC revealed the existence of monocyte subpopulations that produce only TNF-α, IL-1β or both cytokines. Moreover, STF markedly decreased the percentage of CD14+ cells. These data demonstrate that STF are powerful monocyte activators which may have important implications for vaccine development and for understanding the pathogenesis of S. typhi infection.
The gram-negative bacterium Salmonella typhi, a human host-restricted pathogen, is the causative agent of typhoid fever (17, 18, 22). After ingestion, S. typhi reaches the small intestine, where it quickly penetrates the epithelium. A clinically asymptomatic incubation period of 8 to 14 days ensues until clinical onset of typhoid fever, characterized by symptoms that include fever and malaise and by a sustained, low-level bacteremia (17, 18, 22). The mechanisms leading to the bacteremia associated with the onset of typhoid fever, which may include high bacterial load, bacterial virulence, and/or host-mediated immune responses, are not well defined. Proinflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) have been shown to play an important role in the development of fever and other symptoms associated with systemic inflammatory response syndrome (SIRS) (1, 8). These cytokines are released in response to gram-negative and other bacterial infections (1, 31). Lipopolysaccharide (LPS), a constituent of gram-negative bacterial cell walls, has been demonstrated to elicit the production of IL-1β, TNF-α, IL-6, and other cytokines (8, 10, 16). High concentrations of LPS associated with high bacteremia might lead to a severe form of SIRS referred to as endotoxic shock (1, 8). It has been shown that IL-6, as well as the proinflammatory cytokines IL-1β and TNF-α, are involved in the development of SIRS (1, 8). However, it is unlikely that fever and other symptoms of typhoid fever are caused by LPS, since most patients with typhoid do not exhibit increases in circulating levels of endotoxin and volunteers made LPS tolerant by repeated injections of endotoxin developed typhoid fever when challenged with virulent S. typhi (17). Although levels of some cytokines, such as IL-6, IL-8, and TNF-α, appear to be elevated during acute typhoid, the precise role of proinflammatory cytokines in induction and/or perpetuation of fever and other symptoms during typhoid fever remains largely undefined.
Recently, it has been demonstrated that LPS is not the only gram-negative bacterial constituent that elicits the production of proinflammatory cytokines. The ability of Salmonella typhimurium to elicit TNF-α production by human promonocytic cell lines and peripheral blood adherent cells was shown to depend on the expression of the fliC gene, which codes for flagella (5, 6). Furthermore, purified S. typhimurium porins were reported to cause human monocytes to produce TNF-α, IL-1α, IL-1β, IL-6, and gamma interferon (IFN-γ) (11, 12), and S. typhimurium was shown to induce the production of mRNA transcripts for granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1β, IL-6, macrophage inflammatory protein 1β (MIP-1β), and MIP-2 from mouse macrophages (34). The increase in GM-CSF mRNA transcript production, but not that of MIP-1β, was dependent upon S. typhimurium flagellum expression. No information concerning the effects of the human pathogen S. typhi on human monocytes by bacterial components other than LPS is available.
STF induction of cytokine production by human PBMC.
Peripheral blood mononuclear cells (PBMC) were isolated and cultured as previously described (32) from healthy naive volunteers or individuals who had been immunized with the licensed Ty21a vaccine, an attenuated flagellated S. typhi strain (Swiss Serum and Vaccine Institute, Berne, Switzerland). PBMC were cultured for 4 days in the absence or presence of S. typhi flagella (STF) or, as controls, tetanus toxoid (TT) (Wyeth, Marietta, Pa.), bovine serum albumin (BSA) (Fraction V; Sigma, St. Louis, Mo.), or the B-cell epitope malarial multiple repeat of asparagine-alanine-asparagine-proline (NANP50) (Hoffman-La Roche, Basel, Switzerland [15]). Supernatants were collected and the levels of IL-1β, TNF-α, IL-10, IFN-γ, and IL-4 were measured by chemiluminescence enzyme-linked immunosorbent assay (ELISA) following standard procedures (25). The sensitivity levels for the various cytokines tested ranged from 0.2 to 2 pg/ml. Purified STF was prepared by a bulk shearing method from the rough S. typhi strain Ty2R, as previously described (29, 32). This STF preparation consisted of a single flagellin band of ∼55 kDa in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, formed a single strong precipitate band with a rabbit anti-Ty2R flagellar antiserum in an Ouchterlony, and contained less than 24 pg of LPS per ml, as determined by using chromogenic Limulus amebocyte lysate kits (level of sensitivity, 24 pg/ml) (Associates of Cape Cod, Inc.).
