Abstract
Background:
Neonatal herpes simplex virus (HSV) infection is life-threatening, with a mortality of up to 70–80% when disseminated, often due to vague symptoms and delayed treatment. Neonatal screening using dried blood spot (DBS) samples is among the most impactful preventative health measures ever implemented, but screening for HSV has not been investigated.
Methods:
We investigated high throughput multiplexed proteomics on DBS samples collected on days 2–3 of life from a nationwide cohort of neonates with HSV infection (n = 53) and matched controls. We measured 2941 proteins using the Olink Explore 3072 panels and proximity extension assays, followed by differential protein expression by Analysis of Variance with post-hoc correction and functional annotation.
Results:
Here, we show distinct protein profiles in neonates with disseminated HSV disease, with differences in 20 proteins compared to controls. These proteins are associated with innate and adaptive immune responses and cytokine activation.
Conclusions:
Our findings indicate the potential of neonatal screening for disseminated HSV disease to ensure early treatment and reduce the high mortality.
Subject terms: Viral infection, Diagnostic markers, Sepsis
Plain language summary
Herpes simplex virus (HSV) infection in newborns has a 70% risk of death if infection becomes widespread in the body. Initial symptoms are often vague, leading to delayed treatment. Early dried blood spot (DBS) screening of newborns is very effective for identifying disorders present at birth, but its use to identify HSV infection has not been investigated. Here, we analysed DBS samples taken on days 2–3 of life from newborns developing HSV infection in the neonatal period. We identified 20 proteins that differed between those with widespread HSV infection compared to healthy babies. These findings suggest that HSV screening on DBS samples have the potential to detect severe infections early, enabling prompt treatment and reducing the risk of death.
Dungu et al. use high throughput multiplexed proteomics on dried blood spot samples from neonates with herpes simplex virus infection. Distinct protein profiles were seen in proteins associated with innate and adaptive immune responses neonates with disseminated HSV disease compared to controls.
Introduction
Herpes simplex virus (HSV) infection is a life-threatening neonatal infection, with a mortality rate of up to 70–80% when disseminated and neurological damage of up to 50% when affecting the central nervous system (CNS)1–3. In contrast, skin-eye-mouth (SEM) disease is generally less severe, characterised by vesicular lesions on mucocutaneous surfaces, but can progress to more severe forms if left untreated2. Early diagnosis of neonatal HSV infection is difficult because maternal genital herpes is often asymptomatic and the neonatal symptoms vague, particularly in the early stages of infection. In addition, no reliable biomarkers in the neonate exist to identify those who will develop severe HSV disease, often resulting in devastating outcomes due to delayed treatment4–6. Neonatal screening using dried blood spot (DBS) samples is among the most impactful preventative health measures ever implemented for rare but serious diseases with a favourable outcome given early treatment7. However, screening for HSV has not been investigated. HSV often presents clinically in the second or third week of life, and DBS samples are routinely collected on days 2–3 after birth. This timing may enable HSV screening and capture earlier stages of the disease with an improved outcome if treated. However, PCR-based detection of HSV on DBS samples is ineffective8.
Factors that may contribute to the clinical variability of neonatal HSV infection include (1) viral load at the time of exposure and the specific type of HSV strain involved, (2) maternal antibodies which may protect against severe disseminated disease9, and (3) the host response of the neonatal immune system, which exhibits ineffective innate and adaptive responses10,11. Further, inborn errors of immunity, such as impaired toll-like receptor 3 (TLR3)-induced antiviral interferon (IFN) α/β immunity, predispose to severe disease following HSV infection not only in adults and older children12–14 and possibly also in neonates15.
Advances in ‘omics’ technologies have provided new insights into diagnostic markers and disease pathophysiology16,17. For decades, DBS samples have been used in omics methods, particularly in metabolomics and genomics18. However, recent advancements have broadened their feasibility to include proteomics and transcriptomics19–21. Although these approaches are not widely used in routine clinical practice, the accurate measurement of proteins in stored DBS samples using multiplex proximity extension assays (PEA) has been demonstrated19. Additionally, host transcriptional profiles in whole blood samples from neonates with HSV infection, identifying an RNA signature comprising 1322 differentially expressed genes22.
In this study, we investigated high throughput and multiplexed proteomic analysis on DBS samples obtained from neonates prior to developing HSV infection using the Olink Explore 3072 panels and PEA technology. Our aim was to gain insight into screening potential and disease pathophysiology. Our results demonstrate distinct immune-related proteins that differentiate neonates with disseminated disease from controls, indicating the potential of DBS screening approaches for severe neonatal HSV infection.
