Short abstract
This article is a Commentary on Blake‐Mahmud et al. (2025), 245: 885–898.
Keywords: gametophytes, independent generations, physiology, polyploidy, sporophytes
Ferns and lycophytes stand out among land plants for their unique life cycle, featuring two independent generations. By contrast, bryophyte sporophytes are ephemeral and rely on the gametophyte, whereas in seed plants, the gametophyte has been reduced to just a few cells and relies on the sporophyte for resources and protection from the environment. Despite these life cycle differences being well‐known for over a century, most research in ferns and lycophytes is still limited to the sporophyte, leaving a significant gap in our understanding of the natural and evolutionary history of these plants, and largely ignoring the enormous research potential of comparing the two generations. Building on previous research on the distribution and physiology of various fern sporophytes, a paper published in this issue of New Phytologist (Blake‐Mahmud et al., 2025; pp. 885–898) addresses this research gap by examining the stress resistance of fern gametophytes with the added layer of comparing species with different ploidy levels. The study subjected gametophytes from two triads of parental sporophyte diploids and their tetraploid offspring to various drought and heat stress conditions, hypothesizing that tetraploids would exhibit greater stress resistance. Although the results did not show as strong a trend as expected, they confirmed that tetraploids were indeed more stress resistant. Even more interestingly, species with widespread sporophytes apparently do not rely on broadly stress‐tolerant gametophytes, whereas rare taxa exhibited more flexible or robust gametophyte performance. These findings reinforce the critical need to deepen our understanding of gametophyte ecology and evolution across land plants.
‘It is intriguing to consider that a single species, with identical genetic material, might employ coordinated yet contrasting evolutionary strategies across its two generations.’
Fern and lycophyte gametophytes have been historically neglected for several reasons. Their simple and cryptic anatomy, with only a few distinguishing traits at the family level and even fewer at the species level, makes field identification challenging. This difficulty has led to a scarcity of ecological studies, although recent advances in genetic identification via DNA barcoding have begun to change this trend (Nitta & Chambers, 2022). Additionally, their small size – no more than a few centimeters in diameter – and, for some species, subterranean life style renders them less visually apparent compared with sporophytes, which can reach sizes of up to several meters. Gametophytes have long been considered the ‘weaker’ generation, perceived as less capable of coping with environmental stress, and thus frequently considered the limiting factor in population establishment and persistence. However, as Proctor (2007) convincingly argued in a previous commentary in New Phytologist, the notion of a ‘weaker’ generation is in itself conceptually flawed. Rather, the two generations should be viewed as distinct organisms, each with a unique life form and ecological niche, and each potentially limiting different aspects of its species biology (Pittermann et al., 2013).
As more research emerges on the gametophytes of ferns and lycophytes, the notion of them as the limiting generation is increasingly challenged. For instance, within the range of a fern species, the establishment and persistence of gametophytes may be restricted to specific microhabitats (Schneller & Farrar, 2022), potentially limiting the local abundance of the species. Yet, studies on tropical epiphytic ferns reveal that gametophytes can survive for several decades and may be more drought‐tolerant than sporophytes, with some species even exhibiting desiccation tolerance (Watkins Jr et al., 2007). In temperate regions, gametophytes have also been found to endure multiple years, surviving summer droughts and winter frosts (Schneller & Farrar, 2022). These findings suggest that gametophytes are sturdier than previously thought and that, in some cases, environmental factors affecting sporophytes may actually limit their distribution. As a result, an increasing number of species are now documented with gametophytes that have broader distribution ranges than their corresponding sporophytes, with some species even existing exclusively as gametophytes that reproduce vegetatively (Pinson et al., 2017; Nitta et al., 2021). This shift in understanding emphasizes that to truly grasp their evolution and global distribution patterns, we must consider ferns and lycophytes as complete bigenerational organisms.
Adopting this comprehensive view is essential not only because both generations are crucial for species persistence but also because they experience very different environmental pressures (Fig. 1a). Although both generations depend on water availability, their responses to water stress are markedly different. Gametophytes, lacking vascular tissue, a cuticle, and stomata, are poikilohydric, that is they cannot actively regulate their water content and are entirely dependent on environmental conditions (Pittermann et al., 2013). As a result, gametophytes often thrive in sheltered habitats where water stress is reduced, but where light may be limiting. By contrast, sporophytes are homoiohydric in the majority of species, with the ability to regulate their water content. Their vascular tissues allow them to achieve larger sizes and to benefit from increased light availability for photosynthesis but thereby exposing sporophytes to environmental stressors such as wind and higher vapor pressure deficit (VPD). This dichotomy means that gametophytes and sporophytes of a given species are likely subject to different adaptive constraints and may adopt very different strategies to cope with environmental pressures.
