Abstract
Background
Rheumatic mitral stenosis (RMS) is a common valvular heart disease in developing countries. We sought to evaluate the early experience of patients with RMS undergoing transcatheter mitral valve replacement (TMVR).
Methods
In this retrospective study, a total of 5 RMS patients accepted TMVR. All patients underwent computed tomography and echocardiography before having the procedure. After the preprocedural comprehensive evaluations, the surgeons planned to use the Prizvalve (a novel balloon-expandable transcatheter aortic valve system which is now under the clinical registration study) for TMVR. Clinical data were collected at baseline, before discharge, and at the 30-day follow-up.
Results
The median age of the 5 RMS patients was 61 years (range 60–77 years); 60% were male, and the median Society of Thoracic Surgeons score was 13.3% (range 6.2–17.1%). TMVR was successful in all patients. Postoperative transesophageal echocardiography showed that 60.0% (n = 3) of the patients had no paravalvular leakage and 40.0% (n = 2) had trace paravalvular leakage. The median postoperative peak velocity decreased to 1.4 m/s (range 1.1–1.7 m/s), and the median pressure gradient decreased to 3 mmHg (range 2–3 mmHg). No deaths occurred at the 30-day follow-up, and all patients had an improvement of ≥1 on the New York Heart Association functional rating.
Conclusions
Our early experience with TMVR in RMS patients suggests that it is a safe and feasible procedure. The early results of the procedure are acceptable and provide bright prospects and directions for the precision treatment of RMS.
Clinical Trial Registration
ClinicalTrials.gov, identifier (NCT02917980).
Keywords: mitral stenosis, rheumatic valvular heart disease, transcatheter mitral valve replacement, prizvalve, mitral valve
Introduction
Rheumatic valvular heart disease is one of the major diseases affecting human health around the world. Studies have shown that 33.4 million people worldwide suffer from rheumatic valvular heart disease (1). Rheumatic mitral stenosis (RMS) is particularly common in developing countries (2). At present, the main treatment options for rheumatic mitral valve (MV) disease include MV valvuloplasty and MV replacement (3). Evidence-based studies have shown that, compared with MV replacement, MV valvuloplasty has significant advantages in periprocedural risk and the long-term survival rate (4, 5).
However, for patients with end-stage RMS, MV valvuloplasty is a high-risk procedure with a high likelihood of potential complications. It is worth noting that the development of transcatheter aortic valve replacement in recent years has promoted the exploration of transcatheter mitral valve replacement (TMVR). TMVR largely solves the problem of the ineffectiveness of drug therapy and the high risk of surgical treatment (6, 7). TMVR does not require a thoracotomy and cardiopulmonary bypass and has a better therapeutic effect and prognosis, which offers a new choice for patients with MV disease.
The purpose of this study was to evaluate our early experience using Prizvalve (Newmed Medical Technology Co., LTD., Shanghai, China) for transfemoral TMVR in RMS patients (8). To our knowledge, this is the largest single-center cohort of RMS patients treated with TMVR thus far.
Methods
Study population
From September 2022 to June 2023, 5 RMS patients were treated with TMVR at Xijing Hospital. Inclusion criteria included transthoracic echocardiography (TTE), and computed tomography angiography (CTA) confirmed the diagnosis of RMS. In this study, the selection criteria for RMS patients accepting TMVR were as follows: (1) Severe mitral stenosis with orifice area <1.0 cm2; (2) Mitral annular calcification; (3) Preoperative LVOT area ≥150 mm2; (4) The annulus was observed by CT to estimate the anchoring position and effect. Exclusion criteria included other causes of secondary mitral stenosis, including infective endocarditis, congenital heart disease, and previous left cardiac system surgery. This study was carried out in accordance with the principles of the Declaration of Helsinki and met the relevant ethical requirements. All patients provided written informed consent for TMVR and subsequent data collection.