Methods
Study design and population
This nationwide matched case-control study included all neonates aged 0–28 days with HSV-1 and HSV-2 infections from all hospitals with neonatal and paediatric departments in Denmark from 2010 to 2019. Case identification and characteristics have been described previously3. To increase statistical power, two controls without HSV infection were included for each HSV case, matched on gestational age, sex, birth weight and age at DBS sampling. The clinical phenotypes of neonatal HSV infection were based on internationally established classifications2,3.
Sample collection
Parents of all neonates in Denmark are routinely offered DBS newborn screening for rare congenital diseases. For this study, the age at collection was 48 to 72 h postpartum. The DBS samples from the HSV cases and controls were retrieved from the Danish Neonatal Screening Biobank at Statens Serum Institut from January 1, 2022, to April 30, 2022.
Outcomes and measurements
Proteins were extracted from DBS samples according to Olink’s recommendations using 40 μl PBS buffer with 0.05% tween and protease inhibitor. Protein levels were estimated using the Olink Explore 3072 panels (Olink Proteomics Assays, Uppsala, Sweden), which utilise PEA technology to measure 2941 proteins. In PEA, oligonucleotide-labelled antibodies bind to target proteins, allowing for the formation of a unique DNA sequence corresponding to the protein, which is then quantified by sequencing23. The DBS samples were analysed undiluted and non-normalised, following the Olink protocol. Each run included three external controls: the plate control for data normalisation, the sample control to assess potential variation between runs and plates, and the negative control to determine the limit of detection for each assay and to assess potential contamination of assays24. We employed a triplet design (case + control + control) on the same plate, with samples randomised to positions 8 wells apart from each other. Sequencing was performed using the NovaSeq 6000 system (Illumina, San Diego, California, USA). Relative quantification measures and data transformation into normalised protein expression (NPX) values following a log2 scale were performed by BioXpedia (Aarhus, Denmark) according to the manufacturer’s instructions23,24. NPX values were imported to R by the Olink Analyze R package (version 3.4.1). Samples with quality control (QC) and Assay Warnings were excluded. Samples within +/− 3 standard deviations (SD) in Principal Components 1 and 2 calculated from Panel-Assay wise NPX values were considered valid, while outliers (n = 16) were removed.
To identify biological pathways and Key Word sets associated with the clinical phenotypes, we performed functional annotation of the differentially expressed proteins using the Database for Annotation, Visualisation, and Integrated Discovery (DAVID)25. We included only proteins that remained significant in the post-hoc analyses when comparing the individual neonatal HSV phenotypes with controls. For the background list in DAVID, we used the full list of proteins in the Olink Explore 3072 panels.
Statistics and reproducibility
Statistical analyses were performed in R statistical software (version 4.3.1) at the Danish Centre for Neonatal Screening, Department of Congenital Disorders, Statens Serum Institut. Since the study was exploratory in nature, a specific mean NPX difference threshold was not predefined. The threshold for significance was set at an FDR-adjusted p-value of <0.05. Considering the limited sample size, we opted for Analysis of Variance (ANOVA) as statistical model, which precluded the adjustment for matching variables. Since no statistically significant associations were found between the matching variables and NPX levels, we excluded them in our analysis. To identify differentially expressed proteins between cases and controls, ANOVA was performed with the Olink Analyze R package. This allowed us to capture a broad set of proteins with potential differential expression. However, recognising the risk of false positive findings, correction for multiple comparisons was applied using the Benjamini-Hochberg method. Post-hoc correction (Tukey test) was subsequently done to test the significance of pairwise comparisons of the individual HSV phenotypes versus controls. As the data distribution deviated slightly from a normal distribution, we tested the validity of the ANOVA model using permutation testing. Permutation test entailed generating new groupings with constant number of occurrences, iterated variable times per assay until 1000 observations higher or equal to the observed F-statistic or a maximum of 20 million permutations.
