Abstract
We have previously shown by freeze-fracture electron microscopy that serum from infection-immune syphilitic rabbits aggregates the low-density membrane-spanning Treponema pallidum rare outer membrane proteins (TROMPs). The purpose of this study was to determine if a relationship could be demonstrated between acquired immunity in experimental rabbit syphilis, serum complement-dependent treponemicidal antibody, and antibody directed against TROMPs as measured by the aggregation of TROMP particles. Three groups of T. pallidum-infected rabbits were treated curatively with penicillin at 9 days, 30 days, and 6 months postinfection to generate various degrees of immunity to challenge reinfection. Sera from rabbits completely susceptible to localized and disseminated reinfection possessed a low titer of treponemicidal antibody (≤1:1 in killing ≥50% of a treponemal suspension) and showed a correspondingly low level of TROMP aggregation (16.5% of the total number of outer membrane particles counted) similar to normal serum controls (13.4%); the number of particles within these aggregates never exceeded three. Sera from partially immune rabbits, which were susceptible to local reinfection but had no evidence of dissemination, showed an increase in the titer of treponemicidal antibody (1:16) compared to the completely susceptible group (≤1:1). Although no significant increase was observed in the total number of TROMP particles aggregated (18.9%) compared to the number in controls (13.4%), approximately 15% of these aggregates did exhibit a significant increase in the number of particles per aggregate (4 to 5 particles) compared to controls (≤3 particles), indicating a measurable increase in anti-TROMP antibody. Finally, sera from rabbits completely immune to both local and disseminated reinfection possessed both high titers of treponemicidal antibody (1:128) and significant aggregation of TROMP (88.6%); approximately 50% of these aggregates contained four to six particles. The results indicate that complete immunity in experimental rabbit syphilis correlates with antibody that kills T. pallidum and aggregates TROMPs, suggesting that TROMPs are molecules which contribute to the development of acquired immunity.
Syphilis, caused by the noncultivatable spirochete Treponema pallidum subsp. pallidum, continues to be a significant worldwide venereal disease. The chronicity of infection in both human and experimental rabbit syphilis and the slow development of immunity to reinfection have been well-established (10, 11, 19, 20, 21, 24, 38). Several lines of evidence support a major role for humoral immunity, including immune serum passive protection (4, 6, 13, 29, 33, 35–37, 40), inhibition of T. pallidum adherence and invasion of cultured cell monolayers by immune serum (12, 14, 34), immune serum-mediated phagocytosis of T. pallidum by rabbit peritoneal macrophages (18), and immune serum complement-dependent treponemicidal antibody (5, 6, 8, 27, 28). It has been demonstrated that a close quantitative correlation exists between the development of acquired resistance and the level of treponemicidal antibody (5, 6), suggesting that killing antibody plays a key role in the acquisition of protective immunity.
One of the more fascinating physical features of T. pallidum is its strikingly low density of membrane-spanning outer membrane proteins (31, 39) (T. pallidum rare outer membrane proteins [TROMPs]) which are believed to contribute to the pathogenic properties of this organism, including its ability to cause chronic infection and elicit a relatively slow-developing protective immune response. The aggregation of TROMPs in serum from infected and immune syphilitic rabbits (7, 9), as viewed by freeze-fracture electron microscopy, has shown that TROMPs have surface exposure and therefore represent the most likely surface targets for complement-dependent treponemicidal antibody.
In order to measure complement-dependent treponemicidal antibody directed solely against surface targets on T. pallidum, such as TROMPs, we developed a procedure termed the “washed-killing” assay (15). In this system, organisms are preincubated in heat-inactivated test serum and then washed to remove unbound antibody prior to the addition of complement. These studies, which utilized animals with various degrees of resistance to challenge reinfection, showed a quantitative correlation between the titer of killing antibody and level of acquired immunity, suggesting that killing antibody against surface-exposed molecules is a key mechanism of acquired host resistance.