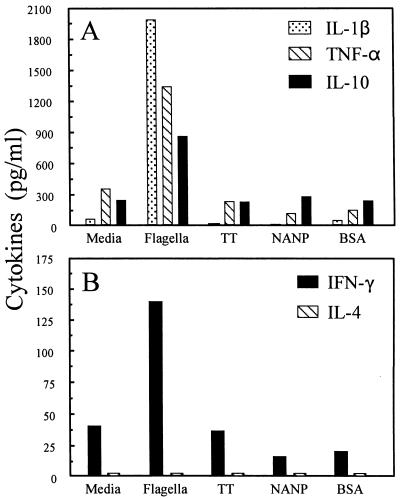
We observed that incubation with STF for 4 days induced high levels (up to 2,000 pg/ml) of the proinflammatory cytokines IL-1β and TNF-α (Fig. 1A), as well as of IL-6 (data not shown). Production of these cytokines was not observed in the presence of the malarial antigen NANP, TT, or BSA (Fig. 1A). Elevated levels of IFN-γ and IL-10, but not IL-4 (Fig. 1), were also induced by STF but not by control antigens. Dose-response studies showed that following 24 h of exposure to antigen, production of both IL-1β and TNF-α was observed in PBMC cultures exposed to concentrations of STF as low as 4 ng/ml (data not shown). Experiments demonstrating that the LPS inhibitor polymyxin B did not affect the ability of STF to induce cytokine production (data not shown), as well as the demonstration that trypsin digestion of STF abrogated its activity (data not shown), confirmed that the induction of proinflammatory cytokines by STF was not the result of LPS present in the STF preparation.
FIG. 1.
Induction of cytokine production by human PBMC after exposure to STF. Cytokine production was measured in culture supernatants 4 days after incubation in the absence (media) or presence of STF (4 μg/ml), TT (2 μg/ml), the malarial repeat antigen NANP (4 μg/ml), or BSA (4 μg/ml). These results are representative of those observed in 10 individual volunteers, all of whom were immunized orally with the attenuated Ty21a S. typhi strain.
Time course of cytokine production after stimulation with STF by PBMC isolated from naive volunteers or individuals immunized with the Ty21a typhoid vaccine and requirement for de novo protein synthesis.
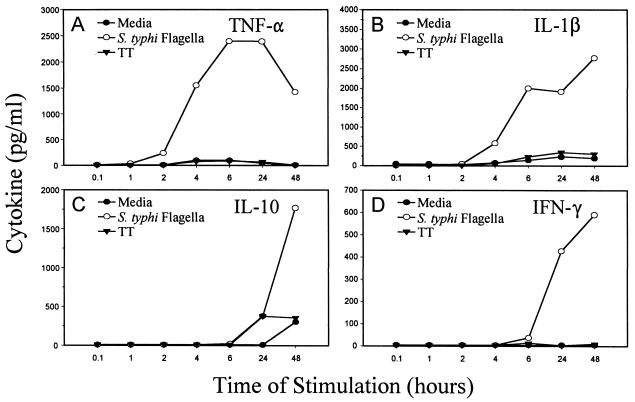
To determine the earliest time at which cytokine production induced by STF could be detected, PBMC from three volunteers immunized with the Ty21a attenuated typhoid oral vaccine were cultured in the absence or presence of STF or TT. Supernatants were collected at 5 min and at 1, 2, 4, 6, 24, and 48 h after addition of the antigen and examined for the presence of IL-1β, TNF-α, IL-10, and IFN-γ. TNF-α was the first cytokine to be detected in the supernatants of cultures stimulated with STF, appearing between 2 and 4 h after stimulation (Fig. 2A). This was followed at 4 to 6 h by production of IL-1β (Fig. 2B). IL-10 production could not be detected until 24 h after stimulation (Fig. 2C). Similar to IL-10 production, IFN-γ secretion was generally not seen until 24 h (Fig. 2D) and tended to have higher variability among volunteers. This is consistent with IFN-γ being a T-cell-derived cytokine, and the variability may reflect the level of S. typhi Ty21a vaccine-induced priming in the individual volunteers. Finally, it was observed that by 48 h, production of TNF-α began to decrease, IL-1β remained constant, and, in contrast, both IFN-γ and IL-10 continued to increase (Fig. 2C and D). Although the cellular origin of IL-10 was not determined, IL-10, produced by cells of the monocyte/macrophage lineage and late in antigen responses by T cells, is an important down-regulator of the immune response (7). Thus, it is possible that late production of IL-10 results in down-regulation of the high levels of proinflammatory cytokines produced very quickly after exposure to STF.