Device
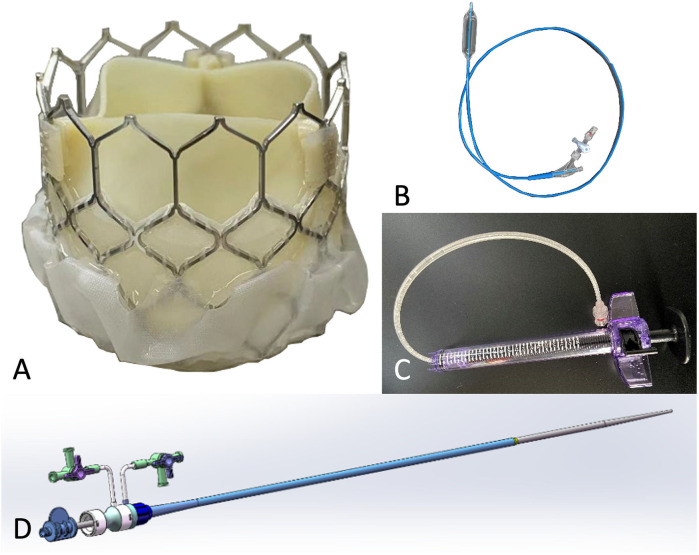
The Prizvalve balloon-expandable valve (Newmed Medical Co., LTD., Shanghai, China) (Figure 1), made of bovine pericardium, was used in the study. The bottom of the stent valve is covered with a polyester membrane, which can reduce the occurrence of paravalvular leakage. There are three marked points in the middle of the valve that can be clearly seen under fluoroscopy to assist in the accurate positioning of the valve. It is available in 4 sizes: 20 mm, 23 mm, 26 mm, and 29 mm.
Figure 1.
Characteristics of the prizvalve balloon-expandable valve (newmed medical Co., LTD., Shanghai, China). (A) Transcatheter heart valve. (B) Balloon. (C) Balloon pressure pump. (D) Expandable arterial sheath.
Preprocedural imaging assessment
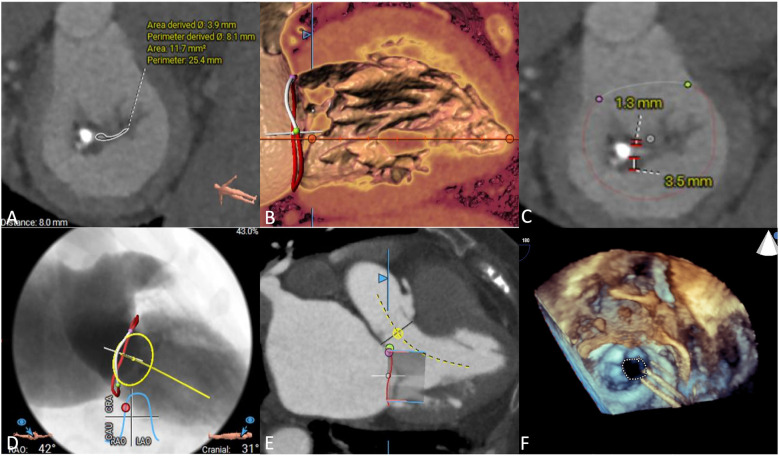
We used CTA to evaluate MV anatomy before the procedures, including evaluation of the mitral annulus, leaflets, left ventricular outflow tract and subvalvular apparatus (Figures 2A–E). Coronary angiography was used to rule out severe coronary artery disease. All patients underwent preprocedural TTE to assess MV and right cardiac system functions (Figure 2F). After assessing each patient's age, frailty, comorbidities, and surgical risk, the cardiac team recommended that all patients undergo TMVR.
Figure 2.
Preprocedural imaging assessments. (A) Mitral annulus measurement, the annular area was 11.7 mm2. (B) Subvalvular apparatus evaluation, chordae tendinae was shown thickened. (C) Leaflets of mitral valve evaluation. (D) Implanted projection angle determination. (E) Neo-left ventricular outflow tract prediction. (F) Rheumatic mitral stenosis was displayed in preprocedural echocardiography.