Ethics and study approvals
The study was approved by the Scientific Research ethics committees for the Capital Region of Denmark (H-21009288) and The Danish Data Protection Agency (P-2020-874) as a nationwide non-interventional case-control study. The Scientific Research ethics committees also granted a waiver of informed consent, based on the rationale that the study imposed no harm, risk or inconvenience upon the participants. There was no contact with the study participants, and the study did not include any DNA or genomic analyses. Permission to disclose patient data was obtained from the Danish Patient Safety Authority (3-3013-2415/1 and 31-1522-43). All ethical regulations relevant to human research participants were followed. The study was registered at ClinicalTrials.gov (NCT05226949).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
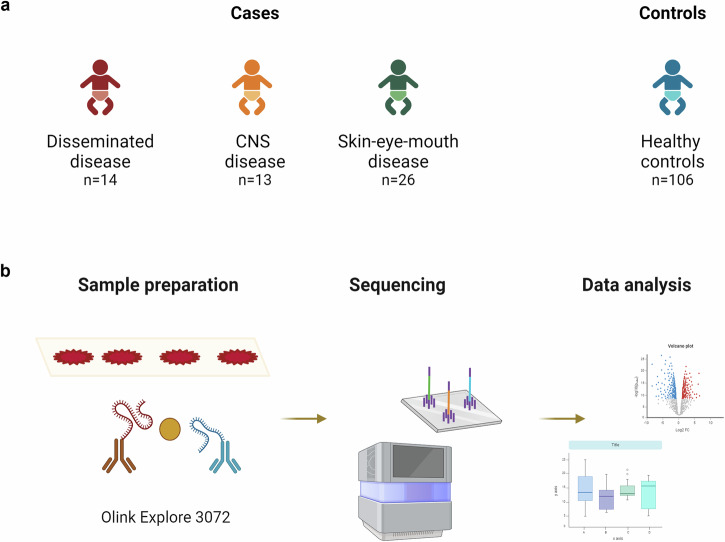
This case-control study included 159 neonates, of whom 53 had HSV infection. Among the neonates with HSV infection, 14 had disseminated disease, 13 had CNS disease, and 26 had SEM disease (Fig. 1a). There were no differences between cases and controls regarding gestational age, birth weight and sex (Supplementary Table 1). The age at DBS sampling was median 2 days (range 2–4). The median age of symptom onset and the proportion of cases with DBS sampling after disease onset is shown in Table 1. Eleven neonates were deceased, all of whom had disseminated disease (Table 1).
Fig. 1. Study cohort overview and proteomics workflow.
a Summary of the study cohort (N = 159), displaying the distribution of HSV phenotypes (n = 53) among cases versus controls. b Laboratory and analytical workflow from preparation of the dried blood spot samples, proximity extension assay using Olink Explore 3072 panels and proximity extension assay technology, library preparation, high-throughput sequencing (NovaSeq 6000, Illumina), and data analysis (Olink Analyze R package) including quality control, normalisation, statistical analysis and functional annotation. Created with BioRender.com.
Table 1.
Characteristics of the neonates with HSV infection (N = 53)
| Disseminated diseasea (n = 14) | CNS diseasea (n = 13) | Skin-eye-mouth diseasea (n = 26) | |
|---|---|---|---|
| Age of symptom onset (days) | 5 (2–10) | 9 (5–15) | 6 (4–12) |
| Symptom onset after DBS sampling | 8 (57%) | 10 (77%) | 20 (77%) |
| Severe neurological sequelae | 0 (0%) | 6 (46%) | 0 (0%) |
| Deceased | 11 (79%) | 0 (0%) | 0 (0%) |
aValues presented are median (interquartile range) and n (%), DBS dried blood spot.
Quality control and data structure
We successfully quantified all 2941 proteins included in the Olink Explore 3072 panels from the DBS samples. QC of the variables used for matching and principal component analysis analyses revealed no differences between cases and controls (Supplementary Fig. 1). The permutation test showed an excellent correlation (R2 = 0.9985) between empirical p-values and the ANOVA results. Post-hoc analysis (Tukey test) was conducted to test the significance of pairwise comparisons (Fig. 1b).
Distinct proteomic profiles of neonatal HSV infection phenotypes
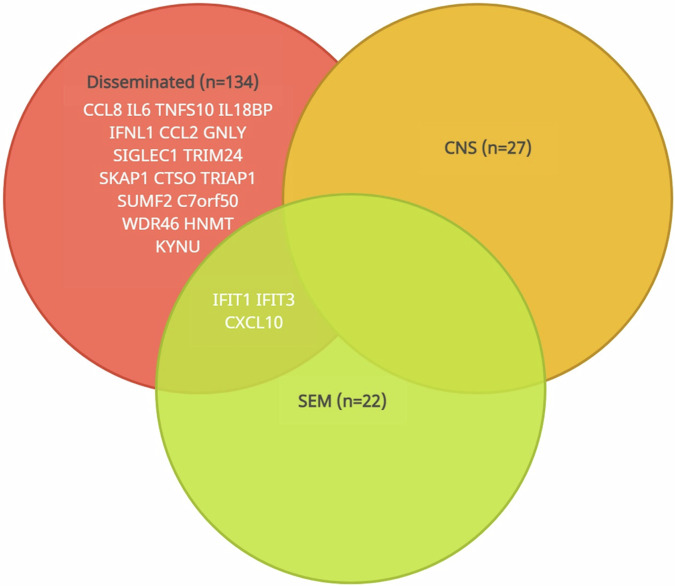
The highest number of significant protein level differences was found in neonates with disseminated disease compared to controls (n = 134, false discovery rate (FDR) adjusted p-value < 0.05, Fig. 2a and Supplementary Data 1). In comparison, 27 and 22 proteins differed when comparing CNS disease versus controls and SEM disease versus controls, respectively (Fig. 1b, c and Supplementary Data 2 and 3). Post-hoc analyses revealed 20 significantly different proteins in neonates with disseminated disease compared to controls (Figs. 3 and 4, Supplementary Fig. 2). The levels of three of these proteins also differed between SEM disease and controls. Protein levels were similar for neonates with CNS disease and controls (Figs. 3 and 4).