Fig. 1.
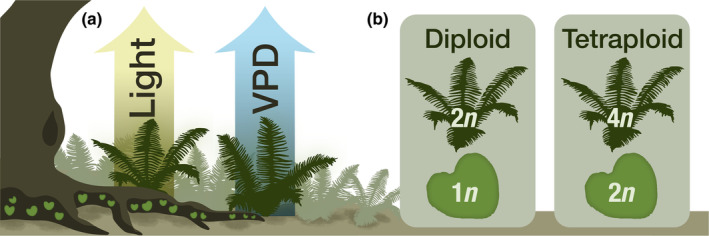
Schematic representation of ecological differences and ploidy levels between conspecific gametophytes and sporophytes. (a) Microclimatic differences between growth sites, with gametophytes typically found in darker, more humid environments and sporophytes in brighter areas with higher vapor pressure deficit (VPD). (b) A comparison of two fern species, highlighting ploidy‐level distribution across generations: one species traditionally termed diploid, with diploid sporophytes and haploid gametophytes, and another termed tetraploid, featuring tetraploid sporophytes and diploid gametophytes.
Blake‐Mahmud et al. illustrated this perfectly, in that the species with as broad distributions as sporophytes do not have the most stress‐tolerant gametophytes, whereas taxa that are less widespread and more local sporophytes have more robust gametophytes. This dichotomy not only highlights once again the necessity of studying both generations of ferns and lycophytes but also reveals the untapped experimental potential of contrasting gametophytes and sporophytes. It is intriguing to consider that a single species, with identical genetic material, might employ coordinated yet contrasting evolutionary strategies across its two generations.
This principle also applies to understanding polyploidization in ferns, where the two generations – gametophyte and sporophyte – have different ploidy levels yet, as a community, we often neglect the research potential of this difference. Polyploidization has played a critical role in the evolution of land plants by freeing duplicated gene copies from their original functions, allowing them to evolve new roles. Even when gene functions remain unchanged, having multiple alleles can enhance the adaptive potential of a species. This has led to the hypothesis that polyploid species may have evolutionary and adaptive advantages over their diploid ancestors (Soltis & Soltis, 2000; Van de Peer et al., 2021). While this idea has been partially supported in angiosperms, it remains underexplored in seed‐free vascular plants such as ferns and lycophytes.
Blake‐Mahmud et al. contribute to this discussion by examining polyploidization at the gametophyte stage, comparing stress resistance between species with haploid and diploid gametophytes. Their findings suggest that diploid gametophytes are somewhat more resilient, but this is just one of many insights that could be gained by comparing ploidy levels in ferns and lycophytes. We emphasize two areas where further research could be particularly fruitful.
First, in angiosperms, polyploidization is often quickly followed by diploidization, accompanied by a reduction in genome size and chromosome number through processes such as gene loss, chromosome loss, and gene silencing (Soltis et al., 2015). In ferns, while genic diploidization also occurs rapidly, it is not necessarily accompanied by genome downsizing (Zhong et al., 2022). Instead, diploidization seems to be driven by processes such as pseudogenization and gene deletion through recombination (Li et al., 2021). This retention of large genomes, known as the ‘polyploidy paradox’ (Soltis & Soltis, 2000), has been recognized for decades, but the evolutionary and adaptive consequences of the maintenance of most of the genomes in ferns in contrast with genome reduction in angiosperms remain poorly understood. To date, there has been little exploration of the potential of comparing the evolutionary fate and regulation of gene families that occur in both ferns and angiosperms, such as those related to photosynthesis or cell growth, which are ubiquitous in land plants.
Second, gametophytes in ferns are haploid relative to the diploid sporophytes of the same species, or diploid in species with tetraploid sporophytes (Fig. 1b). This means that recessive genes that are activated are fully expressed in the haploid gametophyte stage and are subject to purifying selection. While many genes are likely expressed only in one generation, there are also numerous genes that function in both. Exploring these genes offers an exceptional opportunity to understand the adaptive and evolutionary potential of genes at different ploidy levels within a single species rather than through interspecies comparisons, as is usually done when comparing different ploidy levels.