FIG. 2.
Time course of secretion of cytokines after exposure to STF. Human PBMC were cultured in the absence (media) or presence of STF (4 μg/ml) or TT (2 μg/ml). Culture supernatants were collected at various time points, and the concentrations of TNF-α, IL-1β, IL-10, and IFN-γ were determined. These results are representative of those observed with cells obtained from three different individuals immunized orally with the attenuated Ty21a S. typhi strain.
We next investigated whether STF was able to induce production of proinflammatory cytokines in PBMC from unvaccinated volunteers to an extent similar to that observed in cells from Ty21a vaccinees. Results from nine individual volunteers (five vaccinated and four unvaccinated volunteers) tested in parallel showed that following 6 h of exposure to STF, a time at which IFN-γ could not be detected in culture supernatants from immunized volunteers (Fig. 2), significant increases (P < 0.01) in the levels of IL-1β and TNF-α production were observed in both vaccinated and naive individuals (data not shown). IFN-γ is known to be an activator of cells from the monocyte/macrophage lineage that regulates production of proinflammatory cytokines (8), and elevated levels of IFN-γ in serum have been associated with febrile states (2, 14, 30). However, the possibility that IFN-γ production induced by STF plays a significant role in the febrile state characteristic of typhoid fever is unlikely, since the observation that IFN-γ does not reach measurable levels in culture supernatants from immunized volunteers during the first 24 h following exposure to STF rules out the possibility that IFN-γ released by sensitized T lymphocytes induces release of proinflammatory cytokines by monocytes/macrophages in vitro. Moreover, the observation that volunteers immunized with the licensed live oral Ty21a attenuated S. typhi strain also secreted IFN-γ, but not IL-4, following exposure to STF confirms and extends our previous observations on the immune responses elicited in volunteers vaccinated with aroC aroD double deletion mutants of S. typhi (28, 29).
To determine if the production of proinflammatory cytokines by STF was due to the release of preformed cytokines or to de novo protein synthesis, the protein synthesis inhibitors actinomycin D and cycloheximide were added to PBMC cultures in the presence of STF, and IL-1β and TNF-α levels were measured in culture supernatants. We observed that both inhibitors completely abrogated the ability of STF to induce proinflammatory cytokines, indicating that de novo cytokine production was required (data not shown).
Production of proinflammatory cytokines by monocyte cell subsets.