Fig. 2. Differences in protein levels between neonates with HSV infection and controls.
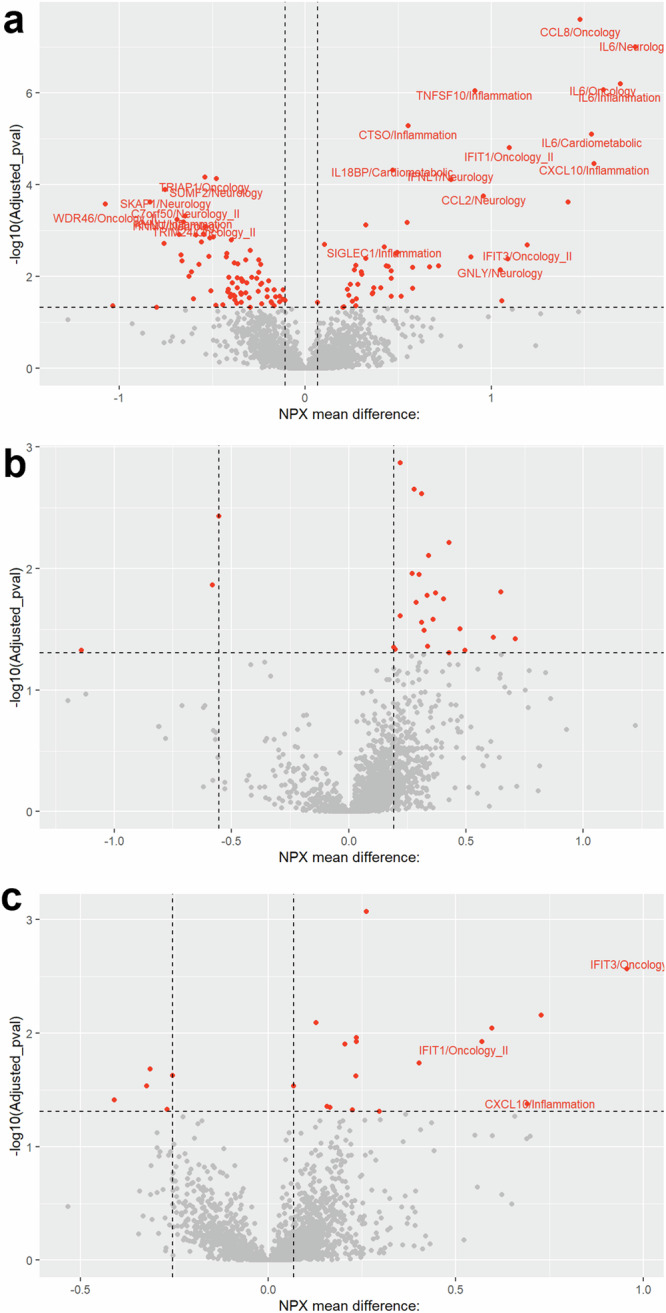
Volcano plots depicting protein differences in neonates with the neonatal HSV phenotypes compared with controls; a disseminated disease (n = 14), b CNS disease (n = 13), and c Skin-eye-mouth disease (n = 26). The x-axis represents the normalised protein expression (NPX) mean difference, and the y-axis represents the log10 p-value. The horizontal dashed line indicates the significance threshold (false discovery rate adjusted p-value < 0.05). The vertical dashed lines indicate the maximum (controls) and minimum (cases) NPX mean difference at significant false discovery rate adjusted p-values. The labelled proteins were significant in the post-hoc analyses when comparing individual HSV phenotypes with controls (n = 20 for disseminated disease, n = 3 for skin-eye-mouth disease).
Fig. 3. Differentially expressed proteins for the neonatal HSV infection phenotypes.
Venn diagram illustrating the significantly different proteins for the neonatal HSV infection phenotypes compared to controls. The labelled proteins were significant in the post-hoc analyses when comparing individual HSV phenotypes with controls (n = 20 for disseminated disease, n = 3 for skin-eye-mouth disease). IFIT1, IFIT3 and CXCL10 were significant for both disseminated and skin-eye-mouth disease. There were no post-hoc significant proteins for CNS disease versus controls. The threshold for significance was set at a false discovery rate adjusted p-value of <0.05. IFIT1 tetratricopeptide repeats 1, IFIT3 tetratricopeptide repeats 3, CXCL10 C-X-C motif chemokine ligand 10.
