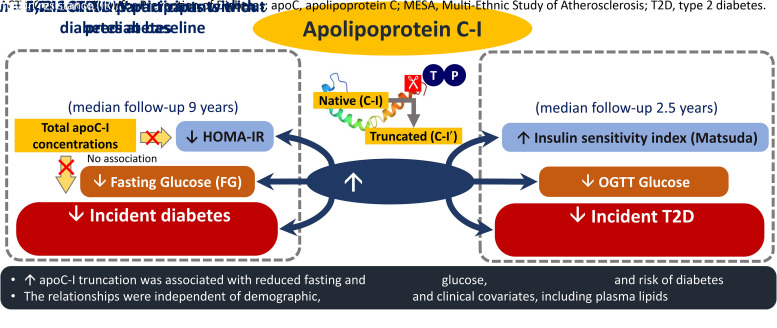
Abstract
OBJECTIVE
Higher truncated-to-native apolipoprotein (apo) C-I proteoform ratios (C-I′/C-I) are associated with favorable cardiometabolic risk profiles, but their relationship with longitudinal changes in insulin resistance (IR) and incident diabetes is unknown.
RESEARCH DESIGN AND METHODS
Plasma apoC-I proteoforms were measured by mass spectrometry immunoassay at baseline in 4,742 nondiabetic participants in the Multi-Ethnic Study of Atherosclerosis (MESA) and 524 participants with prediabetes in the Actos Now for Prevention of Diabetes (ACT NOW) study. The primary outcome was incident diabetes (fasting glucose [FG] ≥7.0 mmol/L or hypoglycemic medication use in MESA; FG ≥7.0 mmol/L or 2-h glucose ≥11.1 mmol/L in an oral glucose tolerance test [OGTT] in ACT NOW). Secondary outcomes were changes in FG and HOMA-IR in MESA, and OGTT-glucose area under the curve (AUCglucose) and Matsuda insulin sensitivity index (ISI) in ACT NOW.
RESULTS
In MESA, a higher C-I′/C-I was associated with lower risk of diabetes (n = 564 events; HR 0.87 [95% CI 0.79, 0.95] per SD; P = 0.0036; median follow-up, 9 years), and smaller increases (follow-up adjusted for baseline) in FG (−0.5%; P < 0.0001) and HOMA-IR (−2.9%; P = 0.011) after adjusting for baseline clinical and demographic covariates, including plasma triglycerides and HDL cholesterol. Total apoC-I concentrations were not associated with changes in FG, HOMA-IR, or incident diabetes. In ACT NOW, higher C-I′/C-I was associated with smaller increases in AUCglucose (−1.8%; P = 0.0052), greater increases in ISI (7.2%; P = 0.0095), and lower risk of diabetes (n = 59 events; 0.66 [95% CI 0.48, 0.91]; P = 0.004; median follow-up, 2.5 years) after adjusting for treatment group and diabetes risk factors, including plasma lipids.
CONCLUSIONS
Our results indicate that apoC-I truncation may contribute to changes in glucose levels, IR, and risk of diabetes.
Graphical Abstract
Type 2 diabetes (T2D), due to its high prevalence and increased risk for serious complications, including excess mortality, is a major global public health challenge (1). Although an extensive body of evidence has demonstrated the multifaceted ways in which insulin resistance (IR) and T2D affect lipid metabolism, contributing to dyslipidemia and cardiovascular risk (2,3), it is becoming increasingly evident that many components of lipid metabolism may, in turn, contribute to IR, increasing risk of and worsening existing T2D (4–6). Consistent with this, concentrations of triglycerides and lipoproteins such as VLDL, LDL, and HDL have been directly linked with development of T2D (7–12). Mendelian randomization studies, however, showed that increases in plasma triglyceride levels or declines in HDL cholesterol (HDL-C) levels alone do not appear to cause T2D (13–16), indicating that the associations reported in the observational studies reflected correlation, residual confounding, or other factors associated with these lipoproteins as potential drivers of T2D risk.
The metabolism of lipoproteins depends on apolipoproteins controlling their assembly and structure, directing their binding to cell-surface receptors and regulating enzymes activity. Apolipoprotein C-I (apoC-I) is a major inhibitor of triglyceride clearance via suppression of lipoprotein and hepatic lipases, and apoE-mediated binding to LDL receptor and LDL receptor–related protein 1 (17,18). ApoC-I also modulates HDL metabolism through inhibition of cholesteryl ester transport protein (CETP) (19). In vivo animal models indicate that apoC-I may also negatively affect insulin action and glucose metabolism (20). In humans, plasma apoC-I concentrations are increased in people with T2D (21,22); however, they have not yet been linked with incident T2D (23).
In circulation, apoC-I appears as a full-length native protein (C-I) and a relatively abundant (∼25% of total) post-translational truncated proteoform (C-I′) (24). We recently demonstrated in the Multi-Ethnic Study of Atherosclerosis (MESA) strong relationships of apoC-I truncation with various measures of lipid and glucose metabolism that were distinct from total apoC-I concentrations (25). Specifically, a higher truncated to native apoC-I ratio (C-I′/C-I) was associated with lower fasting glucose (FG) and triglyceride levels cross-sectionally and with greater reduction in triglyceride levels during follow-up. In contrast, total apoC-I levels were not associated with FG levels or follow-up changes in triglyceride levels, raising the possibility that apoC-I proteoforms may have unique metabolic roles that differ from that previously assigned to total apoC-I concentrations.
In this study, using plasma samples and data from MESA and the Actos Now for Prevention of Diabetes (ACT NOW) study, we tested the hypothesis that apoC-I proteoform composition is associated with changes in measures of IR, glucose regulation, and incident diabetes.
Research Design and Methods
Data and samples used in this study were obtained from the MESA (https://www.mesa-nhlbi.org) administrators in accordance with their published data access policies, including an approved written proposal. Samples and data from ACT NOW were previously approved for use by investigators.
Study Design and Participants
The MESA is a longitudinal, community-based study conducted at six field centers across the U.S. (Supplementary Methods) with individuals aged 45–84 years who were of non-Hispanic White, Black, Hispanic, and Chinese race and ethnicity and who were without known cardiovascular disease at enrollment (26). Institutional review boards at each study site approved the study protocol and informed consent was obtained from all participants.