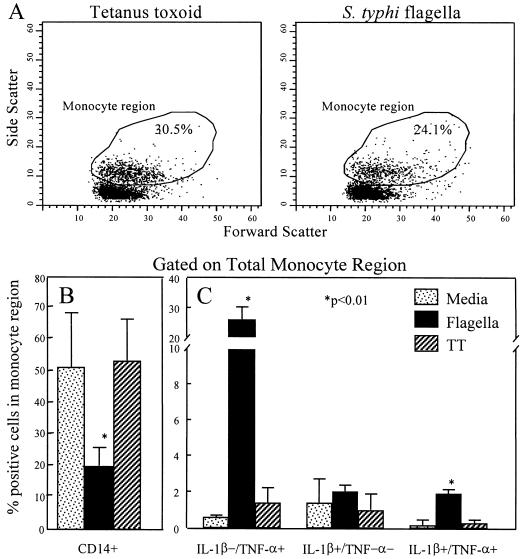
The kinetics of the response and the ability of PBMC from naive and vaccinated individuals to produce proinflammatory cytokines suggest an innate monocyte response. To study this phenomenon in more detail, flow cytometric analysis was carried out to determine whether all monocytes or a subset of these cells were involved in production of proinflammatory cytokines and whether the same cells produce both TNF-α and IL-1β. Intracellular cytokine content was determined by flow cytometry as described previously (9, 20). Briefly, following culture, cells were collected and stained for the surface molecule CD14 by using an allophycocyanin-labeled anti-CD14 monoclonal antibody (Caltag, Burlingame, Calif.), fixed with 4% formaldehyde, permeabilized with 0.1% saponin, and stained intracellularly with fluorescein isothiocyanate-labeled anti-IL-1β and phycoerythrin-labeled anti-TNF-α monoclonal antibodies (Pharmingen). Fluorochrome-labeled monoclonal antibodies of the same isotypes but irrelevant specificities were used as controls. Data are presented as two-color cytograms of IL-1β versus TNF-α of cells gated on the monocyte region (Fig. 3A). In agreement with ELISA data, intracellular cytokine staining showed that by 5 h there was an induction of TNF-α and IL-1β production in cells stimulated with STF (Fig. 3). Of note, incubation with STF resulted in a 50 to 70% decrease in the percentage of cells within the monocyte region expressing CD14 (Fig. 3B) in STF cultures relative to those incubated in the absence (media) or presence of TT (Fig. 3B). These observations are in contrast to reports showing that incubation of monocytes with LPS increases the level of CD14 expression (21), thus indicating an important distinction between the biological effects of LPS and STF. This reduction of CD14+ cells in the presence of STF might be the result of CD14 being modified in such a way that it is no longer recognized by the anti-CD14 monoclonal antibody used in these studies, CD14 being internalized at an accelerated rate or released into the supernatant as soluble CD14. Experiments directed to explore these possibilities appear to rule out the possibility that STF promotes the release of CD14 into the supernatant. While exposure to LPS increased the levels of sCD14 in culture supernatants, no increases in soluble CD14 were observed in supernatants of cell cultures exposed to STF (33).
FIG. 3.
STF induction of increased intracellular levels of IL-1β and/or TNF-α in human monocytes. The percentages of cells containing intracellular IL-1β and TNF-α were determined in PBMC from four individuals 5 h after addition of STF (4 μg/ml) or TT (2 μg/ml). Data are mean percentages of positive cells ± standard deviation (SD) from the four volunteers. (A) Representative example of the high forward scatter/high side scatter region which was used to define the monocyte population. In the experiment shown, 30.5% of the total cells were contained in the monocyte region after stimulation with TT, while 24.1% of the cells were contained within the monocyte region after STF stimulation. In this experiment, the percentage of cells contained within the monocyte region in unstimulated cultures (media) was 35.0%. (B) Percentage of CD14+ cells within the monocyte region in unstimulated cultures (media) or after STF or TT stimulation. (C) Mean percentage of cells containing IL-1β and/or TNF-α ± SD in cells within the monocyte region in the four volunteers. Results shown correspond to four different volunteers, two unvaccinated and two immunized with the attenuated Ty21a S. typhi strain. Asterisks denote P of <0.01 by paired t test, compared with untreated cultures or cultures incubated with TT; two-tailed P values are shown.