In the present study, we tested whether a direct relationship between the status of acquired immunity in experimental rabbit syphilis and antibody against TROMPs can be demonstrated. In order to most closely relate serum killing activity and TROMP aggregation to the degree of protective immunity, sera analyzed in this study were obtained from postchallenge test animals at the time of lesion appearance in the control animals. The results show that when syphilitic lesions appeared in the control animals, complete immunity in the test animals correlated with the presence of high-titered treponemicidal antibody and antibody which significantly aggregates TROMPs. These findings suggest that TROMPs are the primary targets of treponemicidal antibody and are the molecules responsible for eliciting protective immunity.
Syphilitic infection and curative therapy of rabbits.
Fifty rabbits were infected intratesticularly with T. pallidum Nichols and treated with curative doses of penicillin G at various times postinfection in order to generate different degrees of immunity to challenge reinfection as previously described (15). Each animal received 2.5 × 107 T. pallidum cells per testis. The animals were divided into two groups of 17 rabbits each (groups A and B) and one group of 16 rabbits (group C). At 9 days (group A), 30 days (group B), and 6 months (group C) after intratesticular infection, each animal was treated with 25,000 U of aqueous procaine penicillin G/kg of body weight administered intramuscularly twice daily for 10 days (total of 500,000 U/kg of body weight). Ten days after therapy was completed, serum from each of the treated rabbits was shown to be free of penicillin levels capable of killing T. pallidum, based on the ability of this serum to support the viability of the organisms for 16 h in vitro. The efficacy of the treatment was determined 14 days after the completion of therapy with an infectivity test (23), in which a single popliteal lymph node and testis from each animal were removed under anesthesia, suspended in 50% heat-inactivated (56°C for 30 min) normal rabbit serum (NRS) in phosphate buffered saline, and inoculated intratesticularly into normal, serologically nonreactive rabbits. The sensitivity of this assay has been shown to be capable of detecting one to four virulent T. pallidum cells in a transferred tissue inoculum (23). Each of the treated animals was found to be free of infection based on dark-field microscopy-negative aspirates from the testes and nonreactive venereal disease research laboratory and T. pallidum immobilization tests (25, 26) on serum obtained from the recipient rabbits over 6 months.
Immune status of infected and penicillin-treated rabbits.
To determine susceptibility to reinfection, each penicillin-treated rabbit and five serologically nonreactive control rabbits were challenged 35 days after therapy with 103 T. pallidum cells at each of the four intradermal sites as previously described (15). Each rabbit was examined daily for 90 days for lesion appearance and development. All lesions in the test and control animals were observed to appear 11 to 17 days postchallenge. Erythematous, indurated, well-circumscribed lesions progressing to ulceration were considered typical, and atypical lesions were characterized as pale, soft, flat, irregular, and nonprogressive. Aspirates from representative lesions more than 5 mm in diameter were taken from each rabbit at the time of peak lesion development and examined by dark-field microscopy for the presence of motile treponemes. At the end of the 90-day observation period, the animals were euthanized and the second popliteal lymph node and testis were assayed for treponemes by the infectivity test as described above (23). Rabbits in which dark-field microscopy-positive lesions developed within the same incubation period as the controls and which exhibited disseminated infection by infectivity testing were considered susceptible to reinfection. Rabbits were characterized as partially immune if they exhibited dark-field microscopy-negative lesions without disseminated infection or completely immune if lesions and disseminated infection did not develop.
Sera.
Serum from infected, treated, and challenged test rabbits, described above, was obtained 17 days postchallenge when lesions appeared in the control animals at all inoculated sites (11 to 17 days). NRS was obtained from animals with negative venereal disease research laboratory and T. pallidum immobilization tests (25, 26). Immune rabbit serum (IRS) from animals immune to challenge infection with 103 treponemes at four sites was obtained from animals infected for 6 months following their intratesticular injection with a total of 4 × 107 cells of T. pallidum.
Washed-killing treponemicidal assay.