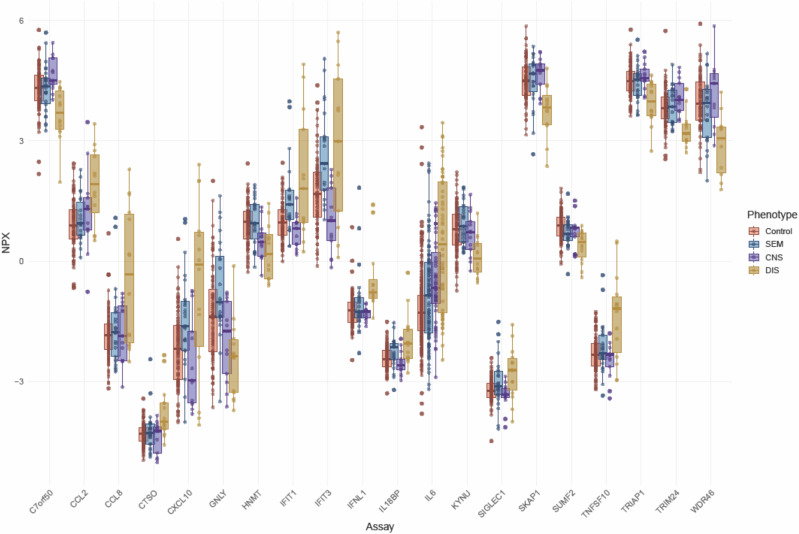
Fig. 4. Boxplot visualisation of NPX levels for post-hoc significant proteins.
Boxplots illustrating the differences in NPX levels for the post-hoc significant proteins (n = 20 for disseminated disease, n = 3 for skin-eye-mouth disease) among the neonatal HSV disease phenotypes and controls (IFIT1, IFIT3 and CXCL10 overlapping for disseminated and skin-eye-mouth disease versus controls). NPX normalised protein expression, DIS disseminated disease, CNS central nervous system disease, SEM skin-eye-mouth disease, IFIT tetratricopeptide repeats 1, IFIT3 tetratricopeptide repeats 3, CXCL10 C-X-C motif chemokine ligand 10.
Biological pathways associated with disease phenotypes
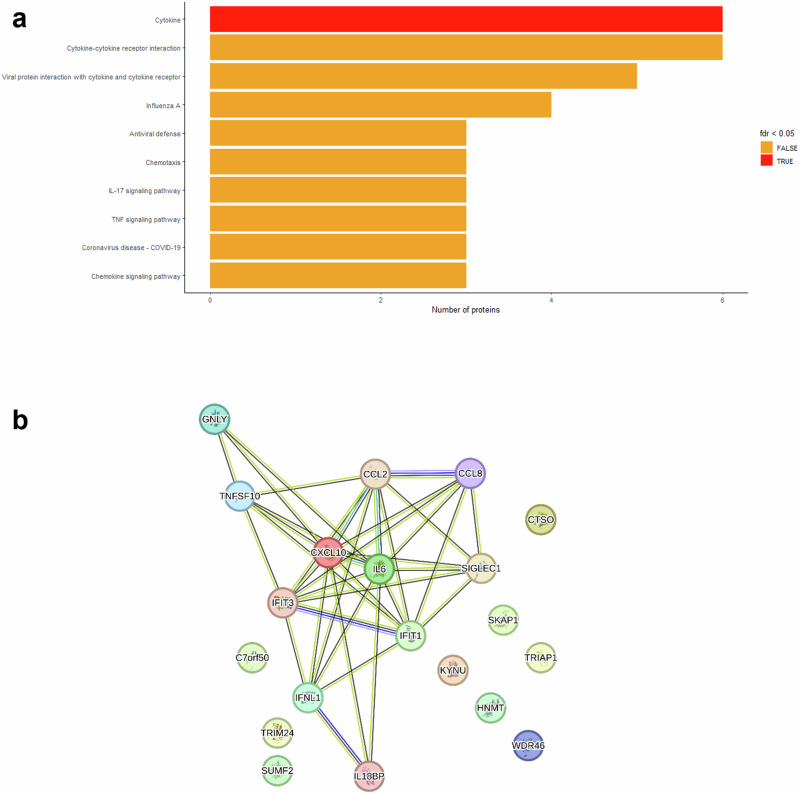
Functional annotation of the post-hoc significant proteins allowed us to identify biological pathways and Key Word sets associated with the clinical phenotypes compared to controls (Fig. 5a and Supplementary Data 4). For neonates with disseminated disease, we identified 33 pathways and Key Word sets, but only the Key Word set “Cytokine” was statistically significant (FDR adjusted p-value < 0.05). Other relevant pathways, such as “Cytokine-cytokine receptor interaction”, “Viral protein interaction with cytokine and cytokine receptor” and “Chemotaxis”, were identified but did not reach statistical significance. In the comparison of SEM disease versus controls, the Key Word set “Tetratricopeptide Repeat was significant. Other relevant pathways, such as “Interferon alpha/beta signalling” and “Cytokine signalling in the immune system” were not significant. The “Antiviral defence” pathway appeared in both comparisons: disseminated disease versus controls and SEM disease versus controls. No pathways or Key Word sets were identified for CNS disease versus controls, as there were no post-hoc significant proteins in this comparison (Supplementary Data 4).
