Abstract
To compare the efficacy and safety of gecacitinib (also known as jaktinib) with hydroxyurea (HU) in treating myelofibrosis (MF) patients. In this multicenter, randomized phase 3 trial (ZGJAK016), intermediate- or high-risk primarily JAK inhibitor naïve MF patients were assigned in a 2:1 ratio to receive either gecacitinib (100 mg twice a day, BID) or HU (500 mg BID). The primary endpoint was the proportion of patients with ≥35% reduction in spleen volume (SVR35) from baseline at week 24. Secondary endpoints included the best spleen response rate, the proportion of patients with a ≥50% reduction in total symptom score (TSS50), anemia improvement, and safety profile. At 24 weeks, the SVR35 was reached by 64.8% of patients on gecacitinib (46/71), compared to 26.5% on HU (9/34), P = 0.0002. The best spleen response rates were also superior for gecacitinib at 81.7%, vs 32.4% for HU, P < 0.0001. The TSS50 rates were 62.0% for gecacitinib- and 50% for HU-treated patients. Among non-transfusion-dependent patients with baseline hemoglobin (HGB) ≤ 100 g/L, 31.0% (13/42) in the gecacitinib group showed a ≥20 g/L increase in HGB, compared to 15.0% (3/20) in HU group. The common grade ≥ 3 treatment-emergent adverse events (TEAEs), including anemia (26.8% vs 44.1%), thrombocytopenia (15.5% vs 32.4%), leukopenia (2.8% vs 20.6%), and neutropenia (1.4% vs 20.6%), were less frequent with gecacitinib than HU. Treatment discontinuation due to TEAEs was lower in gecacitinib (7.0%) compared to HU (11.8%). Gecacitinib demonstrates superior efficacy and a more favorable safety profile compared to HU, making it a promising treatment option for managing MF, particularly in patients with anemia (This trial was registered with ClinicalTrials.gov, (NCT04617028)).
Subject terms: Myeloproliferative disease, Drug development
Introduction
Myelofibrosis (MF) is a BCR::ABL1-negative myeloproliferative neoplasm (MPN) characterized by progressive splenomegaly, systemic symptoms, abnormal blood cell counts, and a risk of leukemic transformation [1, 2]. Patients often suffer from progressive anemia, thrombocytopenia, and debilitating systemic symptoms, including fatigue, dyspnea, night sweats, weight loss, fever, and bone pain. Notably, the incidence of anemia at diagnosis in Chinese patients with primary myelofibrosis (PMF) is 67%, significantly higher than that observed in Western counterparts (35%), underscoring the urgent need to address anemia in this patient population [3].
Mutations in the JAK2, CALR, or MPL genes, found in approximately 90% of patients, lead to hyperactivation of the JAK-STAT pathway, pivotal in the pathogenesis of MF [4]. Advances in understanding this pathway have spurred the development of JAK inhibitors, with ruxolitinib (2011), fedratinib (2019), pacritinib (2022), and momelotinib (2023) approved by the Food and Drug Administration (FDA) for the treatment of MF symptoms and splenomegaly. Momelotinib, in particular, has demonstrated efficacy in ameliorating anemia [5, 6].
In China, ruxolitinib is the only approved JAK inhibitor, yet its efficacy (Chinese population of A2202 study : ≥35% reduction in spleen volume at week 24 was 27%) [7, 8] is slightly lower compared to that in other regions, with Asian (A2202: 31.7%) [9] and Western (COMFORT-I: 41.9%; COMFORT-II: 32%) [10, 11]. Additionally, treatment-related anemia and thrombocytopenia often necessitate dose reductions or discontinuation [12]. Data shows that over 60% of patients require dose reduction or treatment interruption due to adverse events (AEs) [7].
Hydroxyurea (HU) commonly used as cytoreductive therapy in MPN, is also widely used in China with MF [13, 14] The efficacy of HU in treating splenomegaly associated with MF varies across studies, which can be influenced by factors such as the type of MF, dosage, and prior use of HU. One study reported a palpable spleen response rate of 40%, and a symptom response rate of 82% in MF patients treated with HU [15]. In international trials (COMFORT-II and PERSIST-1) comparing JAK inhibitor to the best available therapy (BAT), where HU was used in approximately 50% of cases, no more than 6% of participants achieved ≥ 35% spleen reduction [11, 16]. Additionally, the efficacy of HU is typically limited to a duration of one year, and nearly 50% of patients experience worsening anemia or cytopenia [15].
Gecacitinib (also known as jaktinib), is a novel inhibitor targeting both JAK and ACVR1. By inhibiting the activity of JAK1, JAK2, JAK3, and TYK2, it blocks the JAK-STAT signaling pathway, thereby alleviating inflammation and splenomegaly. The same mechanism as momelotinib, by inhibiting ACVR1 activity, allows gecacitinib to down-regulate hepcidin expression, improve iron metabolism imbalance, and increase hemoglobin, which turns out to reduce the occurrence of anemia and transfusion dependency in patients with MF [17]. Three phase 2 clinical trials (ZGJAK002, ZGJAK006, and ZGJAK017) and one phase 3 clinical trial (ZGJAK016) have been conducted to assess the efficacy and safety of gecacitinib in treating MF [18–21]. The interim results from the ZGJAK016 trial suggest that gecacitinib is superior over HU in reducing spleen size with favorable safety and tolerability, and may offer potential improvements in patient’s quality of life [21].
Here we present the final analysis results of the ZGJAK016 randomized phase 3 trial, evaluating the efficacy and safety of gecacitinib against HU in JAK inhibitor-naïve intermediate- to high-risk MF patients.
Methods
Study design and participants
This double-blind, double-dummy, parallel-controlled, multicenter, phase 3 clinical trial was carried out across 38 sites in China. It included a screening period, followed by a main study period (day 1–week 24), an extension period (post-24 weeks), and a follow-up period. The research protocol was reviewed and approved by the institutional review board/independent ethics committee of the First Affiliated Hospital, Zhejiang University School of Medicine, and each participating center and was conducted in alignment with the International Conference on Harmonization’s Good Clinical Practice guidelines. Written informed consent was obtained from all patients before participation in the trial.