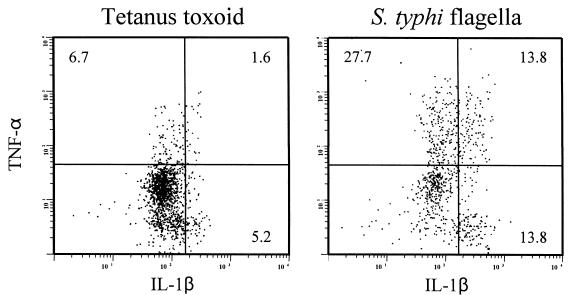
In the presence of the Golgi blocker monensin, stimulation with STF resulted in significant increases in the percentage of cells in the monocyte scatter region (Fig. 3A) expressing TNF-α but not IL-1β (TNF-α+ IL-1β− cells) (26%) and in IL-1β+ TNF-α+ cells (1.9%) (Fig. 3C). In these cultures, IL-1β alone (TNF-α− IL-1β+ cells) was expressed in ∼2% of the cells in the monocyte region. In contrast, when stimulated with TT, only 1.4% of the cells of the monocyte region were TNF-α+ IL-1β−, while both TNF-α− IL-1β+ and TNF-α+ IL-1β+ double-positive cells were present in <1% of the cells. To enhance the detection of IL-1β, whose release is not prevented by monensin, similar experiments were performed in the presence of methylamine (a blocker of IL-1β secretion [26]). In the presence of methylamine and monensin, a higher percentage of cells in the monocyte region (∼14%) became TNF-α+ IL-1β+ or TNF-α− IL-1β+, while ∼28% of the cells were TNF-α+ IL-1β− after exposure to STF (Fig. 4). These percentages of IL-1β-containing cells were markedly higher than those observed in cultures with monensin alone (Fig. 3), indicating that the addition of methylamine resulted in an increase in our ability to identify IL-1β-producing cells. The fact that in the presence of both monensin and methylamine large proportions of cells contained either one or both cytokines suggests that different cell subsets might produce predominantly either IL-1β or TNF-α. Alternatively, these observations might result, at least in part, from differences in the kinetics of cytokine production by individual cells.
FIG. 4.
Flow cytometric analysis showing distribution of human PBMC containing IL-1β and/or TNF-α induced by STF or TT. PBMC from a volunteer immunized with the attenuated Ty21a S. typhi strain were incubated for 5 h with methylamine plus monensin in the absence or presence of TT (2 μg/ml) (left) or STF (4 μg/ml) (right). Cells were then stained intracellularly for IL-1β and TNF-α. Cytograms showing the distribution of cells within the monocyte region containing either IL-1β (lower right quadrants), TNF-α (upper left quadrants), or both cytokines (upper right quadrants) are presented. The percentage of positive cells in each of the quadrants is shown. These results are representative of those observed with cells obtained from three different individuals, one unvaccinated and two immunized with the attenuated Ty21a S. typhi strain.
In sum, we have demonstrated that STF induces de novo secretion of high levels of IL-6 and the proinflammatory cytokines IL-1β and TNF-α, as well as the anti-inflammatory cytokine IL-10, by PBMC from naive volunteers or individuals immunized with the oral typhoid vaccine Ty21a. Interleukin-1β and TNF-α, which have been shown to play major roles in the induction and persistence of fever (31), were rapidly produced (within 6 h) by monocytes at high levels in the presence of STF. These results are consistent with those showing that S. typhimurium components other than LPS (e.g., flagellin and porins) are potent inducers of cytokine production in vitro (5, 6, 11, 12, 34) and with the observations that cytokines that are involved in the induction of fever, including IL-6, IL-8, and TNF-α as well as anti-inflammatory molecules such as soluble TNF-R and IL-1R antagonist, are elevated during acute typhoid (2, 19).
It has been proposed that TNF-α, IL-1β, and IL-6 production induced by LPS from gram-negative bacteria causes fever and other symptoms associated with SIRS in septic individuals (1, 8). Based on our findings, we hypothesize that similar mechanisms might be operational in SIRS associated with typhoid fever. In fact, the observations that the production of high levels of IL-1β, TNF-α, and IL-6 are elicited by STF, and likely other S. typhi components as well, suggest that in a gram-negative bacterium-infected septic individual more than one pathway to SIRS may be operating. Since it is unlikely that fever and other symptoms observed during acute typhoid are caused by LPS (17), it can be hypothesized that organisms such as S. typhi, which persist in the reticuloendothelial system, release bacterial components other than LPS that might be able to induce SIRS without the release of LPS. Alternatively, the combined release of LPS, STF, porins, and other, as-yet-unidentified bacterial components may act in concert to trigger the cascade of events that ultimately leads to SIRS. Many of the efforts in the past to protect against SIRS have focused on interfering with the binding of LPS to LPS-binding protein and/or to CD14 molecules on the surface of monocytes/macrophages (13, 24). However, the data presented in this paper, as well as results from other groups (11, 12, 34), add further support for other strategies being explored in the treatment of SIRS, such as the use of anti-proinflammatory cytokines, inhibition of cytokine synthesis or processing, specific IL-1R blockade with IL-1R blockade with IL-1R antagonist, or administration of soluble cytokine receptors (8, 13).