Fig. 5. Functional annotation and protein network in disseminated HSV disease versus controls.
a Functional annotation of the proteins that remained significant when comparing disseminated HSV disease versus controls in the post-hoc analyses (n = 20). For these proteins, one Key Word set “Cytokine” was statistically significant (false discovery rate adjusted p-value < 0.05). b Protein network visualisation illustrating the relationships between the significant proteins (n = 134) in disseminated HSV disease compared to controls. Nodes represent individual proteins, while edges indicate different types of associations: co-occurrence (blue lines), co-expression (black lines), text mining (yellow lines), neighbourhood (green lines) and known interactions from curated databases (teal lines). The network was created using the STRING database (www.string-db.org, licensed under Creative Commons By 4.0).
Description of protein levels, cytokines, and interferon response
Many of the elevated proteins in neonates with disseminated disease compared to controls were involved in inflammatory response, such as interleukin-6 (IL-6), C-C motif chemokine ligand 8 (CCL8), CCL2, C-X-C motif chemokine ligand 10 (CXCL10), and antiviral defence, e.g., interferon-induced protein with tetratricopeptide repeats 1 (IFIT1), IFIT3, tumor necrosis factor superfamily member 10 (TNFSF10), and interferon lambda-1 (IFN-λ1). Other elevated proteins were granulysin (GNLY), an antimicrobial protein present in cytotoxic T cells and NK cells, and sialic acid-binding immunoglobulin-like lectin 1 (SIGLEC1), which is involved in cell adhesion, binding of macrophages and pathogen recognition and clearance. Other proteins with reduced levels were chromosome 7 open reading frame 50 (C7orf50) and WD repeat-containing protein 46 (WDR46), representing mediators of cell metabolism and RNA processing (Supplementary Table 2). The three overlapping post-hoc significant proteins between SEM and disseminated disease were IFIT1, IFIT3 and CXCL10. For all phenotypes, the levels of IFN gamma (IFN-γ), IFN lambda-2 (IFN-λ2) and IFN omega (IFN-ω), IFN membrane receptors, and other intracellular proteins related to the IFN response, e.g., TLR3, were not different from controls (Supplementary Fig. 3).
Discussion
We investigated proteomic analysis on DBS samples obtained from neonates with HSV infection. Our findings revealed differences in protein levels and enriched pathways between neonates with different HSV phenotypes and healthy controls. Neonates with disseminated disease had different levels of 20 proteins compared to controls. These proteins were associated with innate and adaptive immune responses and cytokine activation. The levels of three proteins, i.e., IFIT1, IFIT3 and CXCL10, also differed between neonates with SEM disease and controls. Protein profiling was similar in neonates with CNS disease and controls.
Our results suggest that the early stages of disseminated HSV disease are characterised by a pronounced cytokine production, as evidenced by the significantly elevated levels of several inflammatory cytokines and annotated pathways associated with cytokine activation. We found indications of high activity in both the innate and adaptive immune systems, with elevated markers of cytotoxic T cells and macrophages, along with proteins and pathways linked to antiviral defence. These findings indicate a highly activated immune response in neonates with disseminated disease, aligning with the RNA signatures identified by Cohen et al.22. Among the antiviral proteins identified, IFIT1 and IFIT3 were elevated in neonates with disseminated and SEM disease. These proteins play important roles in combating viral infections by inhibiting viral translation, recruiting immune cells, and enhancing antigen presentation, and are not expressed in most cells under normal conditions26. The IFIT protein family has been linked to type I IFN responses, and their upregulation in response to various viruses, including HSV26–28. Additionally, CXCL10, an IFN-stimulated gene, supports the notion of an activated innate immune response against HSV infection. The overlap of these proteins between neonates with SEM and disseminated disease suggests a shared host defence. The absence of significantly elevated or reduced proteins in CNS disease may reflect that at the time of DBS sample collection, the infection had yet to be established, resulting in no detectable immune activation. This aligns with the later onset of symptoms in CNS disease compared to disseminated and SEM disease. Additionally, it could suggest that the primary pathogenesis occurs within the CNS, partly protected behind the blood-brain barrier rather than in peripheral blood.