Complement-dependent treponemicidal antibody in sera from two representative rabbits each from groups A, B, and C was measured quantitatively by using the washed-killing assay as described previously (15). The treponemicidal endpoint (TE) was defined as the reciprocal of the highest dilution that exhibited ≥50% treponemal immobilization. Treponemes immobilized under similar conditions have been shown to be killed based upon virulence testing by intradermal injection of rabbits (5). Undiluted sera exhibiting differences in motility of 21 to 49% between the test (with complement) and control (without complement) tubes were considered to have a TE of ≤1. Control criteria included a quantitative IRS with a previously established endpoint. Assays were considered valid when the IRS titers were within 1 dilution of the established endpoint and when residual complement activity could be demonstrated as previously described (41). Each serum was run in duplicate on different days, and tests were considered valid when the endpoints were within 1 dilution of each other. The TE was recorded as the reciprocal of the average of two valid assays.
Freeze-fracture electron microscopy.
Sera from the representative rabbits that were tested for treponemicidal antibody were further analyzed for their ability to aggregate TROMPs following incubation with live T. pallidum cells. Each test serum was set up in quadruplicate as follows. One hundred microliters of a suspension containing approximately 5 × 107 treponemes/ml extracted in heat-inactivated (56°C for 30 min) NRS was combined with 900 μl of heat-inactivated test serum (1:9 ratio of treponemal suspension to test serum as used above for the washed-killing assay). The serum-treponeme mixtures were equilibrated in an atmosphere of 95% N2 and 5% CO2 and incubated at 34°C for 16 h to allow antibody against treponemal surface molecules to bind in the absence of complement (100% of treponemes were observed by dark-field microscopy to be actively motile following the incubation). The suspensions were then centrifuged at 8,000 × g for 10 min to pellet the treponemes, and the treponemal pellets were washed by suspension in 1 ml of phosphate-buffered saline and then centrifuged as described above. Each of the four treponemal pellets from an individual test serum was then fixed for 1 h at room temperature by suspension in 500 μl of 0.1 M sodium cacodylate buffer (pH 7.4) containing 2.5% glutaraldehyde. After fixation, the suspensions were centrifuged at 10,000 × g to pellet the treponemes, and the treponemal pellets were then combined by suspension in 20 μl of 0.1 M sodium cacodylate buffer (pH 7.4) containing 20% glycerol. Freeze-fracture electron microscopy was then performed as previously described (9, 39). Particle enumeration was made by counting the total number of individual and aggregated particles from 20 to 24 concave outer membrane fracture faces (a total of approximately 1 μm2) in sera from each immune-status group, NRS, and IRS. Particle aggregation was defined as two or more adjacent particles. Numbers of particles within an aggregate were determined by both counting the particles and determining the surface area of the aggregate in comparison to the surface area of an individual particle. Standard error comparison was used for all particle enumeration and percent particle aggregation analyses. Significances were based upon Student’s t test.
Status of immunity correlates with antibody that kills T. pallidum.
In order to determine the complement-dependent treponemicidal-antibody level at a time of symptomatic infection following challenge, sera from infected, penicillin-treated, and challenged animals were obtained during the time of lesion appearance in the control animals (17 days). As shown in Table 1, animals remaining susceptible to both symptomatic and disseminated challenge reinfection, as determined from the presence of typical, dark-field microscopy-positive lesions, showed only low-titered (≤1:1) serum treponemicidal activity. Similarly, no treponemicidal activity was present in the serum of normal control animals which showed identical lesions. In comparison, animals which exhibited partial immunity, characterized by atypical dark-field microscopy-negative lesions and the absence of disseminated reinfection following challenge, showed a corresponding increase in the titer (1:16) of treponemicidal activity. Finally, sera from animals completely immune to both local (no lesion appearance) and disseminated challenge reinfection had high-titered (1:128) treponemicidal activity, as did sera from immune control animals (1:128).
TABLE 1.
Immunity correlates with serum treponemicidal activity and TROMP aggregation
| Rabbit serum (n) | Immune statusa | TEb | OM fracture-face observations | Total no. of particles/μm2 | % Aggregationc |
|---|---|---|---|---|---|
| Group A (17) | Susceptible | ≤1:1 | 24 | 54 | 16.5 ± 5.6 |
| Group B (17) | Partial | 1:16 | 20 | 41 | 18.9 ± 6.5 |
| Group C (16) | Complete | 1:128 | 20 | 79 | 88.6 ± 5.4d |
| Normal | Susceptible | 0 | 21 | 48 | 13.4 ± 4.2 |
| Immune | Complete | 1:128 | 20 | 53 | 53.9 ± 7.8d |
Immune status of infected, treated, and challenged rabbits. Susceptible, dark-field microscopy-positive lesions and disseminated infection; partial, dark-field microscopy-negative lesions and no disseminated infection; complete, no lesions or disseminated infection.
