Abstract
1. Explants of mammary gland from mid-pregnant rabbits were cultured with insulin, prolactin and cortisol. 2. Antibodies raised to fatty acid synthetase were used to measure the amount as well as the rate of synthesis and the rate of degradation of the enzyme in the explants over defined periods in organ culture. These measurements were also made after the hormones had been removed from the culture medium. The changes which occur in the activity of fatty acid synthetase are due to changes in the amount of the enzyme present. They are not due to activation or inactivation of the enzyme. 3. The rate of lipogenesis (measured from [1-14C]acetate) in the explants during culture varies independently of the amount of fatty acid synthetase both in the presence and after removal of the hormones. Hence the amount of fatty acid synthetase does not limit lipogenesis. The proportion of medium-chain fatty acids C8:0 and C10:0 (which are characteristic of rabbit milk) synthesized by the explants in the presence of hormones increases at about the same rate as the amount of fatty acid synthetase present. However, when hormones are removed from the medium the proportion of these acids synthesized declines as rapidly as the rate of lipogenesis and not as the amount of fatty acid synthetase presen. 4. The rates of synthesis of fatty acid synthetase and of the total particulate-free supernatant protein in the explants were compared by measuring the incorporation of L-[U-14C]leucine into the protein of the explants. These rates increase by 5-fold and 3.6-fold respectively when explants are cultured with hormones, and they then reach approximately constant rates. When the hormones are removed there is a rapid fall in the rate of synthesis of fatty acid synthetase and of the total particulate-free supernatant protein to values which are similar to those obtained with freshly prepared explanted tissue. 5. In unstimulated explants fatty acid synthetase appears to be degraded with a half-life of 15-21h. During the hormonally stimulated differentiation of the tissue the rate of degradation of the enzyme is considerably decreased or is switched off completely. After the amount of fatty acid synthetase has increased to a maximum the enzyme complex is again degraded with a half-life of 23-29h. The removal of hormones after the explants have been hormonally stimulated for different times results in an increase in the rate of degradation of fatty acid synthetase. However, this increase only occurs if degradation was previously proceeding at a considerably decreased rate. The degradation of the total particulate-free supernatant protein continues throughout the period of differentiation of the explant tissue in culture. It appears to be somewhat decreased during the period of rapid maturation of the tissue during culture.
Full text
PDF



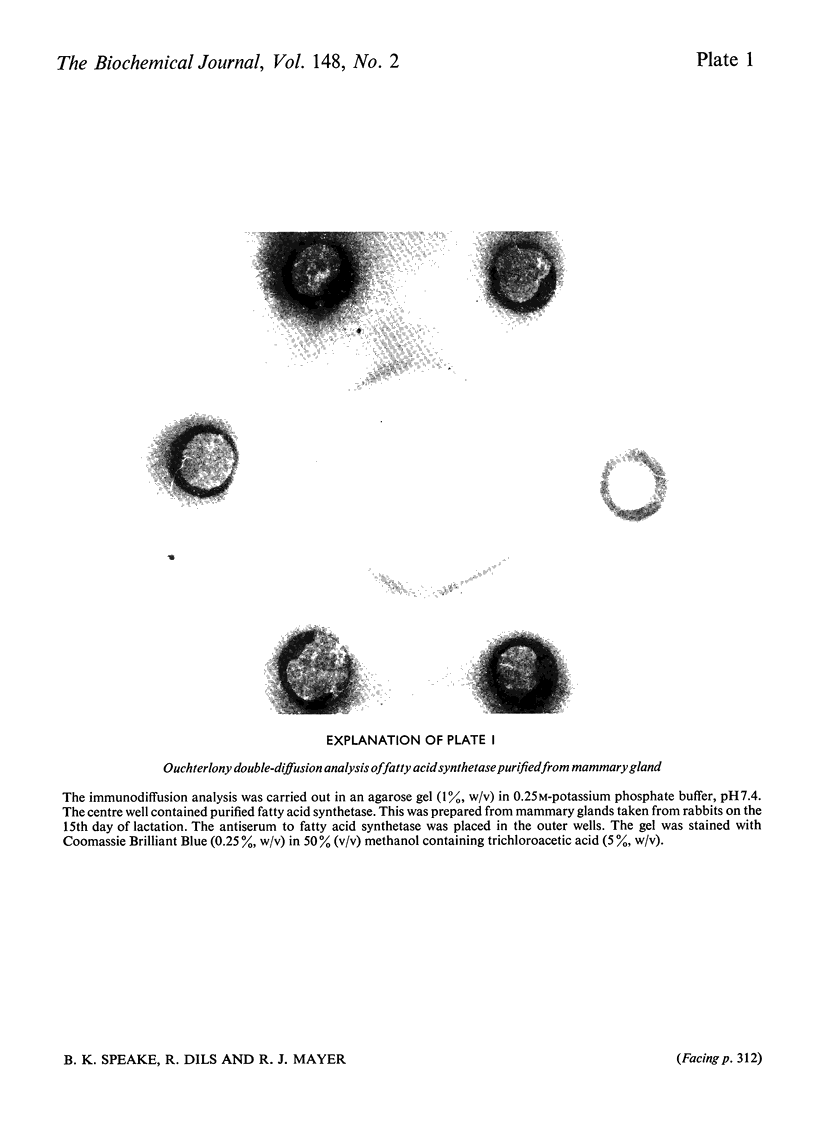








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLMANN D. W., HUBBARD D. D., GIBSON D. M. FATTY ACID SYNTHESIS DURING FAT-FREE REFEEDING OF STARVED RATS. J Lipid Res. 1965 Jan;6:63–74. [PubMed] [Google Scholar]
- Bousquet M., Fléchon J. E., Denamur R. Aspects ultrastructuraux de la glande mammaire de lapine pendant la lactogénèse. Z Zellforsch Mikrosk Anat. 1969;96(3):418–436. [PubMed] [Google Scholar]
- Burton D. N., Collins J. M., Kennan A. L., Porter J. W. The effects of nutritional and hormonal factors on the fatty acid synthetase level of rat liver. J Biol Chem. 1969 Aug 25;244(16):4510–4516. [PubMed] [Google Scholar]
- Carey E. M., Dils R. Fatty acid biosynthesis. V. Purification and characterisation of fatty acid synthetase from lactating-rabbit mammary gland. Biochim Biophys Acta. 1970 Sep 8;210(3):371–387. doi: 10.1016/0005-2760(70)90033-0. [DOI] [PubMed] [Google Scholar]