We identified candidate proteins that could serve as early diagnostic markers in neonates with disseminated disease using DBS samples. Disseminated disease has a mortality rate of up to 70–80%2,3, emphasising the need for earlier treatment with high-dose acyclovir therapy, which has been shown to reduce mortality and morbidity4,5. Early recognition of severe disease is often delayed due to nonspecific symptoms, and conventional markers of organ function, such as alanine aminotransferase and platelet counts, often have limited diagnostic sensitivity in the early disease stages2. Although there have been indications of detecting HSV by PCR on DBS samples, the sensitivity is low8. Combining pathogen detection with proteomics may offer a novel approach for comprehensive screening and risk assessment, differentiating asymptomatic or mild infection from those with life-threatening disease. In our study, more than half of the neonates with disseminated disease had symptom onset after DBS sampling, highlighting the potential of neonatal screening for early detection.
Understanding of the immune dysregulation in neonatal HSV infection is crucial for comprehending the pathophysiology and potential underlying host genetics. Our results indicated a normal IFN response across all phenotypes compared to controls, contrasting with previous studies showing insufficient IFN production or TLR3 pathway deficiencies in HSV encephalitis. Specific genetic variants have been identified that predispose to CNS disease following HSV infection12–14. Furthermore, a study utilising exome sequencing in neonates with disseminated and CNS disease identified gene variants within the TLR3 signalling pathway and other innate viral-sensing receptors. However, the gene variants were not functionally validated15. Studies also suggest that inborn errors in host RNA processing may contribute to severe infection29,30. Additionally, inherent variations in the functionality of neonatal NK cells and T cells have been linked to the development of severe disease31,32.
Several factors limited our study. First, the small number of cases may have decreased precision and increased the risk of type two errors in our protein detection. However, we validated our ANOVA model and data distribution by permutation analyses, which provided confidence in the reliability of our assumptions. Second, the unequal distribution of samples, with more controls than cases and varying ratios across the HSV phenotypes, could influence the ANOVA performance. However, to increase the robustness of the controls’ mean NPX levels, we decided to include more controls than cases. Since the study is exploratory, we accepted some level of statistical uncertainty, and the results should be viewed as preliminary indications of proteins that may be relevant for future biomarker development. Another limitation was the mix of lysed cells and plasma in the DBS samples used for protein analyses. This could lead to changes in protein composition due to different physical and chemical properties, proteases, and protein degradation mechanisms. However, a previous study has validated extraction of reliable protein levels from DBS samples using the Olink Assays19. Additionally, we acknowledge that the protein profile identified may not be specific to disseminated HSV infection. Validation and testing in larger cohorts are necessary to confirm the diagnostic utility of the identified proteins. Last, our protein analyses did not include IFN-α and IFN-β, limiting our ability to conclude on type I IFN response in neonates with HSV infection. However, we observed normal levels of the type I IFN receptor (IFNAR) and elevated IFIT1, IFIT3 and CXCL10 levels, providing some insight into this aspect.
In summary, we found distinct DBS protein profiles in neonates with disseminated HSV disease, with differences in 20 proteins compared to controls. These proteins were associated with innate and adaptive immune responses and cytokine activation, providing insights into the underlying pathophysiology of severe neonatal HSV infection. Our findings indicate the potential of neonatal screening for disseminated HSV disease to ensure early treatment and reduce the high mortality.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The Innovation Fund Denmark (0176–00020B) and Tømrermester Jørgen Holm & hustru Elisa F. Hansens Mindelegat (20006-1991) had no role in the design and conduct of the study.
Author contributions
K.H.S.D., U.N., J.B.G., C.M.H., M.B.H., E.M.C., N.H.V., T.B.H., T.H.M., V.Y. and A.B.D. conceptualised the study. K.H.S.D., U.N., J.B.G. and D.M.H. obtained funding for the study. K.H.S.D. and U.N. obtained clinical details for neonates with HSV infection. K.H.S.D., U.N., C.M.H. and J.B.G. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. K.H.S.D., U.N., C.M.H., J.B.G., E.M.C., N.H.V., V.Y. and A.B.D. analysed data. K.H.S.D., U.N., C.M.H., J.B.G., E.M.C. and N.H.V. drafted the first version of the manuscript. All authors contributed to the data interpretation. All authors revised the manuscript critically for important intellectual content. All authors approved the final version of the work.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The source data behind the figures in the paper can be found in Supplementary Data 5 and 6.
Code availability
The R codes are available at https://github.com/HagenC/Neonatal-HSV-Proteomics/tree/main33.
Competing interests
The authors declare no competing interests. The funders had no role in the design and conduct of the study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s43856-024-00711-8.