The TE was determined as the final serum dilution which immobilized ≥50% of a treponemal suspension incubated for 16 h in the presence of complement.
Percent aggregation (mean ± standard error) was determined from the number of aggregates consisting of two or more particles and the total number of particles counted.
P < 0.0001, compared with the results for the susceptible animals and normal control animals.
Status of immunity correlates with antibody that aggregates TROMPs.
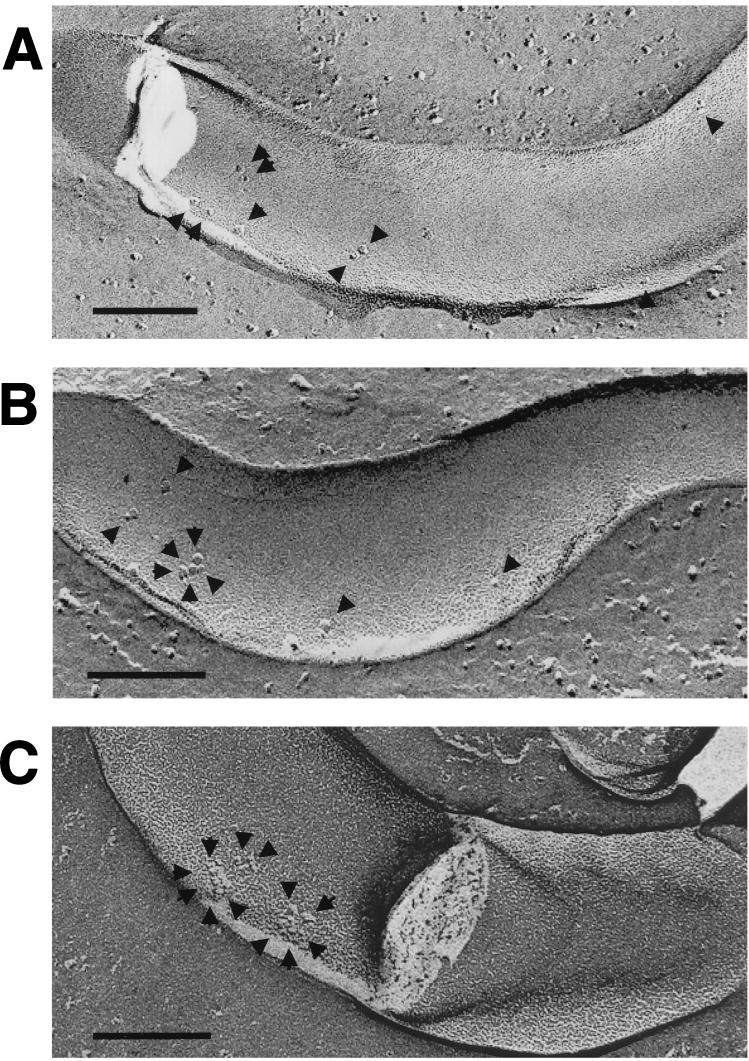
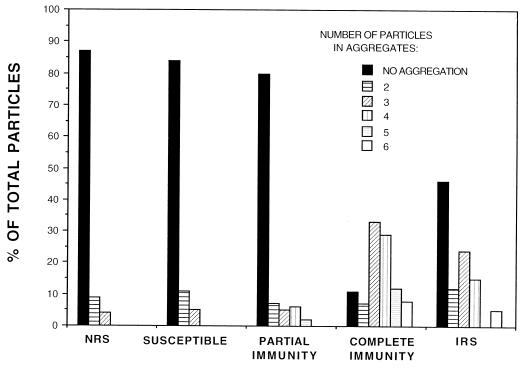
In order to correlate immune status with antibody directed against TROMPs, freeze-fracture electron microscopy was used to view antibody-mediated TROMP aggregation following the incubation of test and control sera with live T. pallidum cells. As shown in Table 1 and Fig. 1, sera from animals susceptible to challenge reinfection and having low-titered (≤1:1) treponemicidal activity as described above showed only a low level of total particle aggregation (16.5% ± 5.6%) similar to the NRS controls (13.4% ± 4.2%). As further shown in Fig. 2, the number of particles within these aggregates never exceeded three particles, again similar to those in the normal serum controls. While sera from animals which exhibited partial immunity showed a corresponding increase in treponemicidal activity as described above (titer of 1:16), no significant increase in total particle aggregation (18.9% ± 6.5%), compared to the susceptible group (16.5% ± 5.6%), was observed (Table 1). However, sera from the partially immune group did show a significant increase in the number of particles within aggregates (P < 0.01), which was found to be four to five particles in approximately 15% of the aggregates observed (Fig. 1 and 2). Finally, sera from animals completely immune to challenge reinfection and having high-titered (1:128) treponemicidal activity as described above showed significant aggregation of the total number of particles observed (88.6% ± 5.4%; P < 0.0001) (Table 1 and Fig. 1). In addition, approximately 50% of these aggregates contained as many as four to six particles (Fig. 2). A similar, significantly high level of particle aggregation (53.9% ± 7.8%; P < 0.0001) and numbers of particles within aggregates (approximately 20% containing four to six particles) was observed with sera from the immune control rabbits (Table 1 and Fig. 2).
FIG. 1.
Freeze-fracture electron microscopy of T. pallidum following incubation in sera from infected and curatively treated rabbits with various degrees of immunity to challenge reinfection. T. pallidum was incubated for 16 h in the absence of complement with serum from a rabbit susceptible to challenge reinfection (A), a rabbit showing partial protection against challenge reinfection (B), and a rabbit completely immune to challenge reinfection (C). Arrows show nonaggregated (A and B) and aggregated (B and C) TROMPs. Bar, 0.1 μm.