Procedures
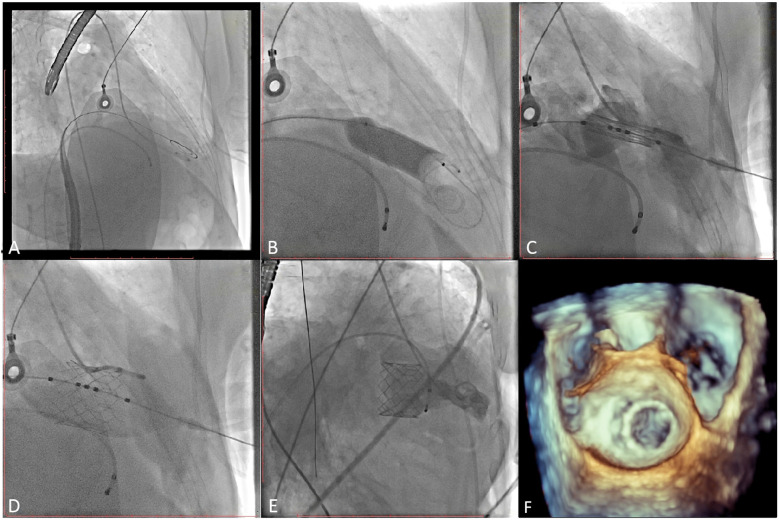
The patient's bilateral femoral artery region was disinfected; the right femoral vein was selected as the puncture point; and the patient was anesthetized with 2 ml of 2% lidocaine administered via local infiltration. After a successful puncture, a 5 Fr arterial sheath tube was placed, and 3,000 units of heparin was injected intravenously. A 6F sheath tube was inserted through the right common jugular vein and the pacemaker catheter was sent to columnae cordis of right ventricle. Subsequently, a 6 Fr sheath was used to lead the 1.5 m Superstiff guide wire (Boston Science Co. Ltd., Boston, USA) to the left atrium through the atrial septal puncture, and the 6 Fr pigtail catheter was inserted to the left ventricle through the guide wire under digital subtraction angiography (DSA) guidance (Figures 3A,B; Supplementary Videos 1, 2). Then, the mitral annulus was marked, and the Prizvalve delivery system was implanted via the guide wire. Pacing to 180 beats/min, and the Prizvalve prothesis was gradually released (Figure 3C; Supplementary Video 3). After the release was completed (Figure 3D; Supplementary Video 4), DSA (Figure 3E; Supplementary Video 5) and transesophageal echocardiography (TEE) (Figure 3F; Supplementary Video 6) showed that the prosthesis was well fixed without regurgitation or paravalvular leakage (PVL).
Figure 3.
Procedural details. (A) The pigtail catheter was inserted into the left ventricle. (B) Pre-dilation was used before delivery system insertion. (C) The prothesis was positioned at mitral valve. (D) The prosthesis was completed release and post-dilation. (E, F) Digital subtracted angiography and transesophageal echocardiography displayed the good position and function of the prosthesis.
Data collection and statistical analysis
The clinical records of all patients were reviewed. TTE was performed 30 days after TMVR to examine the improvement of cardiac function. In addition, all patients underwent CTA at the 30-day follow-up.
SPSS 26.0 software (IBM Corp, Armonk, NY, USA) was used for the statistical analyses. Continuous variables were reported as the median (25th and 75th percentiles), and categorical variables were expressed as frequency and percentage. A double-tail P-value <0.05 was considered statistically significant.
Results
Baseline characteristics
The median age of patients with RMS was 61 years (range 60–77 years); 60.0% (n = 3) were male; and the median Society of Thoracic Surgeons score was 13.3% (range 6.2–17.1%). Detailed baseline characteristics are shown in Table 1. Patient #2 had a history of stroke, and 2 patients had atrial fibrillation. Importantly, the median Wilkins score was 12 (range 10–12). Before TMVR, all patients were evaluated by an interdisciplinary cardiac team and were considered either unable to undergo MV repair or at higher risk due to comorbidities. The results of preprocedural imaging measurements are listed in Table 2. It is worth noting that the median MV area was 0.6 cm2 (range 0.5–0.8 cm2), the median peak velocity was 2.7 m/s (range 2.4–3.0 m/s), and the median mean pressure gradient was 9 mmHg (range 7–11 mmHg). All patients had ≥ moderate mitral regurgitation. Importantly, 4 patients (80.0%) had ≥ moderate tricuspid regurgitation and 3 patients (60.0%) had pulmonary hypertension.
Table 1.