The present study included data from clinical exams conducted in 2000–2002 (exam 1), 2002–2004 (exam 2), 2004–2006 (exam 3), 2005–2007 (exam 4), and 2010–2012 (exam 5). Demographic information, socioeconomic data, medical history, and physical exam measures were obtained through standardized protocols. As described previously (27), physical activity score was defined as quartiles of sedentary behavior in reverse order (i.e., 0 being the most and 3 the least sedentary) plus quartiles of moderate to vigorous physical activity (1 being the least and 4 the most physically active), creating a score ranging from 1 to 7 for each participant.
Blood samples were collected after a 12-h fast, and serum or EDTA-plasma were collected and stored at −70°C, using a standardized protocol (26). Blood biomarkers were measured at the MESA central laboratory at the University of Minnesota. Estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration creatinine-cystatin C equation. Serum insulin concentrations were measured by the radioimmunoassay method (Linco Research, Inc., St. Charles, MO) in the exam 1 samples and by the sandwich immunoassay method (Elecsys 2010; Roche Diagnostics, Indianapolis, IN) in the exam 5 samples.
The ACT NOW study was a prospective, randomized, double-blind, placebo-controlled trial to examine the effectiveness of pioglitazone in prevention of T2D (28). The study was approved by the institutional review boards of all study centers (Supplementary Methods), and all participants gave written informed consent. Participants included 602 adults with impaired glucose tolerance and at least one other risk factor for T2D. At baseline, participants underwent a 2-h 75-g oral glucose tolerance test (OGTT) and then were randomly assigned to pioglitazone or placebo. Follow-up visits were at 2, 4, 6, 8, 10, and 12 months during the first year and then every 3 months thereafter until they reached the primary end point of diabetes, dropped out, were lost to follow-up, or reached study end at 4 years. FG level was determined at each follow-up visit. An OGTT with blood samples collected every 15 min for measurement of plasma glucose and insulin concentrations was performed annually. Plasma insulin level was assayed by radioimmunoassay (Diagnostic Products, Los Angeles, CA). Levels of plasma total cholesterol, HDL-C, and triglycerides were measured using the cholesterol oxidation—DAOS reagent method (WAKO, Richmond, VA) and an enzymatic assay (Stanbio Laboratory, Boerne, TX), respectively. Plasma aliquots for post hoc analyses were stored at −80°C until assayed.
Measurement of apoC-I Proteoforms and Total Plasma Concentrations
ApoC-I proteoforms were measured in baseline plasma samples from both studies by mass spectrometry immunoassay (MSIA), as described previously (25). Mean intra- and interassay coefficients of variation were 4.6% and 5.5% for C-I′, and 1.5% and 1.8% for C-I, respectively. Within-person temporal variability of C-I′/C-I was 3.9% short term (25–30 days) and 10.9% long term (∼10 years) (Supplementary Methods). Total apoC-I concentrations were measured by sandwich ELISA in a subset of MESA participants. Both MSIA and ELISA used identical detection antibodies (Academy Biomedical Co., Houston, TX) and are detailed in the Supplementary Methods.
Study Outcomes
The primary outcome was first occurrence of diabetes, defined as FG ≥7.0 mmol/L (126 mg/dL) or self-reported use of hypoglycemic drugs at follow-up examinations (exams 2–5) in MESA, and as FG ≥7.0 mmol/L or 2-h OGTT glucose ≥11.1 mmol/L (200 mg/dL), confirmed by an OGTT test, in ACT NOW. Secondary outcomes in MESA were longitudinal changes in FG and the HOMA-IR (FG [mmol/L] × fasting insulin [mU/L]/22.5). Participants receiving hypoglycemic medications at follow-up exams were excluded because of modulating effects of these drugs on both FG and insulin levels. In ACT NOW, secondary outcomes included longitudinal changes in glucose response, calculated as glucose area under the curve (AUCglucose) during OGTT by trapezoidal rule, and in Matsuda index of insulin sensitivity (ISI), derived from plasma glucose and insulin measurements obtained during the OGTT (29). Follow-up time was ended at the last OGTT at or prior to diabetes conversion, study end, study dropout, or loss to follow-up.
Statistical Analyses
Statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC). P values <0.05 were considered statistically significant. All nonnormally distributed continuous variables (including apoC-I measures) were natural log-transformed to approximate normality. All continuous variables were scaled to a mean of 0 and an SD of 1 to allow direct comparison of effects in the regression models. The differences in baseline characteristics across quartiles of C-I′/C-I were tested by one-way ANOVA for continuous variables and by Fisher exact test for categories. Baseline characteristics between the groups who did and who did not develop diabetes were compared using Student’s t test for continuous variables and by χ2 or Fisher tests for categorical variables.
Cox proportional hazards regression models were used to assess the association between baseline apoC-I measures and incident diabetes. Proportional hazards assumptions were assessed by inspecting Kaplan-Meier curves for quartiles of apoC-I measures and formally tested by cumulative sums of Martingale residuals with P values of Kolmogorov-type supremum test. Linearity of the relationship of apoC-I measures with diabetes risk was tested by the likelihood ratio test comparing the models with the linear term only with the model including both linear and cubic spline terms. The covariate selection for statistical models was based on previous results in MESA and ACT NOW, and univariate associations of available covariates with C-I′/C-I and incident diabetes in the present study.
In MESA, person-time was calculated as years from exam 1 to the midpoint between the last available examination without diabetes and the examination at which diabetes was first identified, or to the examination at which censoring occurred due to loss to follow-up, death, or the end of follow-up at exam 5. All models were first run unadjusted and then adjusted for study site, age, sex, and race and ethnicity (model 1); then model 1 variables plus income, education, alcohol use, physical activity score, BMI, systolic BP (BP), eGFR, antihypertensive and statin drug use, and without and with further adjustment for FG (model 2); and then model 2 variables plus plasma triglyceride and HDL-C levels to test whether these lipids explain the association of apoC-I proteoform measures with incident diabetes (model 3). Potential heterogeneity between the race and ethnicity groups was assessed by including an interaction term in the models. Separate sensitivity analyses were conducted by 1) defining diabetes cases as reporting diabetes medications only to limit potential diabetes misclassification due to a transient increase in FG; 2) excluding participants developing diabetes by exam 2 to reduce potential for reverse causality; and including participants 3) completing exam 5 to evaluate potential attrition bias; 4) with normal FG at baseline (<5.6 mmol/L) to test whether the associations were present early in the diabetes trajectory; and 5) who were not taking statins, to avoid interference of these medications with apoC-I measures and diabetes risk.