FIG. 2.
Determination of the number of particles in aggregates following incubation of T. pallidum in sera from animals with various degrees of immunity to challenge reinfection, in NRS, and in IRS. The total number of particles from 20 to 24 concave outer membrane fracture faces was determined. Numbers of particles within an aggregate were determined by directly counting the particles and by determining the surface area of an aggregate in comparison to the surface area of an individual particle.
Our previous findings that TROMPs have surface exposure, based on their aggregation in immune serum (9) and the apparent absence of surface exposure of other T. pallidum antigens previously identified (30), prompted the present investigation to address whether a relationship could be established between TROMPs, killing antibody, and host immunity in experimental rabbit syphilis. Killing antibody in this study was detected by the washed-killing assay (15), a procedure developed to measure antibody directed solely against surface-exposed targets on T. pallidum, which presumably would be only TROMPs. A further consideration in this study was the testing of sera from challenged animals at a time when symptomatic infection was observed to occur in the control animals. We believe that sera obtained from the test animals at this time provide the best measurement for establishing a relationship between anti-TROMP antibodies and protective challenge immunity.
The results of the washed-killing assay and freeze-fracture analysis, using sera from infected and cured rabbits that were either susceptible, partially immune, or completely immune to challenge reinfection, demonstrated a positive correlation between the status of immunity and the presence of antibody which kills T. pallidum and aggregates TROMPs. Rabbits that showed complete immunity to challenge reinfection had serum antibody that resulted in both high-titered complement-dependent treponemicidal activity (titer of 1:128) and significant TROMP aggregation, shown by the presence of 88.6% of the outer membrane particles existing in an aggregated state. It was also noted that 50% of the aggregates observed were found to contain four to six particles per aggregate. Both the high-titered killing activity and significant TROMP aggregation was similar to that of sera from immune control animals. It was noted, however, that serum from immune control animals aggregated TROMPs slightly less effectively (53.9%) than that from immune test animals (88.6%). One possible explanation for this observation is that serum from the immune test animals was taken after challenge, whereas no challenge was employed in the immune control animals. Thus, the increase in aggregation found in the sera from the immune test group may reflect an increase in antibody and/or antigen-antibody avidity from a booster immunization after challenge.