Patients aged 18 years or older with a diagnosis of PMF, post-polycythemia vera MF (post-PV-MF), or post-essential thrombocythemia MF (post-ET-MF) were eligible for inclusion. Additional criteria included classification as intermediate-2 or high risk according to the Dynamic International Prognostic Scoring System (DIPSS), palpable splenomegaly ≥5 cm below the left costal margin, peripheral blood blasts ≤ 10%, limited (not exceeding 10 days) or no prior treatment with JAK inhibitors, and a minimum platelet count of 100 × 109/L and neutrophil count of 1 × 109/L. Participants receiving HU or JAK inhibitor therapy were required to discontinue these treatments for at least two weeks prior to randomization. Exclusion criteria comprised a history of splenectomy, spleen-directed radiation therapy within the previous 48 weeks, malignancies within the past five years, or significant clinical and laboratory abnormalities or comorbidities. The inclusion and exclusion criteria are further detailed in the Supplementary trial protocol.
Randomization and masking
Patients were stratified by DIPSS risk level (intermediate-2 or high) and randomized by an Interactive Web Response System (IWRS) in a 2:1 ratio to either gecacitinib (100 mg BID) with HU placebo or HU (500 mg BID) with gecacitinib placebo. To maintain objectivity in the treatment outcomes, the trial was blinded to the investigators, the sponsor, and the participants throughout the study.
Procedures
During the main study period, the eligible patients were treated with either gecacitinib (100 mg BID) with HU placebo or HU (500 mg BID) with gecacitinib placebo, spanning four 6-week treatment cycles (24 weeks total). Patients who demonstrated spleen-related progression, as evaluated by the Independent Review Committee (IRC), were directly moved to the extension period and received open-label gecacitinib 100 mg BID. On the other hand, patients who showed spleen-unrelated progression were to be discontinued from the study and others would complete the main study period before entering the extension period. During the extension period, participants who achieved a 35% reduction in spleen volume from baseline at week 24, as identified by the IRC, continued their initial treatment in a blinded manner until they met the spleen-related progression criterion assessed by the investigator. Those who did not meet this criterion were directly switched to open-label gecacitinib until they met the criteria for treatment termination. During the study, the use of other MF treatments, including bone marrow transplantation, was not allowed. Furthermore, treatments intended to correct anemia were restricted to blood transfusions when clinically indicated.
Spleen volume was measured using CT or MRI, with evaluations conducted by both the IRC and investigators at 12-week intervals. Additional efficacy assessments, like anemia improvement and MPN-Symptom Assessment Form Total Symptom Score (MPN-SAF TSS) [22], were carried out every 6 weeks during the initial 48 weeks and every 12 weeks thereafter. Safety assessments followed a schedule with initial visits at weeks 2, 4, and 6, then every 3 weeks up to week 24, every 6 weeks until week 48, and every 12 weeks after week 48.
Dosing was paused for platelet counts below 50 × 109/L or for grade 3 or higher non-hematologic toxicities related to the investigational drug. Treatment resumed to the original dose once platelet counts were equal to or over 50 × 109/L and related non-hematologic toxicities were reduced to grade 1 or lower.
Study endpoints/outcomes
The primary endpoint of this study was the proportion of patients achieving ≥ 35% reduction in spleen volume (SVR35) from baseline at week 24 as evaluated by the IRC. Key secondary endpoints included SVR35 at week 24 as determined by the investigators, the best spleen response, TSS reduction by 50% or more (TSS50), improvement in anemia at week 24 (including the conversion rate of baseline transfusion-dependent patients to independence, proportion of non-transfusion-dependent patients with baseline hemoglobin ≤ 100 g/L achieving an increase of ≥20 g/L, and reduction in red blood cell (RBC) transfusion frequency and volume by ≥50%), objective response rate, duration of maintenance of ≥35% reduction in spleen volume, progression-free survival, leukemia-free survival and overall survival (OS). Baseline transfusion-dependence was defined as receiving ≥ 2 units of RBC transfusions every 4 weeks, during the 12 weeks before the initiation of investigational drug treatment. Transfusion independence was defined as not requiring RBC transfusion with an HGB level ≥ 85 g/L during any consecutive 12-week interval throughout the main study period. OS was defined as the time from randomization until death. AEs were categorized and graded according to the Common Toxicity Criteria for Adverse Events version 5.0.
Statistical analysis
This study is a phase 3 clinical trial with a superior design and a positive control. The sample size of 105 cases was calculated based on the assumption of an SVR35 rate of 50% for the gecacitinib group and a conservative estimate of 6% for the HU group derived from earlier studies [11, 16, 23]. An interim analysis was planned after the first 70 patients completed the 24-week evaluation or earlier if they terminated the treatment before 24 weeks. To control the overall type I error rate at a two-sided significance level of 0.05, a Lan-DeMets (O’Brien-Fleming) alpha spending function was utilized to determine efficacy boundaries. With a randomization ratio of 2:1 and an anticipated dropout rate of 11%, the power of this study was above 99%, as calculated using Fisher’s exact test.
Efficacy was analyzed on an intention-to-treat basis using the Full Analysis Set, which included all randomized patients who received at least one dose of the assigned drug. Safety was evaluated within the Safety Set, comprising all patients who received at least one dose of gecacitinib or HU.
The SVR35 rate at week 24 was calculated for each group, with 95% confidence intervals determined using the exact Clopper-Pearson method. Differences in response rates between groups and their 95% confidence intervals, as well as P values, were calculated using the stratified Cochran–Mantel–Haenszel test, with stratification factors consistent with randomization. Subgroup analyses were performed for the primary endpoint (24-week SVR35 assessed by IRC) to examine treatment effects across different subgroups with a minimum of 21 cases.