Baseline characteristics.
| Characteristics | Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 |
|---|---|---|---|---|---|
| Age, years | 75 | 61 | 60 | 60 | 77 |
| Sex | Female | Male | Male | Male | Female |
| Body mass index, kg/m2 | 20.6 | 24.5 | 25.2 | 26.0 | 21.7 |
| Diabetes mellitus | No | No | No | No | No |
| Hypertension | Yes | No | No | No | Yes |
| Dyslipidemia | No | No | No | No | No |
| Cerebrovascular disease | No | Yes | No | No | No |
| Peripheral artery disease | No | No | No | No | No |
| Chronic kidney disease | No | No | No | No | Yes |
| Coronary artery disease | No | No | No | No | No |
| Previous PCI | No | No | No | No | No |
| Previous CABG | No | No | No | No | No |
| Atrial fibrillation | No | Yes | Yes | No | No |
| Previously implanted pacemaker | No | No | No | No | No |
| NYHA functional class | III | III | III | III | IV |
| STS score,% | 14.2 | 17.1 | 8.6 | 6.2 | 13.3 |
| Wilkins score | 12 | 12 | 11 | 10 | 12 |
CABG, coronary artery bypass graft; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons.
Table 2.
Preprocedural imaging assessment.
| Characteristics | Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 |
|---|---|---|---|---|---|
| Transthoracic echocardiography | |||||
| Left ventricular ejection fraction, % | 58 | 52 | 37 | 55 | 53 |
| Mitral annular area, cm2 | 0.7 | 0.5 | 0.6 | 0.6 | 0.8 |
| Mitral valve peak velocity, m/s | 2.4 | 3.0 | 2.8 | 2.7 | 2.4 |
| Mean mitral valve pressure gradient, mmHg | 8 | 11 | 10 | 7 | 9 |
| Mean left ventricular outflow tract pressure gradient, mmHg | 24 | 29 | 26 | 26 | 25 |
| Combined with ≥ moderate MR | Yes | Yes | Yes | Yes | Yes |
| Combined with ≥ moderate TR | Yes | Yes | Yes | No | Yes |
| Combined with pulmonary hypertension | No | Yes | Yes | No | Yes |
| Computed tomography angiography | |||||
| Left atrial left–right diameter, mm | 55.27 | 53.50 | 57.42 | 52.68 | 49.76 |
| Left ventricular diameter, mm | |||||
| End-diastolic left ventricle diameter (long axis) | 66.14 | 79.62 | 74.73 | 82.27 | 69.14 |
| End-diastolic left ventricle diameter (short axis) | 45.31 | 46.83 | 46.58 | 56.19 | 50.83 |
| Left-right diameter | 46.62 | 44.57 | 47.03 | 57.17 | 50.50 |
MR, mitral regurgitation; TR, tricuspid regurgitation.
Procedural details
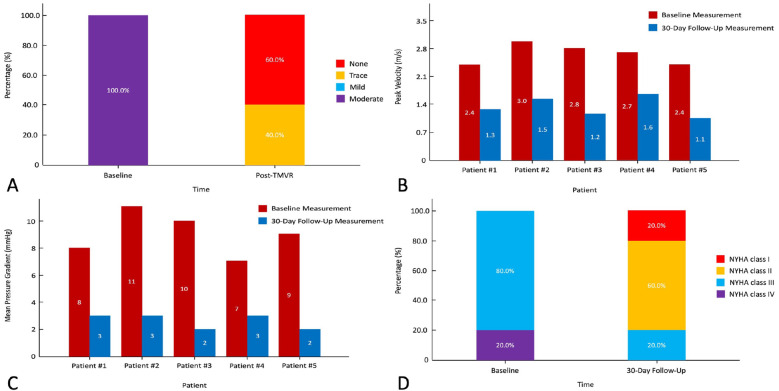
Detailed procedural outcomes are shown in Table 3. All (100%) patients were successfully implanted with the Prizvalve prosthesis. Two 26-mm and three 29-mm prostheses were implanted according to the mitral annulus diameter measured by CTA and TEE. No patient converted to open surgery, and no major adverse cerebrovascular events occurred. Furthermore, there was no annulus rupture or device displacement. The median total operating time was 170 min (range 85–215 min), the median DSA time was 22 min (range 13–35 min), and the median contrast volume was 118 ml (range 89–142 ml). Postoperative transesophageal echocardiography showed that 60.0% (n = 3) of the patients had no PVL and that 40.0% (n = 2) of the patients had trace PVLs (Figure 4A). It is worth noting that patient #3 developed severe hypotension (63/37 mmHg) after the procedures and was given extracorporeal membrane oxygenation. As expected, the median postprocedural peak velocity decreased to 1.4 m/s (range 1.1–1.7 m/s) (Figure 4B), and the median mean pressure gradient decreased to 3 mmHg (range 2–3 mmHg) (Figure 4C).
Table 3.