Rabbits which were only partially immune to reinfection, as determined by atypical lesion development and absence of disseminated infection after challenge, showed a markedly lower level of serum complement-dependent killing activity (titer of 1:16) than sera from the immune animals (titer of 1:128). While no significant increase in total TROMP aggregation was observed (18.9% aggregation of total particles counted), it was found that approximately 15% of these aggregates contained a significant increase in the number of particles per aggregate (four to five particles per aggregate) compared to the susceptible and normal control groups (two to three particles per aggregate). Thus, in accordance with the low-level but detectable killing antibody against T. pallidum, an increase in antibody directed against TROMPs was present in sera from these partially immune animals.
Rabbits which were completely susceptible to reinfection were found to have serum antibody which neither significantly killed T. pallidum (titer of ≤1:1) nor aggregated TROMPs (16.5% aggregation of total particles counted), a finding similar to that obtained with sera from normal control animals. It was also noted that of the 16.5% of particles which were aggregated, none of the aggregates contained more than three particles, again similar to the results in sera from normal control animals.
While a low level of aggregation was observed to occur consistently in sera from susceptible and normal control animals, an obvious question is, why did any aggregation occur under these conditions? It is important to stress that T. pallidum cells used for these experiments were acquired from 10-day-infected rabbits, a time just before infection is normally cleared (14 days) by specific immune mechanisms (2, 16, 17). It has been observed that T. pallidum cells obtained from 14-day-infected rabbits are susceptible to killing with only the addition of complement in the absence of added immune serum antibody (22). It is therefore conceivable that the low level of TROMP aggregation observed in normal control sera is the result of a low level of prebound anti-TROMP antibody on organisms extracted from the infectious rabbit milieu. These observations suggest that this level of anti-TROMP antibody present at or before 10 days after infection, while causing some aggregation, is not sufficient to result in significant complement-dependent killing of T. pallidum or resolution of the local infection. We have found that T. pallidum extracted from 14-day-infected rabbits shows outer membranes with greater amounts of aggregated TROMPs than T. pallidum from 10-day-infected animals. Taken together, these observations suggest that anti-TROMP antibody may have a key role in the resolution of the local primary infection in addition to its likely role in the development of acquired protective immunity.
The implication from this study that TROMPs are the likely targets of high-titered treponemicidal antibody is further supported by a recent study where we found that immunization with purified T. pallidum outer membranes elicits the highest titer of killing activity we have measured to date (7). Moreover, we have found that this antiserum to the T. pallidum outer membrane, when incubated with live organisms, also results in the aggregation of TROMPs. The possibility that molecules other than TROMPs are targets for killing antibody is unlikely given the absence of detectable T. pallidum surface proteins (30). In addition, Radolf et al. (32) have shown that T. pallidum outer membrane lipids are not antigenic in syphilitic human serum, which also possesses high-titered killing activity. Thus, these findings are again consistent with the idea that TROMPs are the primary targets of antibody which kill T. pallidum.
In summary, the results presented in this study provide compelling evidence that TROMPs are the major targets of protective immunity that develop during the course of experimental syphilitic infection. Notwithstanding cell-mediated immunity, our findings support the idea that specific antibody directed against TROMPs is central to the development of protective immunity against challenge reinfection. While the results show that this antibody, in combination with complement, effectively kills T. pallidum, it is certainly conceivable that other antibody-mediated mechanisms, such as opsonization (1, 3, 18) or antibody-dependent cellular cytotoxicity, may play key roles in an antibody protective response. We are hopeful that the future recombinant expression of all TROMP candidates will allow the ultimate identification of those immunogens responsible for protective immunity against syphilis.
Acknowledgments
We thank Cheryl I. Champion and Eldon M. Walker for their invaluable assistance and helpful comments during this study. We also thank Michael E. Kremen and Guido A. Zampighi for their expert freeze-fracture electron microscopy assistance.
This work was supported by U.S. Public Health Service grants AI-12601 (to J. N. Miller and M. A. Lovett) and AI-21352 (to M. A. Lovett).