In a subset of participants with available total apoC-I measurements, we tested the association of incident diabetes with individual apoC-I proteoform concentrations calculated as product of their relative amounts and total apoC-I concentrations (both proteoforms included in the model) to directly compare these two apoC-I measures.
In ACT NOW, all models were first adjusted only for treatment group (pioglitazone or placebo) to reflect the strong effect of pioglitazone on glucose regulation and lipid levels, then adjusted for study site, age, sex, race and ethnicity (model 1), then for BMI and FG (model 2), and then for baseline HDL and triglycerides levels (model 3). Model 3 was further adjusted for ISI to explore whether the association of C-I′/C-I with T2D is independent of insulin sensitivity level.
The association between baseline apoC-I measures and longitudinal changes in continuous variables (follow-up values adjusted for baseline values and time of follow-up) was tested by mixed linear regression for repeated measures with random intercept. Models were run before and after adjusting for baseline covariates as indicated above for each study (models 1, 2, and 3), except for FG (already included as baseline measure or in outcome calculation). In ACT NOW, the associations were also run stratified by treatment group. Potential heterogeneity between the treatment groups was assessed by including interaction term in the models.
Results
A total of 4,742 MESA participants without diabetes at baseline and available follow-up data for diabetes status were included in the analyses (see study flow in Supplementary Fig. 1). In ACT NOW, baseline plasma samples for apoC-I proteoform measurement were available from 524 of 602 original participants.
Association of apoC-I Proteoforms With Characteristics at Baseline
The amount of C-I′ ranged from 13 to 41.5% in MESA (Table 1) and from 13.3 to 37.1% in ACT NOW (Supplementary Table 1). Consistent with our previous report in MESA including participants with prevalent diabetes (25), in those with higher C-I′/C-I, average age was slightly higher as was the percentage of male participants, of Chinese descent, and of White and Black races (Table 1). Higher C-I′/C-I was also associated with lower BMI; lower systolic and diastolic BPs; FG, insulin, and triglyceride levels; and HOMA-IR; and with higher physical activity score and HDL-C and total apoC-I levels (available in 3,427 participants). In ACT NOW, participants with higher C-I′/C-I were also older, more frequently male, and had lower BMI and fasting triglyceride levels (Supplementary Table 1). There was no association of C-I′/C-I with family history of diabetes, FG, or hemoglobin A1c.
Table 1.
Baseline clinical and demographic characteristics by quartiles of C-I′/C-I in MESA
| Variable | Quartile | ||||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | P value | |
| apoC-I′ range (%) | 13.0–23.9 | 23.9–26.5 | 26.5–28.9 | 28.9–41.5 | — |
| C-I′/C-I | 0.28 ± 0.03 | 0.34 ± 0.01 | 0.38 ± 0.01 | 0.46 ± 0.04 | — |
| Age (years) | 61.9 ± 9.8 | 61.5 ± 10 | 62 ± 10.3 | 62.9 ± 10.8 | 0.035 |
| Female sex | 547 (46) | 579 (49) | 596 (50) | 531 (45) | 0.033 |
| Race and ethnicity | |||||
| White | 490 (41) | 453 (38) | 450 (38) | 540 (46) | 0.0003 |
| Black | 268 (23) | 322 (27) | 328 (28) | 358 (30) | 0.0004 |
| Hispanic/Latino | 231 (20) | 262 (22) | 259 (22) | 216 (18) | 0.053 |
| Chinese | 196 (17) | 149 (13) | 149 (13) | 71 (6) | <0.0001 |
| Current tobacco use | 157 (13) | 161 (14) | 147 (12) | 126 (11) | 0.12 |
| Current alcohol use | 688 (58) | 682 (58) | 683 (58) | 689 (58) | 0.98 |
| Bachelor’s degree | 447 (38) | 443 (37) | 417 (35) | 475 (40) | 0.092 |
| Income ≥$50,000/year | 461 (40) | 486 (42) | 476 (41) | 481 (43) | 0.65 |
| Physical activity score (n = 4,676) | 3.86 ± 1.66 | 4.02 ± 1.63 | 4.05 ± 1.60 | 4.14 ± 1.62 | <0.0001 |
| BMI (kg/m2) | 28.9 ± 5.4 | 28.4 ± 5.4 | 27.8 ± 5.2 | 26.8 ± 4.8 | <0.0001 |
| Antihypertensive drug use | 438 (37) | 424 (36) | 384 (32) | 340 (29) | <0.0001 |
| Statin use | 161 (14) | 174 (15) | 178 (15) | 146 (12) | 0.22 |
| Systolic BP (mmHg) | 127 ± 20 | 126 ± 21 | 126 ± 22 | 124 ± 22 | <0.0001 |
| Diastolic BP (mmHg) | 72 ± 10 | 72 ± 10 | 72 ± 10 | 71 ± 10 | 0.021 |
| FG (mmol/L) | 5.08 ± 0.62 | 5.05 ± 0.61 | 4.97 ± 0.57 | 4.86 ± 0.51 | <0.0001 |
| Fasting insulin (pmol/L) (n = 4,735) | 67 ± 38 | 59 ± 32 | 56 ± 33 | 45 ± 24 | <0.0001 |
| HOMA-IR (n = 4,735) | 2.60 ± 1.63 | 2.25 ± 1.38 | 2.10 ± 1.41 | 1.64 ± 1.00 | <0.0001 |
| Triglycerides (mmol/L) | 1.77 ± 1.13 | 1.45 ± 0.77 | 1.36 ± 0.77 | 1.13 ± 0.62 | <0.0001 |
| Total cholesterol (mmol/L) | 4.97 ± 0.92 | 5.06 ± 0.90 | 5.08 ± 0.91 | 5.07 ± 0.88 | 0.0013 |
| HDL cholesterol (mmol/L) | 1.30 ± 0.38 | 1.32 ± 0.37 | 1.33 ± 0.40 | 1.41 ± 0.41 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 91 ± 16 | 90 ± 16 | 89 ± 17 | 88 ± 17 | <0.0001 |
| Total apoC-I (mg/dL) (n = 3,427) | 8.9 ± 3.0 | 9.1 ± 2.9 | 9.3 ± 3.0 | 9.8 ± 3.1 | <0.0001 |
Data are reported as mean ± SD or n (%). Statistical differences were tested by one-way ANOVA for continuous variables and χ2 test for categories. Continuous data were natural log-transformed to approximate normal distribution.