Procedural details.
| Characteristics | Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 |
|---|---|---|---|---|---|
| Intraprocedural outcomes | |||||
| Prosthetic size, mm | 29 | 26 | 26 | 29 | 29 |
| Procedure duration, min | 215 | 205 | 160 | 85 | 170 |
| Fluoroscopy time, min | 35 | 26 | 19 | 13 | 22 |
| Contrast volume, min | 142 | 133 | 114 | 89 | 118 |
| Complications | |||||
| Conversion to surgery | No | No | No | No | No |
| Prosthesis displacement | No | No | No | No | No |
| Annular rupture | No | No | No | No | No |
| Paravalvular leakage | Trace | None | Trace | None | None |
| Third-degree atrioventricular block | No | No | No | No | No |
| Postprocedural transesophageal echocardiography parameters | |||||
| Extracorporeal membrane oxygenation | No | No | Yes | No | No |
| Left ventricular ejection fraction,% | 54 | 50 | 35 | 53 | 55 |
| Mitral valve peak velocity, m/s | 1.4 | 1.6 | 1.2 | 1.7 | 1.1 |
| Mean mitral valve pressure gradient, mmHg | 3 | 3 | 2 | 3 | 2 |
| Mean left ventricular outflow tract pressure gradient, mmHg | 1 | 4 | 1 | 2 | 2 |
Figure 4.
Procedural and follow-up outcomes of patients with rheumatic mitral stenosis. (A) Severity of paravalvular leakage. (B) Peak velocity of the mitral valve. (C) Mean pressure gradient. (D) New York Heart Association functional class. NYHA, New York Heart Association.
Clinical outcomes
The major clinical outcomes and follow-up data are shown in Table 4. Three patients (60.0%) were extubated in the intensive care unit on the first day after the procedure. According to the Acute Kidney Injury Network criteria (9), patient #3 developed stage 3 acute kidney injury. After medication, the patient's symptoms improved significantly, and he recovered before discharge without dialysis. The median length of stay in the intensive care unit and in the hospital was 2 days (range 1–6 days) and 13 days (range 6–22 days), respectively. There were no deaths, no neurological complications (including stroke and transient ischemic attacks), or myocardial infarction/vascular complications during the hospitalization. No patients required permanent pacemaker implants during the periprocedural period. At the 30-day follow-up, one patient (20.0%) was in NYHA Class I and the other 4 patients (80.0%) were in NYHA Class II (Figure 4D). No major adverse events occurred, and 3 patients (60.0%) experienced reverse remodeling of the left cardiac system.
Table 4.
Hospitalization and follow-up outcomes.
| Characteristics | Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 |
|---|---|---|---|---|---|
| Hospitalization outcomes | |||||
| Death | No | No | No | No | No |
| Major adverse cardiovascular events | No | No | No | No | No |
| Stroke | No | No | No | No | No |
| Life-threatening/major bleeding | No | No | No | No | No |
| Major vascular complication | No | No | No | No | No |
| Acute kidney injury stage 2 or 3 | Yes | No | Yes | No | No |
| Permanent pacemaker implant | No | No | No | No | No |
| Extubating time, days | 3 | 1 | 3 | 1 | 1 |
| ICU stay, days | 4 | 1 | 6 | 1 | 2 |
| Hospitalization stay, days | 6 | 7 | 22 | 14 | 13 |
| Follow-up outcomes | |||||
| Death | No | No | No | No | No |
| Major adverse cardiovascular events | No | No | No | No | No |
| NYHA functional class | II | I | II | II | III |
| Left ventricular ejection fraction,% | 56 | 52 | 42 | 55 | 56 |
| Mitral valve peak velocity, m/s | 1.3 | 1.5 | 1.2 | 1.6 | 1.1 |
| Mean mitral valve pressure gradient, mmHg | 3 | 3 | 2 | 3 | 2 |
| Mean left ventricular outflow tract pressure gradient, mmHg | 2 | 3 | 1 | 2 | 1 |
| Left atrial left–right diameter, mm | 53.43 | 51.18 | 57.20 | 51.65 | 50.71 |
| Left ventricular diameter, mm | |||||
| End-diastolic left ventricle diameter (long axis) | 63.89 | 81.17 | 71.23 | 79.88 | 70.33 |
| End-diastolic left ventricle diameter (short axis) | 43.65 | 48.37 | 44.79 | 54.50 | 51.62 |
| Left–right diameter | 43.70 | 49.63 | 45.41 | 53.92 | 52.08 |
ICU, intensive care unit; NYHA, New York Heart Association.