The cutoff date for this data analysis was set at February 15, 2023. Time-to-event outcomes were estimated using the Kaplan–Meier method, providing median times, quartiles, and their 95% confidence intervals, as well as estimated probabilities at specified time points. Patients in the HU group who switched to taking gecacitinib before having the progressive disease were to be censored at the date of the last assessment of their disease prior to crossover. Comparisons between groups were made using the stratified log-rank test, with hazard ratios (HRs) and their 95% confidence intervals estimated from the stratified Cox proportional hazards model. All statistical analyses were conducted using SAS software, version 9.4 or later.
Role of the funding source
The funder was involved in the study design, administration, data analysis, and interpretation. Y.Z. and J.J. wrote the manuscript, and the funder worked in collaboration with the authors on this manuscript. All authors had access to all study data. All authors agreed to be accountable for the accuracy and integrity of the data and all authors approved the final manuscript. The corresponding author had the final responsibility to submit this paper for publication.
Results
Patient characteristics
From January 22, 2021, to August 31, 2022, a total of 105 participants were randomized across 38 centers, with 71 patients allocated to the gecacitinib 100 mg BID group and 34 to the HU 500 mg BID group. All participants were included in both the Full Analysis Set and the Safety Set.
Baseline characteristics were generally balanced between the two groups, with the exception of a higher proportion of patients with grade 3 bone marrow fibrosis (59.2% vs 44.1%) observed in the gecacitinib group, Table 1. The average age of the patient was 63.1 years (SD: 8.76), ranging from 41 years to 79 years, and 44 of them (41.9%) were male. The majority were diagnosed with PMF (73.3%). The proportions of intermediate-2 risk vs high risk were 89.5% vs 10.5% by DIPSS. The mean disease duration at baseline was 1.4 years. Hemoglobin levels below 100 g/L at baseline were reported in 66.2% of the gecacitinib group and 64.7% of the HU group. JAK2V617F mutation was present in 59.2% of the gecacitinib vs 58.8% in the HU group. 42.3% of the gecacitinib group had received prior HU therapy, compared with 50.0% in the HU group.
Table 1.
Baseline characteristics.
| Gecacitinib | HU | |
|---|---|---|
| (n = 71) | (n = 34) | |
| Age, years | 63.5 (8.02) | 62.1 (10.19) |
| Gender—male | 29 (40.8%) | 15 (44.1%) |
| ECOG performance status | ||
| 0 | 20 (28.2%) | 8 (23.5%) |
| 1 | 47 (66.2%) | 26 (76.5%) |
| 2 | 4 (5.6%) | 0 |
| Disease subtype | ||
| PMF | 50 (70.4%) | 27 (79.4%) |
| Post-PV-MF | 9 (12.7%) | 3 (8.8%) |
| Post-ET-MF | 12 (16.9%) | 4 (11.8%) |
| Prior HU therapy | 30 (42.3%) | 17 (50.0%) |
| Prior JAK inhibitors | 1 (1.4%) | 2 (5.9%) |
| MF grading | ||
| MF-0 | 0 | 2 (5.9%) |
| MF-1 | 2 (2.8%) | 2 (5.9%) |
| MF-2 | 27 (38.0%) | 14 (41.2%) |
| MF-3 | 42 (59.2%) | 15 (44.1%) |
| NA | 0 | 1 (2.9%) |
| DIPSS risk status | ||
| Intermediate-2 | 63 (88.7%) | 31 (91.2%) |
| High | 8 (11.3%) | 3 (8.8%) |
| MF duration, years | 1.5 (2.54) | 1.3 (2.36) |
| Spleen volume by MRI/CT, cm3 | 1514.8 (820.79) | 1549.7 (734.75) |
| MPN-SAF TSS | 21.2 (14.54) | 21.4 (14.43) |
| Mutations | ||
| JAK2 V617F | 42 (59.2%) | 20 (58.8%) |
| CALR | 19 (26.8%) | 9 (26.5%) |
| TYPE-1 | 8 (11.3%) | 6 (17.6%) |
| TYPE-2 | 6 (8.5%) | 1 (2.9%) |
| Others | 5 (7.0%) | 2 (5.9%) |
| MPL W515L/K | 4 (5.6%) | 4 (11.8%) |
| WBC, 109/L | 20.6 (17.5) | 24.6 (21.1) |
| HGB, g/L | 97.1 (27.5) | 96.8 (24.7) |
| HGB < 100 g/L | 47 (66.2) | 22 (64.7) |
| PLT, 109/L | 349.7 (267.7) | 407.2 (372.2) |
| RBC transfusion dependency | 6 (8.5%) | 3 (8.8%) |
Data presented as mean (SD) or n (%).
ECOG Eastern Cooperative Oncology Group, DIPSS dynamic international prognostic scoring system, TSS total symptom score, WBC white blood cell count, HGB hemoglobin level, PLT platelet count.