Association of apoC-I Proteoforms with Incident Diabetes
In MESA, diabetes developed in 580 participants over a median follow-up time of 9.0 years. As shown in Supplementary Table 2, those who developed diabetes were, on average, younger; more likely men; of Black or Hispanic race and ethnicity; had a smaller proportion of those with a bachelor’s degree as well as higher annual income; and higher BMI, BP, eGFR, and levels of FG, insulin, and triglycerides; and lower HDL-C levels.
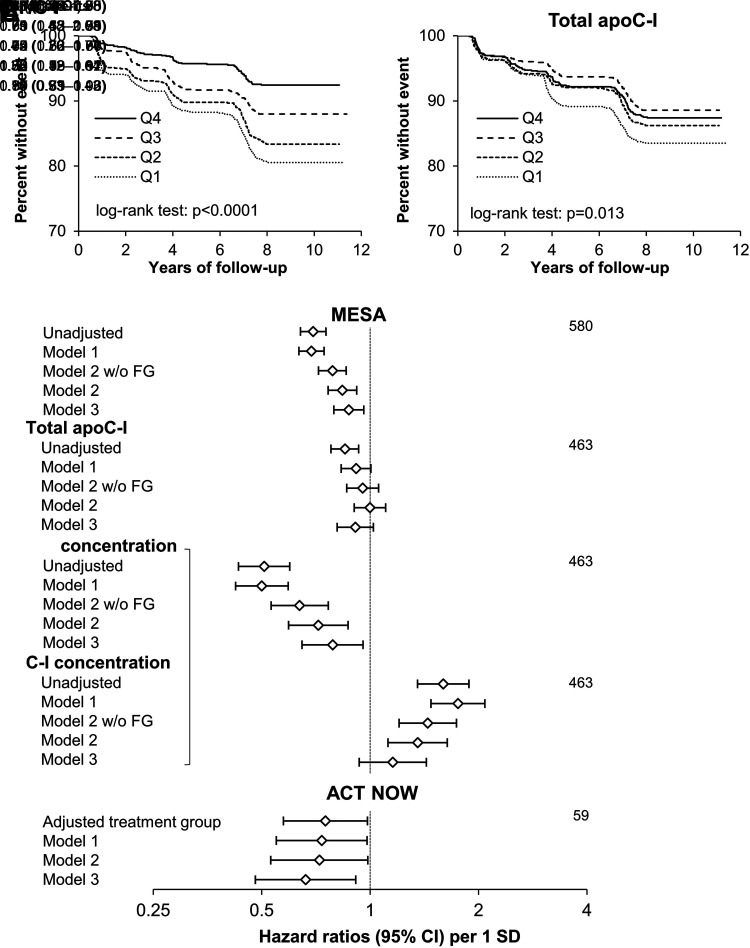
Comparison of Kaplan-Meier curves showed that higher C-I′/C-I quartiles were associated with longer diabetes-free survival, and this relationship appeared stronger than for total apoC-I (Fig. 1A and B). Accordingly, risk of diabetes was lower with higher C-I′/C-I (hazard ratio [HR] 0.79 [95% CI 0.72, 0.86] per SD) and C-I′ concentrations (0.64; 95% CI 0.53, 0.77), but increased with higher C-I concentrations (1.45; 95% CI 1.20, 1.74) after adjustment for demographic, socioeconomic, and cardiometabolic (without FG) covariates (P < 0.0001 for all; Fig. 1C). The inverse associations of C-I′/C-I and C-I′ concentrations with incident diabetes were attenuated but still statistically significant after further adjustment for baseline FG (0.84 [95% CI 0.76, 0.92], P = 0.0002; 0.72 [95% CI 0.59, 0.87], P = 0.0006, respectively), and additional adjustment for HDL-C and triglyceride levels (0.87 [95% CI 0.79, 0.96], P = 0.0051; 0.79 [95% CI 0.65, 0.96], P = 0.016, respectively) (Fig. 1C). The association of total apoC-I concentrations with diabetes risk was present only in the unadjusted model (0.84 [95% CI 0.78, 0.90]; P < 0.0001) and was abolished after adjustment for demographic, socioeconomic, and cardiometabolic covariates (0.95 [95% CI 0.86, 1.06]; P = 0.36) (Fig. 1C).
Figure 1.
Association of baseline C-I′/C-I and total apoC-I with incident diabetes. A and B) Kaplan-Meier curves for quartiles of C-I′/C-I and total apoC-I concentrations in MESA. C) Cox proportional hazards risk models of diabetes risk in MESA and ACT NOW. In MESA, the models were unadjusted and then adjusted for study site, age, sex and race and ethnicity (model 1); model 1 variables plus education and income levels, physical activity score, BMI, systolic BP, eGFR, use of antihypertensive and statin drugs, and without (w/o) and with (w/) FG (model 2); and model 2 variables (with FG) plus fasting triglycerides and HDL-C levels (model 3). C-I′ and C-I proteoform concentrations were tested in the same models as indicated by the bracket. In ACT NOW, the models were adjusted for treatment group and then further adjusted for study site, age, sex, and race and ethnicity (model 1); and model 1 variables plus BMI and FG (model 2); and model 2 variables plus triglycerides and HDL-C (model 3).
The relationship of C-I′/C-I with incident diabetes showed no statistically significant heterogeneity with race and ethnicity (P = 0.32 for interaction), and was largely unchanged with diabetes defined as new use of diabetes medications, after excluding those who developed diabetes by exam 2, or when restricted to participants completing exam 5, those with normal FG, or those without statin use at baseline (HR 0.83–0.90; Supplementary Table 3).
In ACT NOW, T2D developed in 59 participants during median follow-up of 2.5 years. Baseline C-I′/C-I was inversely associated with risk of T2D after adjusting for cardiometabolic risk factors (0.72 [95% CI 0.53, 0.99] per SD; P = 0.040) and further adjustments for plasma lipids (0.66 [95% CI 0.48, 0.91]; P = 0.012) (Fig. 1C) and baseline ISI (HR 0.62 [95% CI 0.45, 0.85; P = 0.003; not shown in figure).