Discussion
The early clinical outcomes of this study found that TMVR with Prizvalve was safe and feasible in patients with RMS. China is ranked second in the world for the incidence of rheumatic valvular disease (1, 10). RMS is characterized by comprehensive pathological changes in the mitral annulus, chordae tendineae, and the papillary muscle at the same time. The pathological changes are relatively complex, resulting in various manifestations of MV dysfunction. Importantly, the lack of calcification of the mitral annulus in patients with RMS may lead to increased difficulty in positioning the prosthesis. Therefore, for the treatment of such patients, ensuring the integrity of the prosthesis morphology plays an important role in maintaining left ventricular function (11).
Unlike transcatheter aortic valve replacement, TMVR devices are more difficult to develop. Due to the asymmetric saddle-shaped MV anatomy, the MV is greatly deformed when the heart is beating, and the pressure gradient is relatively high, so the prosthesis is easy to shift and has the risk of PVL. Furthermore, the MV is adjacent to the left ventricular outflow tract, and the prosthesis may cause left ventricular outflow tract obstruction (12). However, TMVR has several distinct advantages over transcatheter MV repair. First, TMVR is suitable for different types of MV disease and different pathological changes. Second, TMVR has better operability and a higher success rate than transcatheter MV repair. Third, the hemodynamic improvement immediately after the procedure is better and the effect is more stable (13). This study shows that although the long-term follow-up is still in progress, TMVR has demonstrated certain technical advantages in the treatment of elderly patients with RMS who are at high risk and cannot tolerate a thoracotomy.
In our experience, it is crucial to accurately determine the annulus diameter before TMVR. If the size of the prosthesis is not large enough, the anatomical and pathophysiological uniqueness of RMS may lead to an increased incidence of PVL and device displacement. It is worth noting that careful attention should be paid to positioning when the device is implanted. If the device is positioned inaccurately, PVL (14) and/or left ventricular outflow tract obstruction (15) may develop.
This study has some limitations. First, it a single-center study with a small sample size, so more samples need to be accumulated in the future to confirm the safety and efficacy of TMVR. Second, procedures were completed by interventionists. There is a lack of standardized procedures for the selection of device type, prosthetic size, procedural approach, and postprocessing methods related to TMVR, as well as the process of device deployment. The heterogeneity may affect the reliability of the conclusions obtained. In addition, the follow-up period is relatively short; there is a need to explore more long-term follow-up observations. Finally, we did not use the dedicated devices (such as Highlife, Tendyne, and Intrepid, etc.) to treat such patients. The reasons are as follows: First of all, unfortunately, the dedicated devices are not currently being used in China. In this study, we used the PrizValve balloon-expandable valve, and the clinical outcomes with small sample size indicated that the bioprosthesis may allow such patients to obtain a larger effective orifice area after procedures. In addition, based on the clinical outcomes obtained in this study, the clinical development of TMVR using the PrizValve balloon-expandable valve in MS patients may carry out in the next step to further evaluate the clinical safety and efficacy of the device in patients with MS.
Conclusions
Overall, TMVR may be an alternative treatment for RMS patients with high surgical risk. Our early experience with TMVR in patients with RMS shows that these procedures are feasible and that early clinical outcomes are reliable. The specific anatomical challenges of RMS may require more in-depth analysis to ensure better results. In addition, further sample collection and follow-up are needed to determine the safety and efficacy of this approach.
Acknowledgments
We would like to thank Make Medical Technology Co., LTD (Xi'an, China) for supplying the 3-dimensional printed models and Protext Editorial Services, USA, for English language editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China (2020YFC2008100); the Development and Transformation of New Technology and Construction of Precision Diagnosis and Treatment System for Transcatheter Interventional Diagnosis and Treatment of Structural Heart Diseases (2022YFC2503400); National Natural Science Foundation (82370375); Research on Key Techniques of Minimally Invasive Treatment for Valvular Heart Diseases (2023-YBSF-105); Xijing Hospital Booster Foundation (XJZT24LY42).