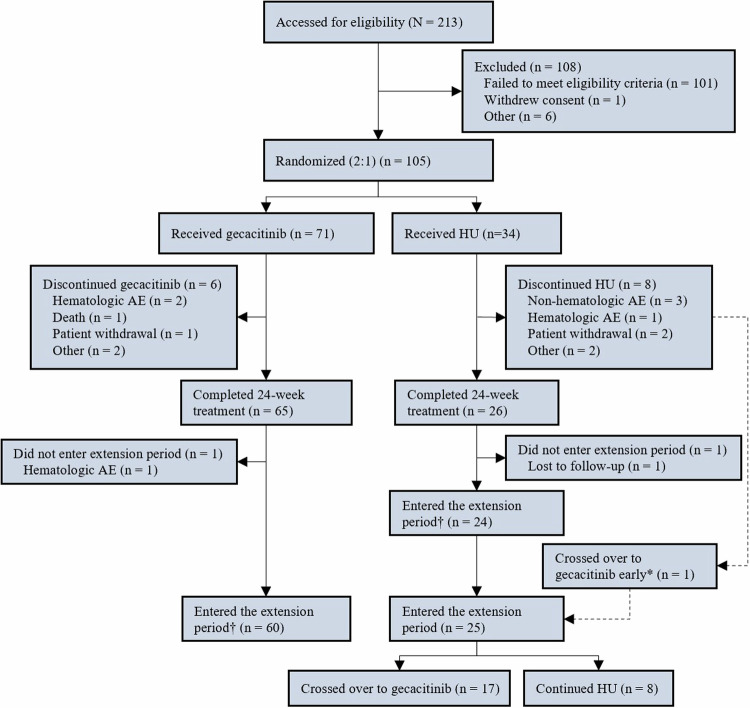
The median follow-up time was 17.5 months in the gecacitinib group and 15.9 months in the HU group. In total, 91 of the 105 patients (86.7%) completed the 24-week main study period, with 65 (91.5%) from the gecacitinib group and 26 (76.5%) from the HU group, as depicted in Fig. 1. Reason for treatment discontinuation in the gecacitinib group (n = 6) were hematologic adverse event (n = 2), death (n = 1), patient withdrawal (n = 1), and other reasons (n = 2). In the HU group (n = 8), reasons included non-hematologic AE (n = 3), hematologic adverse event (n = 1), patient withdrawal (n = 2), and other reasons (n = 2). A total of 85 patients (81.0%) entered the extension phase, with 60 (84.5%) from the gecacitinib group and 25 (73.5%) from the HU group, with 17 patients (50.0%) crossed over from HU to gecacitinib treatment. No patients underwent bone marrow transplantation in the study.
Fig. 1. Patient disposition.
*One patient who discontinued the main study period early due to a ≥25% increase in spleen volume entered the extension period and crossed over to gecacitinib. †As of February 15, 2023, five patients (four in the gecacitinib group and one in the HU group) completing the main study period did not yet enter the extension period. HU hydroxyurea, AE adverse event.
Primary endpoint
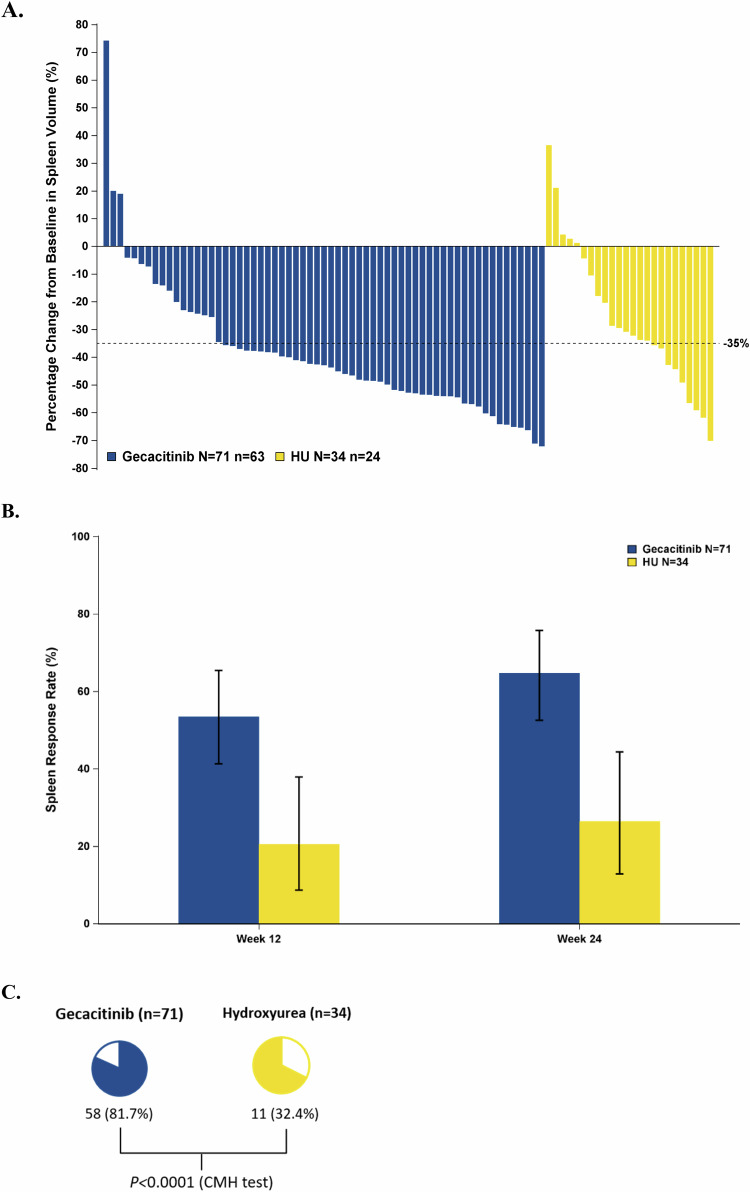
The gecacitinib group exhibited a 24-week SVR35 rate of 64.8% (46/71), significantly higher than the HU group’s 26.5% (9/34), with a difference of 36.5% (95% CI: 19.0–53.9%; P = 0.0002; Table 2). Among evaluable patients at week 24, most patients treated with gecacitinib had a spleen volume reduction, Fig. 2A. Across all predefined subgroups, gecacitinib’s 24-week SVR35 efficacy rates surpassed those of HU, Supplementary Fig. 1.
Table 2.
Summary of primary and key secondary efficacy endpoint analyses at week 24.
| Gecacitinib | HU | P value | |
|---|---|---|---|
| (n = 71) | (n = 34) | ||
| SVR35 rate assessed by IRC at week 24, % | 64.8 | 26.5 | 0.0002 |
| TSS50 rate at week 24, % | 62 | 50 | 0.2065 |
| Transfusion-dependent to independent, % | 16.7 | 0 | 0.4386 |
| Non-transfusion-dependent baseline HGB ≤ 100 g/L exhibited a ≥20 g/L HGB increase, % | 31 | 15 | 0.1827 |
| ≥50% Reduction in transfusion frequency, % | 63.6 | 60 | >0.9999 |
| ≥50% Reduction in transfusion units, % | 54.5 | 40 | 0.4795 |
| Mean absolute change in HGB at week 24, g/L | 1.27 | −3.5 | NA |
| Mean absolute change in PLT at week 24, 109/L | −93.28 | −182.56 | NA |
| Clinical improvement, % | 70.4 | 41.2 | NA |
HGB hemoglobin level, PLT platelet count, NA not available.