REFERENCES
- 1.Baker-Zander S A, Lukehart S A. Macrophage-mediated killing of opsonized Treponema pallidum. J Infect Dis. 1992;165:69–74. doi: 10.1093/infdis/165.1.69. [DOI] [PubMed] [Google Scholar]
- 2.Baker-Zander S A, Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Am J Pathol. 1980;101:387–403. [PMC free article] [PubMed] [Google Scholar]
- 3.Baker-Zander S A, Shaffer J M, Lukehart S A. Characterization of the serum requirement for macrophage-mediated killing of Treponema pallidum spp. pallidum: relationship to the development of opsonizing antibodies. FEMS Immunol Med Microbiol. 1993;6:273–279. doi: 10.1111/j.1574-695X.1993.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 4.Bishop N H, Miller J N. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J Immunol. 1976;117:191–196. [PubMed] [Google Scholar]
- 5.Bishop N H, Miller J N. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976;117:197–207. [PubMed] [Google Scholar]
- 6.Bishop N H, Miller J N. Humoral immune mechanisms in acquired syphilis. In: Schell R F, Musher D M, editors. Pathogenesis and immunology of treponemal infection. New York, N.Y: Marcel Dekker; 1983. pp. 241–269. [Google Scholar]
- 7.Blanco, D. R., C. I. Champion, M. A. Lewinski, E. S. Shang, S. G. Simkins, J. N. Miller, and M. A. Lovett. Immunization with Treponema pallidum outer membrane vesicles induces high titer complement-dependent treponemicidal activity and aggregation of T. pallidum rare outer membrane proteins (TROMPs). Submitted for publication. [PubMed]
- 8.Blanco D R, Miller J N, Hanff P A. Humoral immunity in experimental syphilis: the demonstration of IgG as a treponemicidal factor in immune rabbit serum. J Immunol. 1984;133:2693–2697. [PubMed] [Google Scholar]
- 9.Blanco D R, Walker E M, Haake D A, Champion C I, Miller J N, Lovett M A. Complement activation limits the rate of in vitro treponemicidal activity and correlates with antibody-mediated aggregation of Treponema pallidum rare outer membrane protein (TROMP) J Immunol. 1990;144:1914–1921. [PubMed] [Google Scholar]
- 10.Chesney A M. Immunity in syphilis. Medicine. 1926;5:463–547. [Google Scholar]
- 11.Chesney A M. Acquired immunity in syphilis. Harvey Lect. 1931;25:103–128. [Google Scholar]
- 12.Fitzgerald T J, Johnson R C, Miller J N, Sykes J A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977;18:467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graves S, Alden J. Limited protection of rabbits against infection with Treponema pallidum by immune sera. Br J Vener Dis. 1979;55:399–403. doi: 10.1136/sti.55.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes N S, Muse K E, Collier A M, Baseman J B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977;17:174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewinski M A, Miller J N, Champion C I, Walker E M, Borenstein L A, Gayek R J, Lovett M A, Blanco D R. Treponemicidal antibody measured by the “washed-killing” assay correlates with immunity in experimental rabbit syphilis. Sex Transm Dis. 1995;22:31–38. doi: 10.1097/00007435-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lukehart S A, Baker-Zander S A, Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980;124:454–460. [PubMed] [Google Scholar]
- 17.Lukehart S A, Baker-Zander S A, Lloyd R M C. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980;124:461–467. [PubMed] [Google Scholar]
- 18.Lukehart S A, Miller J N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978;121:2014–2024. [PubMed] [Google Scholar]
- 19.Magnuson H J, Rosenau B J, Clark J W. The rate of development and degree of acquired immunity in experimental syphilis. Am J Syph. 1948;32:418–436. [PubMed] [Google Scholar]
- 20.Magnuson H J, Rosenau B J, Clark J W. The duration of acquired immunity in experimental syphilis. Am J Syph. 1949;33:297–302. [PubMed] [Google Scholar]
- 21.Magnuson J J, Thomas E W, Olansky S, Kaplan B I, DeMello L, Cutler J C. Inoculation of syphilis in human volunteer. Medicine. 1956;35:33–82. doi: 10.1097/00005792-195602000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Miller, J. N. Unpublished data.