Association of Baseline apoC-I Proteoforms With Longitudinal Changes in Blood Glucose Levels and Estimates of IR and Insulin Sensitivity
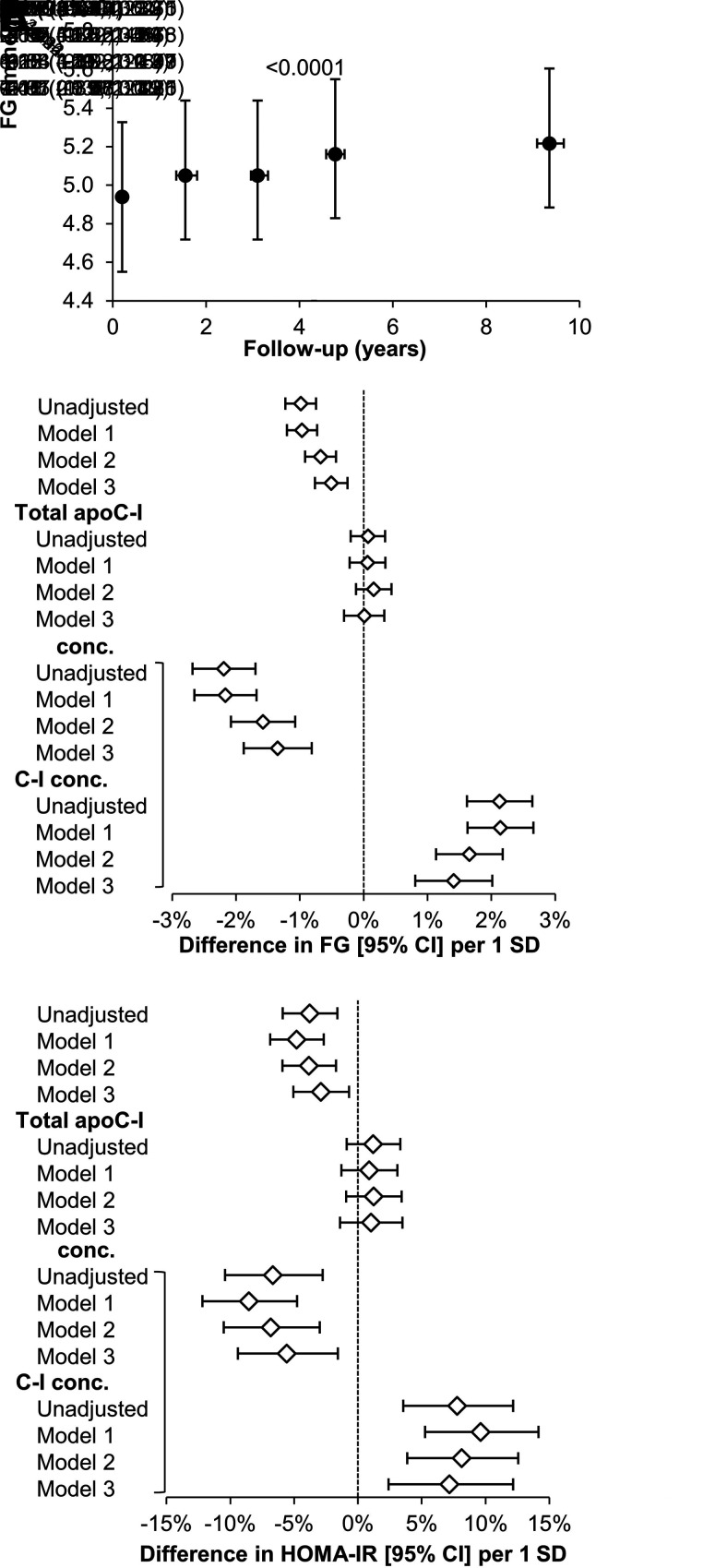
In MESA, FG levels increased over time and were higher at follow-up compared with baseline (Fig. 2A). Baseline C-I′/C-I and C-I′ concentrations were inversely associated with changes in FG (exams 2–5 adjusted for baseline) (estimated differences per SD difference in apoC measures: −0.51% [95% CI −0.76, −0.25], P = 0.0001; and −1.4% [95% CI −1.9, −0.81], P < 0.0001, respectively), whereas C-I concentrations were positively associated with changes in FG (1.4% [95% CI 0.81, 2.0]; P < 0.0001) (Fig. 2B). Similarly, both C-I′/C-I and C-I′ concentrations were inversely associated with change in HOMA-IR (exam 5 adjusted for baseline) (−2.9% [95% CI −5.1, −0.70], P = 0.011; and −5.6% [95% CI −9.4, −1.6], P = 0.0069, respectively), whereas C-I concentrations were positively associated with change in HOMA-IR (7.1% [95% CI 2.4, 12]; P = 0.0029) (Fig. 2C). There was no association between baseline total apoC-I concentrations and follow-up changes in FG and HOMA-IR.
Figure 2.
Relationship of baseline apoC-I measures with longitudinal changes in FG and HOMA-IR in MESA. A) FG from exam 1 (baseline) to exam 5 in the entire cohort. Data are reported as median (interquartile range) and P value is for overall trend. B and C) Relationships of baseline apoC-I measures with changes in FG (exams 2–5 adjusted for baseline and time of follow-up) and HOMA-IR (exam 5 value adjusted for baseline). Mixed linear regression models for repeated measures were run unadjusted and then adjusted for study site, age, sex and race and ethnicity (model 1); model 1 variables plus family income and education levels, physical activity score, BMI, systolic BP, use of antihypertensive and statin drugs, and eGFR (model 2); and model 2 variables plus fasting triglycerides and HDL-C levels (model 3). C-I′ and C-I proteoform concentrations were tested together in the same models (indicated by the bracket).
In ACT NOW, C-I′/C-I was inversely associated with change in AUCglucose and positively associated with change in ISI after adjustment for diabetes risk factors, including HDL-C and triglyceride levels (−1.8% [95% CI −3.1, −0.6], P = 0.0052; and 7.2% [95% CI 1.7, 13], P = 0.0095, respectively) (Table 2). The associations of C-I′/C-I with changes in AUCglucose and ISI were similar in the two treatment groups.