Fig. 2. Change in spleen volume.
A Percentage change in spleen volume compared to baseline at week 24. B Proportion of patients achieving SVR35 (≥35% reduction in spleen volume) at each visit. C Proportion of patients achieving the best spleen response. HU hydroxyurea, CMH test stratified Cochran–Mantel–Haenszel test (Note: patients without week 24 spleen volume data [8 from the gecacitinib group and 10 from the HU group] are not included on the waterfall plot).
Secondary endpoints
Spleen response
At 12 weeks, the gecacitinib group exhibited a rapid spleen response with an SVR35 compared to the HU, 53.5% (38/71) vs 20.6% (7/34), P = 0.0017, Fig. 2B and Table 2. The gecacitinib group reached an 81.7% (58/71) best overall spleen response rate, markedly greater than the HU group’s 32.4% (11/34; P < 0.0001), Fig. 2C and Table 2. The median percentage of the largest spleen volume reduction from baseline was 51.7% in gecacitinib-treated patients vs 30.0% in the HU group.
TSS
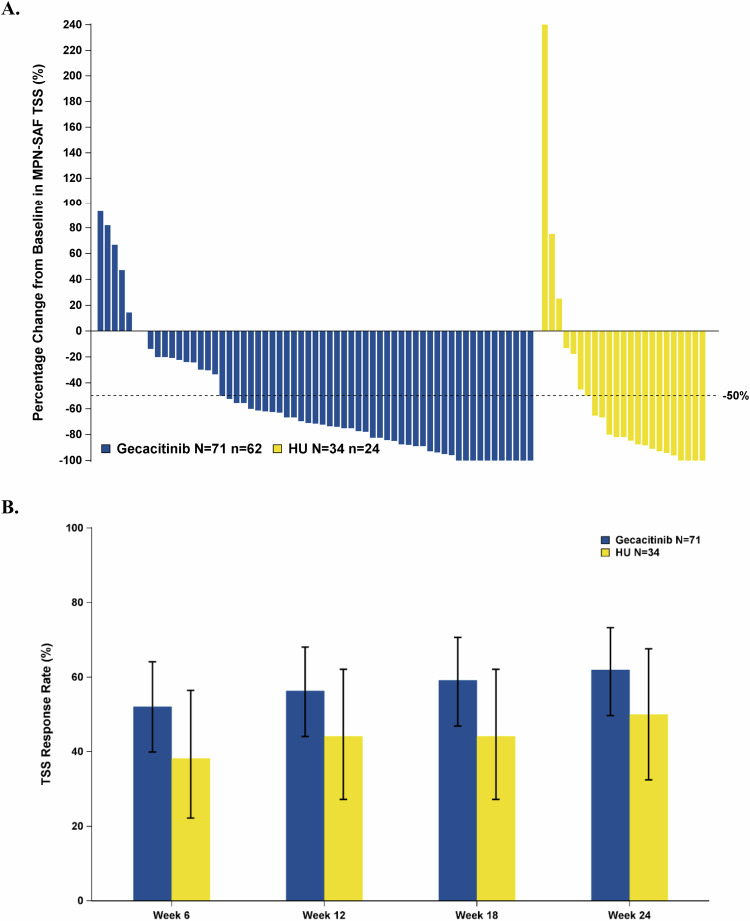
At week 24, 62.0% (44/71; 95% CI: 49.7–73.2%) of gecacitinib patients reported a TSS50, compared to 50.0% (17/34; 95% CI: 32.4–67.6%) in the HU group, P = 0.2065; Fig. 3A and Table 2. Besides, more patients achieved TSS50 in the gecacitinib group than in the HU Group at each visit, starting from week 6, Fig. 3B.
Fig. 3. Change in MPN-SAF TSS.
A Percentage change in TSS compared to baseline at week 24. B Proportion of patients achieving TSS50 (≥50% reduction in TSS) at each visit. HU hydroxyurea (Note: patients without week 24 TSS data or with TSS 0 at baseline [9 from the gecacitinib group and 10 from the HU group] are not included on the waterfall plot).
In the gecacitinib group, 47.9% (34/71) achieved both SVR35 and TSS50 responses at week 24, compared with 20.6% (7/34) in the HU group (post-hoc analysis). Moreover, the gecacitinib group showed a 63.4% (45/71) achievement of both SVR35 and TSS50 at any timepoint during the study, vs the 26.5% (9/34) in the HU group (post-hoc analysis).