- Carey E. M., Dils R. The pattern of fatty acid synthesis in lactating rabbit mammary gland studied in vivo. Biochem J. 1972 Feb;126(4):1005–1007. doi: 10.1042/bj1261005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig M. C., Nepokroeff C. M., Lakshmanan M. R., Porter J. W. Effect of dietary change on the rates of synthesis and degradation of rat liver fatty acid synthetase. Arch Biochem Biophys. 1972 Oct;152(2):619–630. doi: 10.1016/0003-9861(72)90258-5. [DOI] [PubMed] [Google Scholar]
- Forsyth I. A., Myres R. P. Human prolactin. Evidence obtained by the bioassay of human plasma. J Endocrinol. 1971 Sep;51(1):157–168. doi: 10.1677/joe.0.0510157. [DOI] [PubMed] [Google Scholar]
- Forsyth I. A. Reviews of the progress of dairy science. Section A. Physiology. Organ culture techniques and the study of hormone effects on the mammary gland. J Dairy Res. 1971 Oct;38(3):419–444. [PubMed] [Google Scholar]
- Forsyth I. A., Strong C. R., Dils R. Interactions of insulin, corticosterone and prolactin in promoting milk-fat synthesis by mammary explants from pregnant rabbits. Biochem J. 1972 Oct;129(4):929–935. doi: 10.1042/bj1290929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON D. M., HUBBARD D. D. Incorporation of malonyl CoA into fatty acids by liver in starvation and alloxan-diabetes. Biochem Biophys Res Commun. 1960 Nov;3:531–535. doi: 10.1016/0006-291x(60)90169-8. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Malamud D. Decreased protein catabolism during stimulated growth. FEBS Lett. 1974 Sep 15;46(1):308–311. doi: 10.1016/0014-5793(74)80394-7. [DOI] [PubMed] [Google Scholar]
- Hübscher G., West G. R., Brindley D. N. Studies on the fractionation of mucosal homogenates from the small intestine. Biochem J. 1965 Dec;97(3):629–642. doi: 10.1042/bj0970629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. D., Walther B. T., Rutter W. J. Protein synthesis during the secondary developmental transition of the embryonic rat pancreas. J Biol Chem. 1972 Jun 25;247(12):3941–3952. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYNEN F., HOPPER-KESSEL I., EGGERER H. ZUR BIOSYNTHESE DER FETTSAEUREN. 3. DIE FETTSAEURENSYNTHETASE DER HEFE UND DIE BILDUNG ENZYMGEBUNDENER ACETESSIGSAEURE. Biochem Z. 1964 Jul 8;340:95–124. [PubMed] [Google Scholar]
- Lakshmanan M. R., Nepokroeff C. M., Porter J. W. Control of the synthesis of fatty-acid synthetase in rat liver by insulin, glucagon, and adenosine 3':5' cyclic monophosphate. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3516–3519. doi: 10.1073/pnas.69.12.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- Mayne R., Barry J. M. Biochemical changes during development of mouse mammary tissue in organ culture. J Endocrinol. 1970 Jan;46(1):61–70. doi: 10.1677/joe.0.0460061. [DOI] [PubMed] [Google Scholar]
- Moretti R. L., Abraham S. Effects of insulin on glucose metabolism by explants of mouse mammary gland maintained in organ culture. Biochim Biophys Acta. 1966 Aug 24;124(2):280–288. doi: 10.1016/0304-4165(66)90191-7. [DOI] [PubMed] [Google Scholar]
- Mori T., Kitagawa H., Riggs T. R. Action of insulin on amino acid uptake by the immature rat uterus in vitro. Biochim Biophys Acta. 1974 Sep 6;363(2):249–260. doi: 10.1016/0005-2736(74)90064-9. [DOI] [PubMed] [Google Scholar]
- Murphy G., Walker D. G. Enzyme synthesis in the regulation of hepatic "malic" enzyme activity. Biochem J. 1974 Oct;144(1):149–160. doi: 10.1042/bj1440149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens I. S., Vonderhaar B. K., Topper Y. J. Concerning the necessary coupling of development to proliferation of mouse mammary epithelial cells. J Biol Chem. 1973 Jan 25;248(2):472–477. [PubMed] [Google Scholar]