Table 2.
Relationship of baseline C-I′ to C-I with longitudinal changes in AUCglucose and Matsuda ISI in ACT NOW
| Outcome | Group | n | Adjusted for treatment or unadjusted | Model 1* | Model 2† | Model 3‡ |
|---|---|---|---|---|---|---|
| AUCglucose | All | 417 | −1.8 (−2.9, −0.6) | −2.0 (−3.1, −0.8) | −1.9 (−3.1, −0.7) | −1.8 (−3.1, −0.6) |
| PIO | 211 | −2.0 (−3.6, −0.4) | −2.3 (−3.9, −0.7) | −2.2 (−3.8, −0.5) | −1.6 (−3.3, 0.2) | |
| PBO | 206 | −1.5 (−3.2, 0.2) | −1.5 (−3.3, 0.3) | −1.4 (−3.3, 0.4) | −1.8 (−3.7, 0.1) | |
| ISI | All | 417 | 9.1 (3.9, 15) | 9.3 (4.0, 15) | 8.6 (3.3, 14) | 7.2 (1.7, 13) |
| PIO | 211 | 7.9 (0.7, 16) | 8.7 (1.4, 17) | 8.3 (0.9, 16) | 6.3 (−1.2, 15) | |
| PBO | 206 | 11 (3.4, 19) | 12 (3.9, 21) | 11 (3.4, 20) | 10 (2.3, 19) |
The associations of C-I′/C-I with changes (follow-up value adjusted for baseline value and time of follow-up) in AUCglucose and ISI were tested by mixed linear regression for repeated measures with random intercept and are expressed as % difference (95% CI) per 1 SD increase in C-I′/C-I. Adjusted for treatment group (All) or unadjusted (stratified analyses). PBO, placebo; PIO, pioglitazone.
*Further adjusted for study site, age, sex, and race and ethnicity.
†Model 1 variables plus BMI.
‡Model 2 variables plus triglycerides and HDL-C.
Conclusions
Our results suggest a role for post-translational modification of apoC-I in regulating glucose metabolism. Increased apoC-I truncation was associated with lower risk of future diabetes and smaller increases in fasting and postprandial IR during follow-up. The associations were observed in two independent cohorts, including a general nondiabetic population as well as cohort at high risk of T2D, and appeared only partially accounted for by variation in plasma lipid levels. In contrast, our data in MESA showed that total apoC-I levels were not associated with changes in IR or incident diabetes upon adjusting for typical diabetes risk factors.
In a few cross-sectional studies, total apoC-I levels in plasma were higher in individuals with T2D (21,22). In our previous analysis in MESA that included participants with and without diabetes, total apoC-I levels were positively associated with FG in univariate models; however, the association was absent upon adjustment for demographic characteristics (25). Similarly, in the present study, the association of total apoC-I with diabetes risk disappeared after adjusting for diabetes risk factors, confirming a previous observation from individuals with prediabetes (23). Overall, these results indicate that total apoC-I may associate with diabetes risk indirectly through its relationships with standard clinical and demographic risk factors. Consistent with this, rodent models using genetic overexpression of human APOC1, leading to increased total apoC-I concentrations, also have not demonstrated a clear relationship with risk of diabetes (20,30). In contrast, our results showed very different relationships of the native and truncated apoC-I proteoforms with various measures of glucose metabolism, indicating that measurement of total apoC-I may obscure the very different physiologic roles of its proteoforms and the level of metabolic risk for individuals.
Truncation of apoC-I is catalyzed by dipeptidyl peptidase 4 (DPP-4) (31). In humans, increased DPP-4 activity is associated with the onset of IR, prediabetes, and T2D (32). Pharmacologic inhibition of DPP-4 reduces glycemia, primarily through inhibiting cleavage of endogenous incretins (33). The inverse association of apoC-I truncation with IR and risk of diabetes thus appears to counter the effects of the enzyme responsible for its formation and suggests that apoC-I truncation is influencing glucose metabolism by mechanisms that are independent of, and perhaps overcoming, the deleterious effects of increased DPP-4 on glucose metabolism. It also raises the possibility that reduced apoC-I truncation may offset, at least in part, benefits of DPP-4 inhibitors on lipid and glucose metabolism and may help explain their neutral effect on cardiovascular risk (34).
Partial attenuation of inverse relationships of C-I′/C-I or C-I′ concentrations with incident diabetes after adjusting for plasma lipids supports changes in lipid metabolism as one potential link between apoC-I truncation and diabetes risk. ApoC-I reduces triglyceride clearance mainly via inhibition of lipoprotein lipase (LPL). LPL deficiency in humans is characterized by severe hypertriglyceridemia, IR, and diabetes despite a lean phenotype (35). In contrast, global or adipose tissue–specific overexpression of LPL ameliorated IR and hyperglycemia in animal models of obesity (36,37). It is plausible that greater relative abundance of C-I′, through its less potent inhibition of LPL, may improve triglyceride clearance in adipose tissue and reduce harmful flux of triglycerides and their metabolites into skeletal muscle and liver (4,5). In contrast, the positive associations of C-I with incident diabetes and prospective worsening of fasting glycemia and IR are very similar to those previously observed for apoC-III, a potent inhibitor of triglyceride clearance (38). Inhibition of apoC-III with antisense oligonucleotides ameliorated IR and improved glycemic control in individuals with T2D (39). Thus, reducing levels of apolipoproteins or their specific proteoforms that inhibit triglyceride clearance may be a potential strategy to improve glycemic control and reduce diabetes risk. Besides inhibiting triglyceride clearance, apoC-I increases HDL-C levels through CETP inhibition (19). Consistent with this effect, our previous analyses showed positive associations of total and native apoC-I levels with HDL-C (25). In contrast, neither C-I′/C-I nor C-I′ was associated with changes in HDL-C in adjusted models (25). Studies in humans showed inverse association between HDL-C and risk of T2D (7,11). The increase in HDL-C level may counteract the diabetogenic effect of increased triglycerides and thus explain the lack of association between total apoC-I and diabetes risk. The independent relationship of apoC-I proteoform composition with diabetes risk could be further explained by factors related to lipid metabolism that are not fully reflected by changes in plasma lipids, such as changes in VLDL or LDL particle size (12), and potential nonlipid factors such as modifying proinflammatory action of apoC-I (40).