Anemia improvement
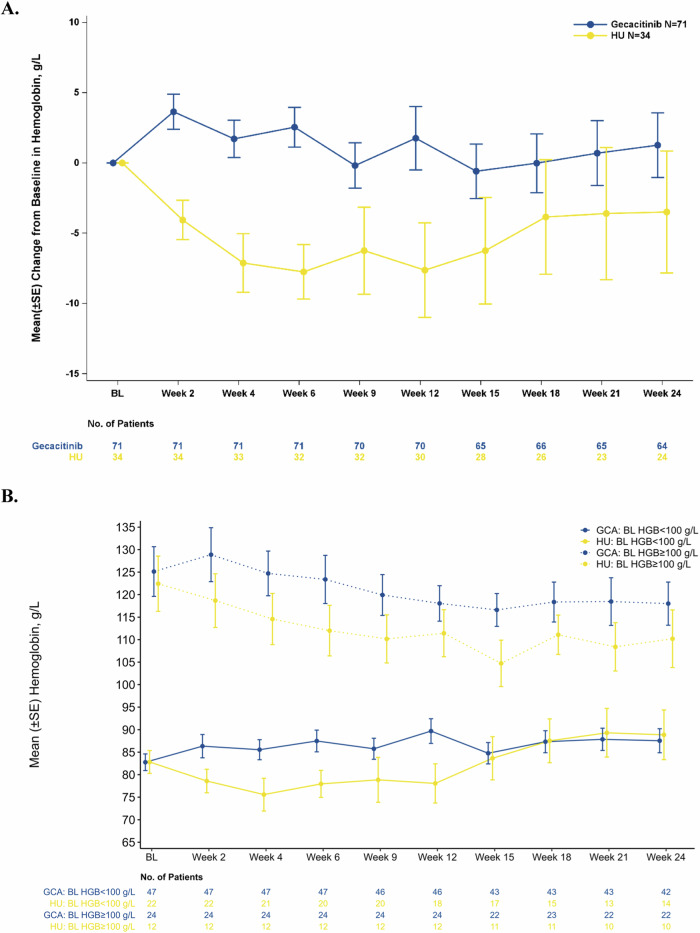
One of six transfusion-dependent gecacitinib patients at baseline became independent post-treatment, whereas none in the HU group archived this; P = 0.4386; Table 2. Among patients who were non-transfusion-dependent and had HGB ≤ 100 g/L at baseline, 31.0% (13/42) in the gecacitinib group experienced an increase of at least 20 g/L in HGB post-treatment, compared to 15.0% (3/20) in the HU group; Table 2. Among the 11 gecacitinib patients requiring RBC transfusions at baseline, seven and six patients, respectively, showed a reduction in transfusion frequency and units by ≥50% from baseline; in the HU group, these numbers were three and two out of five patients, P > 0.9999 and P = 0.4795 respectively; Table 2. Gecacitinib demonstrated a positive effect compared to HU, as indicated by the mean absolute change in HGB relative to baseline at each visit, particularly at week 24 (1.27 g/L vs −3.50 g/L), Fig. 4A and Table 2. A steady increase in mean HGB levels over time has been observed in patients with baseline levels < 100 g/L, while mean HGB levels have remained stable in patients with baseline levels ≥ 100 g/L, showing only a minor overall decrease, Fig. 4B. Using a mixed-effects model for repeated measures, a significant difference in HGB change from baseline between the two treatment groups during the main study period was observed (P = 0.0118).
Fig. 4. Change in hemoglobin.
A Change in hemoglobin (HGB) relative to baseline at each visit. B Mean hemoglobin levels in patients by baseline levels of <100 g/L or ≥100 g/L (main study period). GCA gecacitinib, HU hydroxyurea, BL HGB baseline hemoglobin level.
Platelet changes
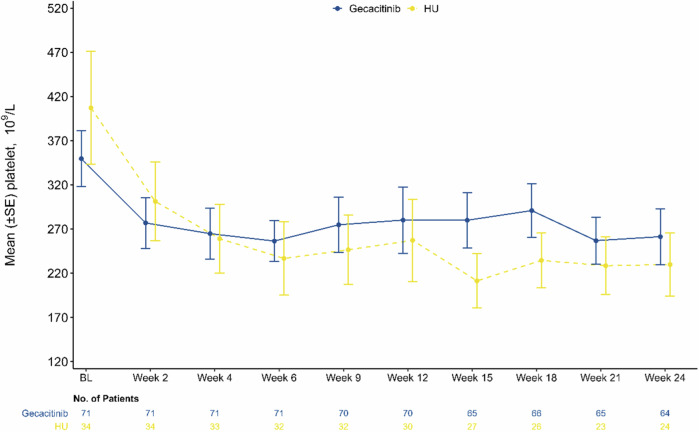
Even with lower initial platelet levels (mean [SD]: 349.70 [267.67] × 109/L vs 407.24 [372.18] × 109/L), gecacitinib group exhibited less fluctuation in platelet levels at each visit, and from week 4 onward, the mean platelet levels surpassed those of the HU group (−93.28 × 109/L vs −182.56 × 109/L) (Fig. 5 and Table 2).
Fig. 5. Change in platelet count.
Change in platelet relative to baseline at each visit. HU hydroxyurea, BL baseline.
Objective response rate
At week 24, one patient of each group achieved partial remission; furthermore, 70.4% (50/71) of patients in the gecacitinib group experienced clinical improvement, vs 41.2% (14/34) in the HU group, Table 2.
In the gecacitinib group, progressive splenomegaly, defined as an increase of ≥25% from the nadir, including baseline measurements, was observed at a rate of 25.4% (18/71; Supplementary Table 1). In comparison, the incidence in the HU group was 11.8% (4/34). Within the gecacitinib group, three (4.2%) experienced leukemic transformation (Supplementary Table 2). In contrast, no patients in the HU group exhibited these leukemic events. Notably, among the HU group, 17 of 34 patients subsequently crossed over to receive gecacitinib, with 14 of these patients demonstrating no signs of progressive disease at the time of crossover. Censoring prior to crossover for patients who had not yet experienced disease progression may be associated with a smaller number of patients with progressive disease in the HU group.
A total of 12 patients, with 8 in the gecacitinib group and 4 in the HU group died. The OS rate of the gecacitinib group at 18 months was 85.4%, compared to 79.5% in the HU group, with median OS not reached in either group, Supplementary Fig. 2.
Efficacy was durable with continued gecacitinib. At week 48 in the interim analysis population, patients in the gecacitinib group showed a 66.0% (31/47) SVR35 rate, a 59.6% (28/47) TSS50 rate, an 85.1% (40/47) best overall spleen response rate, and a 46.4% (13/28) rate of ≥20 g/L HGB increase among non-transfusion-dependent patients with baseline HGB ≤ 100 g/L. Among patients with SVR35, 74.7% maintained a response for at least 48 weeks.
Safety
The average drug exposure during the main study period was 173.5 (SD: 30.7) days for the gecacitinib group and 154.9 (57.9) days for the HU group. Adverse event occurrence was 98.6% in the gecacitinib group, with ≥grade 3 AEs in 49.3%; the HU group reported 100% occurrence with 64.7% at ≥grade 3. The incidence of serious AEs was 26.8% for gecacitinib and 41.2% for HU.