- 23.Miller J N, editor. Spirochetes in body fluids and tissues: manual of investigative methods. Springfield, Ill: Charles C Thomas; 1971. pp. 14–25. [Google Scholar]
- 24.Miller J N, Fazzan F P, Whang S J. Studies on immunity in experimental syphilis. II. Treponema pallidum immobilization (TPI) antibody and the immune response. Br J Vener Dis. 1963;39:199–203. doi: 10.1136/sti.39.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Centers for Disease Control. Manual of serologic tests for syphilis. Atlanta, Ga: U.S. Department of Health, Education, and Welfare, Public Health Service, NCDC; 1964. [Google Scholar]
- 26.National Centers for Disease Control. Manual of serologic tests for syphilis. Atlanta, Ga: U.S. Department of Health, Education, and Welfare, Public Health Service, N.C.D.C.; 1969. [Google Scholar]
- 27.Nelson R A, Jr, Diesendruck J A. Studies on immobilizing antibodies in syphilis. I. Techniques of measurement and factors influencing immobilization. J Immunol. 1951;66:667–685. [PubMed] [Google Scholar]
- 28.Nelson R A, Jr, Mayer M M. Immobilization of Treponema pallidum in vitro by antibody produced in syphilitic infection. J Exp Med. 1949;89:369–393. doi: 10.1084/jem.89.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perine P L, Weiser R S, Klebanoff S J. Immunity to syphilis. I. Passive transfer in rabbits with hyperimmune serum. Infect Immun. 1973;8:787–790. doi: 10.1128/iai.8.5.787-790.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radolf J D. Treponema pallidum and the quest for outer membrane proteins. Mol Microbiol. 1995;6:1067–1073. doi: 10.1111/j.1365-2958.1995.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 31.Radolf J D, Norgard M V, Shulz W W. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci USA. 1989;86:2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radolf J D, Robinson E J, Bourell K W, Akins D R, Porcella S F, Weigel L M, Jones J D, Norgard M V. Characterization of outer membranes isolated from Treponema pallidum, the syphilis spirochete. Infect Immun. 1995;63:4244–4252. doi: 10.1128/iai.63.11.4244-4252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sepetijian M, Salussola D, Thivolet J. Attempt to protect rabbits against experimental syphilis by passive immunization. Br J Vener Dis. 1973;49:335–337. doi: 10.1136/sti.49.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas D D, Navab M, Haake D A, Fogelman A M, Miller J N, Lovett M A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci USA. 1988;85:3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Titus R G, Weiser R S. Experimental syphilis in the rabbit: passive transfer of immunity with immunoglobulin G from immune serum. J Infect Dis. 1979;140:904–913. doi: 10.1093/infdis/140.6.904. [DOI] [PubMed] [Google Scholar]
- 36.Turner T B. Protective antibodies in the serum of syphilitic rabbits. J Exp Med. 1939;69:867–890. doi: 10.1084/jem.69.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner T B, Hardy P H, Neuman B, Nell E E. Effect of passive immunization on experimental syphilis in the rabbit. Johns Hopkins Med J. 1973;133:241–251. [PubMed] [Google Scholar]
- 38.Turner T B, Nelson R A., Jr The relationship of treponemal immobilizing antibody to immunity in syphilis. Trans Assoc Am Physicians. 1950;63:112–117. [PubMed] [Google Scholar]
- 39.Walker E M, Zampighi G A, Blanco D R, Miller J N, Lovett M A. Demonstration of rare protein in the outer membrane of Treponema pallidum subsp. pallidum by freeze-fracture analysis. J Bacteriol. 1989;171:5005–5011. doi: 10.1128/jb.171.9.5005-5011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wicher K, Zabek J, Wicher V. Effect of passive immunization with purified specific or cross-reacting immunoglobulin G antibodies against Treponema pallidum on the course of infection in guinea pigs. Infect Immun. 1992;60:3217–3223. doi: 10.1128/iai.60.8.3217-3223.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams C A, Chase M W, editors. Methods Immunology and Immunochemistry. New York, N.Y: Academic Press; 1977. [Google Scholar]