Strengths of this study include the large sample size, follow-up duration up to 12 years, the diverse racial and ethnic background of the cohort, and ability to precisely measure proteoforms of apoC-I. We thereby were able to analyze regression models including a variety of key covariates. Importantly, we confirmed the inverse association between C-I′/C-I and development of diabetes in a smaller ACT NOW cohort, which required rigorous adjudication of T2D, and provide validation of our findings in MESA.
Limitations of our study include its observational design limiting inference regarding causality and relying on the schedule of MESA exams to determine the diabetes outcome. The enrollment of individuals without cardiovascular disease might have led to selection of relatively healthy individuals, which could reduce the cohort’s risk for diabetes, especially in the older participants. Lack of a strict diabetes adjudication protocol (i.e., reliance on single FG measurement from each visit) might have led to diabetes misclassification due to transient hyperglycemia. Although our findings were similar when diabetes diagnosis was based on initiation of diabetes medication, we cannot exclude a self-report bias in the later analysis. We were unable to distinguish type of diabetes in MESA, but incident type 1 diabetes is relatively uncommon in middle-aged or older adults, so we assume a predominance of T2D. Finally, the availability of follow-up data on serum insulin levels at only exam 5 might have led to attrition bias in assessment of the relationship between apoC-I measures and HOMA-IR. Nevertheless, these associations were consistent with those found for incident diabetes in the study and with the role of IR in T2D pathophysiology. Importantly, we observed very similar and consistent relationships between C-I′/C-I with incident T2D and longitudinal changes in the ISI in the ACT NOW cohort. Because of smaller size and relatively low number of T2D events in ACT NOW, we were unable to match the robustness of models in MESA. Finally, as indicated in our previous analyses (25), apoC-I proteoform composition changes with increasing age. Whether this change modulates propensity for diabetes is unknown.
Despite consistent results in two different cohorts, our findings remain hypothesis-generating. Studies are necessary to establish whether apoC-I truncation is causally related or indirectly reflects other contributing mechanisms to diabetes risk. Mendelian randomization studies of genetic variants associated with apoC-I truncation and outcomes such as T2D, hyperglycemia, and IR may be useful initial steps.
In conclusion, the findings of this study indicate posttranslational modification of apoC-I may contribute to risk of diabetes. Specifically, higher C-I′ and lower C-I concentrations, but not total apoC-I concentration, were independent predictors of incident diabetes and were related to future changes in plasma glucose and measures of whole-body IR. Identifying the underlying mechanisms of these associations will undoubtedly provide novel insights into our understanding how disturbances in lipid metabolism contribute to impairment of glucose regulation.
This article contains supplementary material online at https://doi.org/10.2337/figshare.27041416.
Article Information
Acknowledgments. The authors thank the other investigators, the staff, and the participants of the MESA and ACT NOW studies for their valuable contributions. A full list of participating MESA investigators and institutions can be found at https://www.mesa-nhlbi.org. A full list of ACT NOW investigators and institutions has been published previously (28).
Funding. This study was supported by the National Heart, Lung, and Blood Institute (grant R01-HL-138969) and National Institutes of Health (grant R24-DK090958). The MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute; and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. ACT NOW was supported by Takeda Pharmaceuticals.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
The contents of this article do not represent the views of the Department of Veterans Affairs or the U.S. Government.
Author Contributions. J.K. and P.D.R. researched data, contributed to discussion, and wrote the first draft of the manuscript. Y.H. and J.F. researched data. D.B., M.J.B., and A.G.B. reviewed and edited the manuscript. D.N., D.S., and R.L.M. researched data and reviewed and edited the manuscript. J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in part at the 84th ADA Scientific Sessions, Orlando, FL, 21–25 June 2024.
Handling Editors. The journal editors responsible for overseeing the review of the manuscript were Steven E. Kahn and Kristina M. Utzschneider.
Funding Statement
This study was supported by the National Heart, Lung, and Blood Institute (grant R01-HL-138969) and National Institutes of Health (grant R24-DK090958). The MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute; and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. ACT NOW was supported by Takeda Pharmaceuticals.
References
- 1. Gregg EW, Buckley J, Ali MK, et al. Global Health and Population Project on Access to Care for Cardiometabolic Diseases . Improving health outcomes of people with diabetes: target setting for the WHO Global Diabetes Compact. Lancet 2023;401:1302–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krauss RM. Lipids and lipoproteins in patients with type 2 siabetes. Diabetes Care 2004;27:1496–1504 [DOI] [PubMed] [Google Scholar]
- 3. Taskinen M-R. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia 2003;46:733–749 [DOI] [PubMed] [Google Scholar]
- 4. Szendroedi J, Yoshimura T, Phielix E, et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A 2014;111:9597–9602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ussher JR, Koves TR, Cadete VJJ, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 2010;59:2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kane JP, Pullinger CR, Goldfine ID, Malloy MJ.. Dyslipidemia and diabetes mellitus: role of lipoprotein species and interrelated pathways of lipid metabolism in diabetes mellitus. Curr Opin Pharmacol 2021;61:21–27 [DOI] [PubMed] [Google Scholar]
- 7. Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG.. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ 1995;310:560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dotevall A, Johansson S, Wilhelmsen L, Rosengren A.. Increased levels of triglycerides, BMI and blood pressure and low physical activity increase the risk of diabetes in Swedish women. A prospective 18-year follow-up of the BEDA study. Diabet Med 2004;21:615–622 [DOI] [PubMed] [Google Scholar]