Vaccines designed to elicit strong host immune responses to a defined antigen(s) are currently under development. Among these are vaccines utilizing bacterial vectors such as attenuated S. typhi (4, 23) and STF carrying foreign antigens (27). In conjunction with genes encoding foreign antigens, cytokine genes are also being added to modify the response to the antigens in a desired, protective way (3). It is therefore of critical importance to fully understand the immune response to such vectors. The strong proinflammatory response in the presence of STF demonstrated here suggests that bacterial components of attenuated live vector vaccines, such as S. typhi, may profoundly affect the immune responses to both the vector and the foreign antigen through the production of an array of potent immunoregulatory cytokines. Further research into this possibility will undoubtedly impact the design of future vaccines based on live vector vaccines. We have described here an initial characterization of the cytokine responses to only one of many bacterial constituents that elicit immune responses. A complete understanding of the human immune response to vaccination with attenuated live vector vaccines will allow researchers to more accurately predict the outcome of immunization with live vector vaccines or combinations of antigens and cytokines.
Acknowledgments
This work was supported by grant RO1 AI36525 from the National Institute of Allergy and Infectious Diseases, NIH.
We thank Myron M. Levine and Alan S. Cross for critical review of the manuscript. We also thank David Maneval for assistance in preparing the STF antigen.
REFERENCES
- 1.Bone R C. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome) JAMA. 1992;268:3452–3455. [PubMed] [Google Scholar]
- 2.Butler T, Ho M, Acharya G, Tiwari M, Gallati H. Interleukin-6, gamma interferon, and tumor necrosis factor receptors in typhoid fever related to outcome of antimicrobial therapy. Antimicrob Agents Chemother. 1993;37:2418–2421. doi: 10.1128/aac.37.11.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrier M J, Chatfield S N, Dougan G, Nowicka U T, O’Callaghan D, Beesley J E, Milano S, Cillari E, Liew F Y. Expression of human IL-1 beta in Salmonella typhimurium. A model system for the delivery of recombinant therapeutic proteins in vivo. J Immunol. 1992;148:1176–1181. [PubMed] [Google Scholar]
- 4.Chatfield S N, Dougan G. Attenuated Salmonella as a live vector for expression of foreign antigens. Part i. Expressing bacterial antigens. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 331–341. [Google Scholar]
- 5.Ciacci-Woolwine F, Blomfield I C, Richardson S H, Mizel S B. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect Immun. 1998;66:1127–1134. doi: 10.1128/iai.66.3.1127-1134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciacci-Woolwine F, Kucera L S, Richardson S H, Iyer N P, Mizel S B. Salmonellae activate tumor necrosis factor alpha production in a human promonocytic cell line via a released polypeptide. Infect Immun. 1997;65:4624–4633. doi: 10.1128/iai.65.11.4624-4633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, De Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello C A, Gelfand J A, Wolff S M. Anticytokine strategies in the treatment of the systemic inflammatory response syndrome. JAMA. 1993;269:1829–1835. [PubMed] [Google Scholar]
- 9.Elson L H, Nutman T B, Metcalfe D D, Prussin C. Flow cytometric analysis for cytokine production identifies T helper 1, T helper 2, and T helper 0 cells within the human CD4+ CD27− lymphocyte subpopulation. J Immunol. 1995;154:4294–4301. [PubMed] [Google Scholar]
- 10.Engervall P, Granstrom M, Andersson B, Kalin M, Bjorkholm M. Endotoxemia in febrile patients with hematological malignancies. Relationship of type of bacteremia, clinical findings and serum cytokine pattern. Infection. 1997;25:2–7. doi: 10.1007/BF02113498. [DOI] [PubMed] [Google Scholar]
- 11.Galdiero F, Cipollaro de L’Ero G, Benedetto N, Galdiero M, Tufano M A. Release of cytokines induced by Salmonella typhimurium porins. Infect Immun. 1993;61:155–161. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galdiero M, Cipollaro de L’Ero G, Donnarumma G, Marcatili A, Galdiero F. Interleukin-1 and interleukin-6 gene expression in human monocytes stimulated with Salmonella typhimurium porins. Immunology. 1995;86:612–619. [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie E M, Kruse-Elliott K. Endotoxic shock. Part II. A review of treatment. J Vet Intern Med. 1990;4:306–314. doi: 10.1111/j.1939-1676.1990.tb03128.x. [DOI] [PubMed] [Google Scholar]