- Philippidis H., Ballard F. J. The development of gluconeogenesis in rat liver: experiments in vivo. Biochem J. 1969 Jul;113(4):651–657. doi: 10.1042/bj1130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidis H., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. The initial synthesis of proteins during development. Phosphoenolpyruvate carboxylase in rat liver at birth. Biochem J. 1972 Mar;126(5):1127–1134. doi: 10.1042/bj1261127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B. The kinetics of disappearance of labeled leucine from the free leucine pool of rat liver and its effect on the apparent turnover of catalase and other hepatic proteins. J Biol Chem. 1971 Nov;246(21):6587–6591. [PubMed] [Google Scholar]
- Rutter W. J., Pictet R. L., Morris P. W. Toward molecular mechanisms of developmental processes. Annu Rev Biochem. 1973;42:601–646. doi: 10.1146/annurev.bi.42.070173.003125. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Effects of altered dietary and hormonal conditions. Biochem J. 1970 Sep;119(2):221–242. doi: 10.1042/bj1190221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. Hormonal regulation of amino acid accumulation in human fetal liver explants. Effects of dibutyryl cyclic AMP, glucogon and insulin. Biochim Biophys Acta. 1974 Sep 5;362(2):276–289. doi: 10.1016/0304-4165(74)90220-7. [DOI] [PubMed] [Google Scholar]
- Scornik O. A. Decreased in vivo disappearance of labelled liver protein after partial hepatectomy. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1063–1066. doi: 10.1016/0006-291x(72)90941-2. [DOI] [PubMed] [Google Scholar]
- Silpananta P., Goodridge A. G. Synthesis and degradation of malic enzyme in chick liver. J Biol Chem. 1971 Sep 25;246(18):5754–5761. [PubMed] [Google Scholar]
- Smith S., Easter D. J., Dils R. Fatty acid biosynthesis. 3. Intracellular site of enzymes in lactating-rabbit mammary gland. Biochim Biophys Acta. 1966 Dec 7;125(3):445–455. [PubMed] [Google Scholar]
- Strong C. R., Carey E. M., Dils R. The synthesis of medium-chain fatty acids by lactating-rabbit mammary gland studied in vitro. Biochem J. 1973 Jan;132(1):121–123. doi: 10.1042/bj1320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong C. R., Dils R. Fatty acid biosynthesis in rabbit mammary gland during pregnancy and early lactation. Biochem J. 1972 Aug;128(5):1303–1309. doi: 10.1042/bj1281303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong C. R., Forsyth I., Dils R. The effects of hormones on milk-fat synthesis in mammary explants from pseudopregnant rabbits. Biochem J. 1972 Jul;128(3):509–519. doi: 10.1042/bj1280509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J. J., Lyles T. O., Roncari D. A., Vagelos P. R. Fatty acid synthetase of developing brain and liver. Content, synthesis, and degradation during development. J Biol Chem. 1973 Apr 10;248(7):2502–2513. [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Regulation of mammalian fatty-acid synthetase. The roles of carbohydrate and insulin. Proc Natl Acad Sci U S A. 1974 Mar;71(3):889–893. doi: 10.1073/pnas.71.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKIL S. J., TITCHENER E. B., GIBSON D. M. Evidence for the participation of biotin in the enzymic synthesis of fatty acids. Biochim Biophys Acta. 1958 Jul;29(1):225–226. doi: 10.1016/0006-3002(58)90177-x. [DOI] [PubMed] [Google Scholar]
- Wallyn C. S., Vidrich A., Airhart J., Khairallah E. A. Analysis of the specific radioactivity of valine isolated from aminoacyl-transfer ribonucleic acid of rat liver. Biochem J. 1974 Jun;140(3):545–548. doi: 10.1042/bj1400545. [DOI] [PMC free article] [PubMed] [Google Scholar]