- 9. Tirosh A, Shai I, Bitzur R, et al. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care 2008;31:2032–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waldman B, Jenkins AJ, Davis TME, et al.; FIELD Study Investigators . HDL-C and HDL-C/ApoA-I predict long-term progression of glycemia in established type 2 diabetes. Diabetes Care 2014;37:2351–2358 [DOI] [PubMed] [Google Scholar]
- 11. Abbasi A, Corpeleijn E, Gansevoort RT, et al. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: the PREVEND study. J Clin Endocrinol Metab 2013;98:E1352–1359 [DOI] [PubMed] [Google Scholar]
- 12. Mackey RH, Mora S, Bertoni AG, et al. Lipoprotein particles and incident type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis. Diabetes Care 2015;38:628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Silva NM, Freathy RM, Palmer TM, et al. Mendelian randomization studies do not support a role for raised circulating triglyceride levels influencing type 2 diabetes, glucose levels, or insulin resistance. Diabetes 2011;60:1008–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmad S, Mora S, Ridker PM, Hu FB, Chasman DI.. Gene-based elevated triglycerides and type 2 diabetes mellitus risk in the Women's Genome Health Study. Arterioscler Thromb Vasc Biol 2019;39:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haase CL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R.. HDL cholesterol and risk of type 2 diabetes: a Mendelian randomization study. Diabetes 2015;64:3328–3333 [DOI] [PubMed] [Google Scholar]
- 16. Fall T, Xie W, Poon W, et al.; GENESIS Consortium . Using genetic variants to assess the relationship between circulating lipids and type 2 diabetes. Diabetes 2015;64:2676–2684 [DOI] [PubMed] [Google Scholar]
- 17. Rouland A, Masson D, Lagrost L, Vergès B, Gautier T, Bouillet B.. Role of apolipoprotein C1 in lipoprotein metabolism, atherosclerosis and diabetes: a systematic review. Cardiovasc Diabetol 2022;21:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conde-Knape K, Bensadoun A, Sobel JH, Cohn JS, Shachter NS.. Overexpression of apoC-I in apoE-null mice: severe hypertriglyceridemia due to inhibition of hepatic lipase. J Lipid Res 2002;43:2136–2145 [DOI] [PubMed] [Google Scholar]
- 19. Gautier T, Masson D, Jong MC, et al. Apolipoprotein CI deficiency markedly augments plasma lipoprotein changes mediated by human cholesteryl ester transfer protein (CETP) in CETP transgenic/ApoCI-knocked out mice. J Biol Chem 2002;277:31354–31363 [DOI] [PubMed] [Google Scholar]
- 20. Muurling M, van den Hoek AM, Mensink RP, et al. Overexpression of APOC1 in obob mice leads to hepatic steatosis and severe hepatic insulin resistance. J Lipid Res 2004;45:9–16 [DOI] [PubMed] [Google Scholar]
- 21. Bouillet B, Gautier T, Aho LS, et al. Plasma apolipoprotein C1 concentration is associated with plasma triglyceride concentration, but not visceral fat, in patients with type 2 diabetes. Diabetes Metab 2016;42:263–266 [DOI] [PubMed] [Google Scholar]
- 22. Bouillet B, Gautier T, Blache D, et al. Glycation of apolipoprotein C1 impairs its CETP inhibitory property: pathophysiological relevance in patients with type 1 and type 2 diabetes. Diabetes Care 2014;37:1148–1156 [DOI] [PubMed] [Google Scholar]
- 23. Croyal M, Wargny M, Chemello K, et al. Plasma apolipoprotein concentrations and incident diabetes in subjects with prediabetes. Cardiovasc Diabetol 2022;21:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bondarenko PV, Cockrill SL, Watkins LK, Cruzado ID, Macfarlane RD.. Mass spectral study of polymorphism of the apolipoproteins of very low density lipoprotein. J Lipid Res 1999;40:543–555 [PubMed] [Google Scholar]
- 25. Koska J, Furtado J, Hu Y, et al. Plasma proteoforms of apolipoproteins C-I and C-II are associated with plasma lipids in the Multi-Ethnic Study of Atherosclerosis. J Lipid Res 2022;63:100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881 [DOI] [PubMed] [Google Scholar]
- 27. Joseph JJ, Echouffo-Tcheugui JB, Golden SH, et al. Physical activity, sedentary behaviors and the incidence of type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA). BMJ Open Diabetes Res Care 2016;4:e000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeFronzo RA, Tripathy D, Schwenke DC, et al.; ACT NOW Study . Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 29. Matsuda M, DeFronzo RA.. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 30. Jong MC, Voshol PJ, Muurling M, et al. Protection from obesity and insulin resistance in mice overexpressing human apolipoprotein C1. Diabetes 2001;50:2779–2785 [DOI] [PubMed] [Google Scholar]
- 31. Skinner NE, Wroblewski MS, Kirihara JA, Nelsestuen GL, Seaquist ER.. Sitagliptin results in a decrease of truncated apolipoprotein C1. Diabetes Ther 2015;6:395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng T, Qin L, Chen B, et al. Association of plasma DPP4 activity with mild cognitive impairment in elderly patients with type 2 diabetes: results from the GDMD study in China. Diabetes Care 2016;39:1594–1601 [DOI] [PubMed] [Google Scholar]
- 33. Mulvihill EE, Drucker DJ.. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev 2014;35:992–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ.. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017;136:849–870 [DOI] [PubMed] [Google Scholar]
- 35. Mingrone G, Henriksen FL, Greco AV, et al. Triglyceride-induced diabetes associated with familial lipoprotein lipase deficiency. Diabetes 1999;48:1258–1263 [DOI] [PubMed] [Google Scholar]
- 36. Walton RG, Zhu B, Unal R, et al. Increasing adipocyte lipoprotein lipase improves glucose metabolism in high fat diet-induced obesity. J Biol Chem 2015;290:11547–11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kitajima S, Morimoto M, Liu E, et al. Overexpression of lipoprotein lipase improves insulin resistance induced by a high-fat diet in transgenic rabbits. Diabetologia 2004;47:1202–1209 [DOI] [PubMed] [Google Scholar]
- 38. Aroner SA, Yang M, Li J, et al. Apolipoprotein C-III and high-density lipoprotein subspecies defined by apolipoprotein C-III in relation to diabetes risk. Am J Epidemiol 2017;186:736–744 [DOI] [PubMed] [Google Scholar]
- 39. Digenio A, Dunbar RL, Alexander VJ, et al. Antisense-mediated lowering of plasma apolipoprotein C-III by volanesorsen improves dyslipidemia and insulin sensitivity in type 2 diabetes. Diabetes Care 2016;39:1408–1415 [DOI] [PubMed] [Google Scholar]
- 40. Berbée JFP, Coomans CP, Westerterp M, Romijn JA, Havekes LM, Rensen PCN.. Apolipoprotein CI enhances the biological response to LPS via the CD14/TLR4 pathway by LPS-binding elements in both its N- and C-terminal helix. J Lipid Res 2010;51:1943–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]