Whether through molecular, physiological, or ecological studies, it is evident that plant scientists should no longer overlook the inclusion of both gametophytes and sporophytes in the study of ferns and lycophytes. The sheer size of fern genomes and the high level of gene duplication have long hindered genomic studies, particularly when compared to angiosperms, but recent breakthroughs have led to the successful assembly of whole genomes for several species (e.g. Li et al., 2018; Zhong et al., 2022). We are now poised at the threshold of a new era of research on seed‐free vascular plant evolution, with far‐reaching implications for our understanding of land plants as a whole. However, while genomic research will undoubtedly be crucial, the true depth of understanding will come from integrating these insights with physiological and ecological data, as Blake‐Mahmud et al. have compellingly shown.
Acknowledgements
The Swiss National Science Foundation (grant no. 310030_188498) for funding, and Ingrid Olivares and Scott McAdam for reviewing the manuscript. Open access funding provided by Universitat Zurich.
This article is a Commentary on Blake‐Mahmud et al. (2025), 245: 885–898.
Contributor Information
Michael Kessler, Email: michael.kessler@systbot.uzh.ch.
Daniela Aros‐Mualin, Email: darosmualin@gmail.com.
References
- Blake‐Mahmud J, Sessa EB, Visger CJ, Watkins JE Jr. 2025. Polyploidy and environmental stress response: a comparative study of fern gametophytes. New Phytologist 245: 885–898. [DOI] [PubMed] [Google Scholar]
- Li FW, Brouwer P, Carretero‐Paulet L, Cheng S, De Vries J, Delaux PM, Eily A, Koppers N, Kuo L‐Y, Zheng L et al. 2018. Fern genomes elucidate land plant evolution and cyanobacterial symbioses. Nature Plants 4: 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, McKibben MT, Finch GS, Blischak PD, Sutherland BL, Barker MS. 2021. Patterns and processes of diploidization in land plants. Annual Review of Plant Biology 72: 387–410. [DOI] [PubMed] [Google Scholar]
- Nitta JH, Chambers SM. 2022. Identifying cryptic fern gametophytes using DNA barcoding: a review. Applications in Plant Sciences 10: e11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta JH, Watkins JE Jr, Holbrook NM, Wang TW, Davis CC. 2021. Ecophysiological differentiation between life stages in filmy ferns (Hymenophyllaceae). Journal of Plant Research 134: 971–988. [DOI] [PubMed] [Google Scholar]
- Pinson JB, Chambers SM, Nitta JH, Kuo LY, Sessa EB. 2017. The separation of generations: biology and biogeography of long‐lived sporophyteless fern gametophytes. International Journal of Plant Sciences 178: 1–18. [Google Scholar]
- Pittermann J, Brodersen C, Watkins JE Jr. 2013. The physiological resilience of fern sporophytes and gametophytes: advances in water relations offer new insights into an old lineage. Frontiers in Plant Science 4: e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MCF. 2007. Ferns, evolution, scale and intellectual impedimenta. New Phytologist 176: 504–506. [DOI] [PubMed] [Google Scholar]
- Schneller JJ, Farrar DR. 2022. Photographic analysis of field‐monitored fern gametophyte development and response to environmental stress. Applications in Plant Sciences 10: e11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis PS, Marchant DB, Van de Peer Y, Soltis DE. 2015. Polyploidy and genome evolution in plants. Current Opinion in Genetics & Development 35: 119–125. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE. 2000. The role of genetic and genomic attributes in the success of polyploids. Proceedings of the National Academy of Sciences, USA 97: 7051–7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Peer Y, Ashman TL, Soltis PS, Soltis DE. 2021. Polyploidy: an evolutionary and ecological force in stressful times. Plant Cell 33: 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JE Jr, Mack MC, Sinclair TR, Mulkey SS. 2007. Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytologist 176: 708–717. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Liu Y, Wu W, Chen J, Sun C, Liu H, Shu J, Ebihara A, Yan Y, Zhou R et al. 2022. Genomic insights into genetic diploidization in the homosporous fern Adiantum nelumboides . Genome Biology and Evolution 14: evac127. [DOI] [PMC free article] [PubMed] [Google Scholar]