AEs leading to dose reduction or treatment interruption were reported in 25.4% of gecacitinib patients, with 7.0% discontinuing treatment permanently; corresponding rates for HU were 32.4% and 11.8%, Supplementary Table 3.
The common grade ≥ 3 treatment-emergent adverse events (TEAEs, occurring in ≥20%) were anemia (26.8% in gecacitinib vs 44.1% in HU), thrombocytopenia (15.5% vs 32.4%), leukopenia (2.8% vs 20.6%), and neutropenia (1.4% vs 20.6%). Common non-hematological TEAEs of any grade, including upper respiratory tract infection (15.5% vs 17.6%), blood bilirubin increase (12.7% vs 20.6%), fever (12.7% vs 17.6%), and diarrhea (14.1% vs 17.6%), were also favorable with gecacitinib, Table 3.
Table 3.
TEAEs reported in ≥10%.
| Gecacitinib | HU | |||
|---|---|---|---|---|
| (n = 71), n (%) | (n = 34), n (%) | |||
| Any grade | ≥3 | Any grade | ≥3 | |
| Hematological | ||||
| Thrombocytopenia | 27 (38.0%) | 11 (15.5%) | 19 (55.9%) | 11 (32.4%) |
| Anemia | 26 (36.6%) | 19 (26.8%) | 19 (55.9%) | 15 (44.1%) |
| Leukopenia | 10 (14.1%) | 2 (2.8%) | 12 (35.3%) | 7 (20.6%) |
| Leukocytosis | 9 (12.7%) | 3 (4.2%) | 0 | 0 |
| Neutropenia | 7 (9.9%) | 1 (1.4%) | 9 (26.5%) | 7 (20.6%) |
| Lymphopenia | 2 (2.8%) | 1 (1.4%) | 8 (23.5%) | 4 (11.8%) |
| Non-hematological | ||||
| Blood creatinine increased | 14 (19.7%) | 0 | 2 (5.9%) | 0 |
| Alanine aminotransferase increased | 12 (16.9%) | 1 (1.4%) | 1 (2.9%) | 0 |
| Upper respiratory tract infection | 11 (15.5%) | 0 | 6 (17.6%) | 0 |
| Diarrhea | 10 (14.1%) | 0 | 6 (17.6%) | 0 |
| Aspartate aminotransferase increased | 10 (14.1%) | 0 | 1 (2.9%) | 0 |
| Blood bilirubin increased | 9 (12.7%) | 0 | 7 (20.6%) | 0 |
| Pyrexia | 9 (12.7%) | 0 | 6 (17.6%) | 0 |
| Hypertension | 9 (12.7%) | 5 (7.0%) | 0 | 0 |
| Hyperuricemia | 8 (11.3%) | 0 | 3 (8.8%) | 0 |
| Urinary tract infection | 6 (8.5%) | 0 | 4 (11.8%) | 1 (2.9%) |
| Weight decreased | 4 (5.6%) | 0 | 5 (14.7%) | 0 |
Data are n (%). Shown are any grade event occurring in more than 10% of patients in either group.
Adverse drug reactions (ADRs) of special interest showed lower cytopenia rates in the gecacitinib group (56.3% vs 76.5%) with respective anemia rates of 26.8% and 52.9%. Infectious pneumonia occurred in 5.6% of gecacitinib patients vs 2.9% in HU, and peripheral neuropathy was reported in 2.8% of gecacitinib patients, with no cases in the HU group. Opportunistic infection-related AEs were reported in 9.9% for gecacitinib and 8.8% for HU, with 1.4% and 2.9% being drug-related, respectively.
By the cutoff date, five deaths were reported during the study or within 28 days after treatment discontinuation. The causes of death included septicemia, infectious pneumonia, multiple organ failure, gastrointestinal bleeding, and adenocarcinoma of the cardia, all of which occurred in the gecacitinib group. One death occurred during the main study period and was considered drug-unrelated by the investigator, while four deaths occurred in the extension period, one (infectious pneumonia) was deemed drug-related, and three were considered unrelated.
Discussion
Our study, conducted through a randomized controlled trial, has established that gecacitinib not only outperforms HU in reducing splenomegaly but also effectively mitigates anemia in patients with MF, with a trend towards symptom improvement, while maintaining an acceptable safety profile. Gecacitinib monotherapy is capable of addressing the multifaceted clinical requirements of MF patients, offering efficient symptom management, enhancing patient adherence, and ensuring safety.
Compared with phase 3 trials in western patients with MF, the baseline characteristics in this study presented lower HGB levels and smaller spleen volumes, consistent with the features of Chinese patients [3]. In contrast to other phase III studies of JAK inhibitors (such as COMFORT I/II, SIMPLIFY-1, and JAKARTA), our study utilized DIPSS rather than the International Prognostic Scoring System (IPSS). Research by Hernández-Boluda JC et al. has demonstrated that the proportion of patients classified as high risk is markedly reduced when assessed using DIPSS as opposed to IPSS [24]. In our study, the proportions of patients reclassified as intermediate-2 and high risk at baseline according to IPSS were 26.7% and 72.4%, respectively.
Given HU’s prevalent use in MF treatment in China, it served as the control in our study [14]. In comparison to HU, gecacitinib adeptly addresses the three primary clinical challenges in MF patients: splenomegaly, constitutional symptoms, and anemia. Data analysis from 105 patients at week 24 confirmed the interim findings from 70 patients [21], with statistically significant group differences (24-week SVR35 rate of 64.8% vs 26.5%, P = 0.0002). The Gecacitinib group exhibited a notably greater decrease in spleen volume than those on HU (−51.7% vs −30.0%), signifying its superior efficacy in ameliorating splenomegaly. This reduction in spleen volume was paralleled by a tendency toward improvement in constitutional symptoms. A 14.6% greater proportion of TSS50 achievement with gecacitinib at week 6 underscored its potential for prompt and effective treatment impact.