Abbreviations
CTA, computed tomography angiography; DSA, digital subtracted angiography; MV, mitral valve; PVL, paravalvular leakage; RMS, rheumatic mitral stenosis; TMVR, transcatheter mitral valve replacement; TTE, transthoracic echocardiography.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by Ethics Committee of Xijing Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PJ: Conceptualization, Writing – original draft. HG: Conceptualization, Data curation, Writing – original draft. YM: Conceptualization, Data curation, Writing – original draft. MZ: Data curation, Writing – original draft. YL: Writing – review & editing. JY: Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1424105/full#supplementary-material
The pigtail catheter was inserted into the left ventricle.
Pre-dilation.
Position.
Release.
Postprocedural digital subtracted angiography.
Postprocedural transesophageal echocardiography.
References
- 1.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. (2017) 377:713–22. 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 2.Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. (2007) 357:470–6. 10.1056/NEJMoa065085 [DOI] [PubMed] [Google Scholar]
- 3.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2021) 143:e227–72. 10.1161/CIRCULATIONAHA.120.051941 [DOI] [PubMed] [Google Scholar]
- 4.Fu J, Li Y, Zhang H, Han J, Jiao YQ, Du J, et al. Outcomes of mitral valve repair compared with replacement for patients with rheumatic heart disease. J Thorac Cardiovasc Surg. (2021) 162:72–82.e7. 10.1016/j.jtcvs.2020.01.053 [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Zhou C, Gu H, Zheng Z, Hu S. Mitral valve repair versus replacement in patients with rheumatic heart disease. J Heart Valve Dis. (2013) 22:333–9. [PubMed] [Google Scholar]
- 6.Ahmed MI, Aban I, Lloyd SG, Gupta H, Howard G, Inusah S, et al. A randomized controlled phase IIb trial of beta (1)-receptor blockade for chronic degenerative mitral regurgitation. J Am Coll Cardiol. (2012) 60:833–8. 10.1016/j.jacc.2012.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorilli PN, Herrmann HC, Szeto WY. Transcatheter mitral valve replacement: latest advances and future directions. Ann Cardiothorac Surg. (2021) 10:85–95. 10.21037/acs-2020-mv-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao Y, Liu Y, Ma Y, Jin P, Li L, Yang J. Mitral valve-in-valve implant of a balloon-expandable valve guided by 3-dimensional printing. Front Cardiovasc Med. (2022) 9:894160. 10.3389/fcvm.2022.894160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. (2007) 11:R31. 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remenyi B, ElGuindy A, Smith SC, Yacoub M, Holmes DR, Jr. Valvular aspects of rheumatic heart disease. Lancet. (2016) 387:1335–46. 10.1016/S0140-6736(16)00547-X [DOI] [PubMed] [Google Scholar]
- 11.Athanasiou T, Chow A, Rao C, Aziz O, Siannis F, Ali A, et al. Preservation of the mitral valve apparatus: evidence synthesis and critical reappraisal of surgical techniques. Eur J Cardiothorac Surg. (2008) 33:391–401. 10.1016/j.ejcts.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 12.Goode D, Dhaliwal R, Mohammadi H. Transcatheter mitral valve replacement: state of the art. Cardiovasc Eng Technol. (2020) 11:229–53. 10.1007/s13239-020-00460-4 [DOI] [PubMed] [Google Scholar]
- 13.Sorajja P, Moat N, Badhwar V, Walters D, Paone G, Bethea B, et al. Initial feasibility study of a new transcatheter mitral prosthesis: the first 100 patients. J Am Coll Cardiol. (2019) 73:1250–60. 10.1016/j.jacc.2018.12.066 [DOI] [PubMed] [Google Scholar]
- 14.Sorajja P, Vemulapalli S, Feldman T, Mack M, Holmes DR, Jr, Stebbins A, et al. Outcomes with transcatheter mitral valve repair in the United States: an STS/ACC TVT registry report. J Am Coll Cardiol. (2017) 70:2315–27. 10.1016/j.jacc.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 15.Wang DD, Eng MH, Greenbaum AB, Myers E, Forbes M, Karabon P, et al. Validating a prediction modeling tool for left ventricular outflow tract (LVOT) obstruction after transcatheter mitral valve replacement (TMVR). Catheter Cardiovasc Interv. (2018) 92:379–87. 10.1002/ccd.27447 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The pigtail catheter was inserted into the left ventricle.
Pre-dilation.
Position.
Release.
Postprocedural digital subtracted angiography.
Postprocedural transesophageal echocardiography.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.