References
- 1.Looker, K. J. et al. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob. Health5, e300–e309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samies, N. L., James, S. H. & Kimberlin, D. W. Neonatal herpes simplex virus disease: updates and continued challenges. Clin. Perinatol.48, 263–274 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Dungu, K. H. S et al. Herpes simplex virus infection among neonates suspected of invasive bacterial infection: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed.108, 655–660 (2023). [DOI] [PubMed]
- 4.Kimberlin, D. W. et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics108, 230–238 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Shah, S. S., Aronson, P. L., Mohamad, Z. & Lorch, S. A. Delayed acyclovir therapy and death among neonates with herpes simplex virus infection. Pediatrics128, 1153–1160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long, S. S. Diagnosis and management of undifferentiated fever in children. J. Infect.72, S68–S76 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Nørgaard-Pedersen, B. & Hougaard, D. M. Storage policies and use of the Danish newborn screening biobank. J. Inherit. Metab. Dis.30, 530–536 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Lewensohn-Fuchs, I., Osterwall, P., Forsgren, M. & Malm, G. Detection of herpes simplex virus DNA in dried blood spots making a retrospective diagnosis possible. J. Clin. Virol.26, 39–48 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Brown, Z. A. et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N. Engl. J. Med.324, 1247–1252 (1991). [DOI] [PubMed] [Google Scholar]
- 10.Basha, S., Surendran, N. & Pichichero, M. Immune responses in neonates. Expert Rev. Clin. Immunol.10, 1171–1184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olin, A. et al. Stereotypic immune system development in newborn children. Cell174, 1277–1292.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mørk, N. et al. Mutations in the TLR3 signaling pathway and beyond in adult patients with herpes simplex encephalitis. Genes Immun.16, 552–566 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Casrouge, A. et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science314, 308–312 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Mielcarska, M. B., Bossowska-Nowicka, M. & Toka, F. N. Functional failure of TLR3 and its signaling components contribute to herpes simplex encephalitis. J. Neuroimmunol.316, 65–73 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Cummings, L. et al. Rare genetic variants in immune genes and neonatal herpes simplex viral infections. Pediatrics147, e20200687 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Tsao, Y. T. et al. Differential markers of bacterial and viral infections in children for point-of-care testing. Trends Mol. Med.26, 1118–1132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanash, S. Disease proteomics. Nature422, 226–232 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Hollegaard, M. V. et al. Archived neonatal dried blood spot samples can be used for accurate whole genome and exome-targeted next-generation sequencing. Mol. Genet. Metab.110, 65–72 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Björkesten, J. et al. Stability of proteins in dried blood spot biobanks. Mol. Cell Proteomics16, 1286–1296 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bybjerg-Grauholm, J. et al. RNA sequencing of archived neonatal dried blood spots. Mol. Genet. Metab. Rep.10, 33–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grauholm, J. et al. Gene expression profiling of archived dried blood spot samples from the Danish neonatal screening biobank. Mol. Genet. Metab.116, 119–124 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Cohen, S. H. et al. 82. Blood gene expression profiles in neonates with herpes simplex virus (HSV) infection. Open Forum Infect. Dis.8, S53–S53 (2021). [Google Scholar]
- 23.Olink. Olink explore overview [Internet]. Available from: https://7074596.fs1.hubspotusercontent-na1.net/hubfs/7074596/01-User%20Manuals%20for%20website/1187-olink-explore-overview-user-manual.pdf
- 24.Olink. Olink data normalization and standardization [Internet]. Available from: https://www.olink.com/content/uploads/2021/09/olink-data-normalization-white-paper-v2.0.pdf
- 25.Huang, D. W. et al. DAVID bioinformatics resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res.35, W169–W175 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamond, M. S. & Farzan, M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol.13, 46–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou, X. et al. Interferon induced IFIT family genes in host antiviral defense. Int. J. Biol. Sci.9, 200–208 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fensterl, V. & Sen, G. C. Interferon-induced Ifit proteins: their role in viral pathogenesis. J. Virol.89, 2462–2468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, S. Y. et al. Inborn errors of RNA lariat metabolism in humans with brainstem viral infection. Cell172, 952–965.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lafaille, F. G. et al. Human SNORA31 variations impair cortical neuron-intrinsic immunity to HSV-1 and underlie herpes simplex encephalitis. Nat. Med.25, 1873–1884 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jouanguy, E. et al. Human inborn errors of immunity to herpes viruses. Curr. Opin. Immunol.62, 106–122 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gantt, S. & Muller, W. J. The immunologic basis for severe neonatal herpes disease and potential strategies for therapeutic intervention. Clin. Dev. Immunol.2013, 369172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HagenC. 10.5281/zenodo.14280562. github/HagenC/Neonatal-HSV-Proteomics. Available from: https://github.com/HagenC/Neonatal-HSV-Proteomics/releases/tag/v.1.0.0
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The source data behind the figures in the paper can be found in Supplementary Data 5 and 6.
The R codes are available at https://github.com/HagenC/Neonatal-HSV-Proteomics/tree/main33.