- 14.Harpaz R, Edelman R, Wasserman S S, Levine M M, Davis J R, Sztein M B. Serum cytokine profiles in experimental human malaria. Relationship to protection and disease course after challenge. J Clin Investig. 1992;90:515–523. doi: 10.1172/JCI115889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrington D A, Clyde D F, Losonsky G, Cortesia M, Murphy J R, Davis J, Baqar S, Felix A M, Heimer E P, Gillessen D, et al. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987;328:257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- 16.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman S L. Typhoid fever. In: Strickland G T, editor. Hunter’s tropical medicine. W. B. Philadelphia, Pa: Saunders Company; 1991. pp. 344–359. [Google Scholar]
- 18.Jones B D, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 19.Keuter M, Dharmana E, Gasem M H, van der Ven-Jongekrijg J, Djokomoeljanto R, Dolmans W M, Demacker P, Sauerwein R, Gallati H, van der Meer J W. Patterns of proinflammatory cytokines and inhibitors during typhoid fever. J Infect Dis. 1994;169:1306–1311. doi: 10.1093/infdis/169.6.1306. [DOI] [PubMed] [Google Scholar]
- 20.Kierszenbaum F, Mejia Lopez H, Tanner M K, Sztein M B. Trypanosoma cruzi-induced decrease in the level of interferon-gamma receptor expression by resting and activated human blood lymphocytes. Parasite Immunol. 1995;17:207–214. doi: 10.1111/j.1365-3024.1995.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 21.Landmann R, Knopf H P, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine M M. Typhoid fever vaccines. In: Plotkin S A, Mortimer E A, editors. Vaccines. W. B. Philadelphia, Pa: Saunders Company; 1994. pp. 597–633. [Google Scholar]
- 23.Levine M M, Galen J E, Sztein M B, Beier M, Noriega F R. Attenuated Salmonella as a live vector for expression of foreign antigens. Part iii. Salmonella expressing protozoal antigens. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 351–361. [Google Scholar]
- 24.Lugowski C. Immunotherapy in gram-negative bacterial infections. Acta Biochim Pol. 1995;42:19–24. [PubMed] [Google Scholar]
- 25.Pasetti, M. F., R. J. Anderson, F. R. Noriega, M. M. Levine, and M. B. Sztein. Attenuated ΔguaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin. Immunol., in press. [DOI] [PubMed]
- 26.Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1β, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stocker B A, Newton S M. Immune responses to epitopes inserted in Salmonella flagellin. Int Rev Immunol. 1994;11:167–178. doi: 10.3109/08830189409061724. [DOI] [PubMed] [Google Scholar]
- 28.Sztein M B, Tanner M, Polotsky Y, Orenstein J M, Levine M M. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J Immunol. 1995;155:3987–3993. [PubMed] [Google Scholar]
- 29.Sztein M B, Wasserman S S, Tacket C O, Edelman R, Hone D, Lindberg A A, Levine M M. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 30.Waage A, Steinshamn S. Cytokine mediators of septic infections in the normal and granulocytopenic host. Eur J Haematol. 1993;50:243–249. doi: 10.1111/j.1600-0609.1993.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 31.Watkins L R, Maier S F, Goehler L E. Immune activation: the role of proinflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- 32.Wyant T L, Tanner M K, Sztein M B. Potent immunoregulatory effects of Salmonella typhi flagella on antigenic stimulation of human peripheral blood mononuclear cells. Infect Immun. 1999;67:1338–1346. doi: 10.1128/iai.67.3.1338-1346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyant, T. L., M. K. Tanner, and M. B. Sztein. Unpublished data.
- 34.Yamamoto Y, Klein T W, Friedman H. Induction of cytokine granulocyte-macrophage colony-stimulating factor and chemokine macrophage inflammatory protein 2 mRNAs in macrophages by Legionella pneumophila or Salmonella typhimurium attachment requires different ligand-receptor systems. Infect Immun. 1996;64:3062–3068. doi: 10.1128/iai.64.8.3062-3068.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]