Compared with the phase 2 ZGJAK002 study [18], the SVR35 rate at week 24 was higher in the gecacitinib group (64.8% vs 54.8%). One potential explanation is that unlike the former, only dose interruption but no dose reduction strategy was planned to be used for safety in this study. Although there were individual patients who reported dose reduction, the median gecacitinib dose intensity was 3.8 tablets/day, higher than that in the ZGJAK002 study (3.4 tablets/day in the 100 mg BID group).
On the other hand, HU demonstrated a higher SVR35 response at week 24 than anticipated in this study (~26% vs 6%). It is important to note that this study involved a patient population with limited prior exposure to HU (gecacitinib 42.3% vs HU 50.0%), and both groups had a relatively short median disease duration of 0.2 years prior to randomization. Additionally, as a double-blind study, the protocol mandated the maintenance of a fixed dosage of HU to maintain consistency with the treatment regimen of the experimental group. The selection of the HU 500 mg BID dosage in this study was based on a comprehensive assessment of the physical conditions of patients with higher-risk MF and their baseline complete blood cell counts [15].
Gecacitinib is a deuterated derivative of momelotinib, developed with the aim of improving the pharmacokinetic properties of the compound [25]. Given the shared mechanism of ACVR1 inhibiting, gecacitinib was also associated with anemia improvement. HGB measurements also favored gecacitinib, by which HGB levels increased from week 2 especially in patients with baseline levels < 100 g/L (Fig. 4B), pointing to its potential for ameliorating anemia by mitigating hematopoietic suppression.
At the 48-week interim analysis, except for a slight fluctuation in TSS50, which was still around 60%, gecacitinib’s treatment benefits with respect to SVR35, best spleen response, and improvement in HGB increased compared to 24 weeks. This indicates that continuous treatment with gecacitinib can bring sustained long-term benefits.
Gecacitinib demonstrated a safety profile, with lower incidences of ≥grade 3 AEs/ADRs and AEs/ADRs leading to drug discontinuation compared to HU (Supplementary Table S3). In the gecacitinib group, the AE of infections, mainly upper respiratory tract infection, and no second cancer were reported. An infectious screening was required before study entry but prophylaxis was not to be needed. The incidences of common TEAEs were comparable to that in the ZGJAK002 study [18] regardless of gecacitinib exposure, and so was the rate of AEs leading to drug discontinuation. Gecacitinib was less associated with myelosuppression in Chinese MF patients compared with other JAK inhibitors, such as ruxolitinib [7]. The occurrence rates of the non-hematological TEAEs peripheral neuropathy, which is common in patients with momelotinib, and diarrhea, nausea, and vomiting, which are common with fedratinib and pacritinib, were relatively low with gecacitinib (peripheral neuropathy 2.8%, diarrhea 14.1%, nausea 7.0%, and vomiting 2.8%) [26]. This safety profile of gecacitinib underscores its suitability for long-term use in MF patients. In another study of longer follow-up of gecacitinib treatment (median follow-up of 30.7 months), long-term safety advantages have also been reported [27]. It should be noted that, like other JAK inhibitors, gecacitinib has a certain immunosuppressive activity, it is recommended to conduct timely screening and monitoring of relevant risks [28].
This study, despite providing valuable insights into the efficacy and safety of gecacitinib in JAK-naïve MF patients in China, has several limitations. First, this study is limited to a Chinese population, which may affect the generalizability of the results to a broader and more diverse patient group. Additionally, the stringent criteria for RBC transfusion in anemic patients in China [3] resulted in the inclusion of a limited number of transfusion-dependent patients, hindering a comprehensive evaluation of gecacitinib’s efficacy in reversing transfusion dependency. Furthermore, early crossover in the HU group may have affected the comparison of progression-free and leukemia-free survival between the two treatment groups. Finally, the study’s focus on HU as a comparator somewhat narrows its international clinical relevance. Further research, especially head-to-head comparisons with other JAK inhibitors, is warranted to better understand gecacitinib’s benefits in MF patients.
In conclusion, the final analysis of this phase 3 study reveals that gecacitinib consistently surpasses HU in spleen reduction, symptom improvement, and anemia amelioration in MF patients, with no new safety concerns emerging. These findings endorse gecacitinib as a potential new front-line treatment option for MF patients, particularly those suffering from anemia.
Supplementary information
Acknowledgements
This study was supported by Suzhou Zelgen Biopharmaceuticals Co., Ltd, and the Natural Science Foundation of China (82100159 to YZ). We thank all the participating patients and their families, the investigators, and the teams who participated in this trial.
Author contributions
JJ and LW conceived and designed the study, YZ, HZ, SS, JZ, LY, AH, QL, XD, SG, YL, YL, YC, WW, HZ, GH, MH, QJ, ZJ, HJ, JW, NX, LY, CZ, ZZ, CJ, XL, LL, YX, DW, FZ, JZ, XZ, and JJ were study investigators and thus collected data and contributed to its analysis and interpretation. JJ and ZX were also study co-leading investigators. YZ and JJ contributed to the manuscript writing, and all authors critically reviewed and approved the final version before submission.
Funding
Suzhou Zelgen Biopharmaceuticals Co., Ltd, and the Natural Science Foundation of China (82100159 to Y.Z.).
Data availability
There was no data-sharing plan set out at the start of this study. Specific requests for non-identifiable data for valid academic reasons as judged by the trial management group will be granted, with appropriate data sharing agreement, and should be addressed to the chief investigator.
Competing interests
Liqing Wu, Hewen Yin, and Binhua Lv are employees of Suzhou Zelgen Biopharmaceuticals Co., Ltd., which provided funding for this research, and reported stock and other ownership of Suzhou Zelgen Biopharmaceuticals Co., Ltd. However, the views expressed in this publication are those of the author and not necessarily those of Suzhou Zelgen Biopharmaceuticals Co., Ltd. No other potential conflicts of interest were reported.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yi Zhang, Hu Zhou, Shanshan Suo, Junling Zhuang.
These authors jointly supervised this work: Jie Jin, Zhijian Xiao.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-024-01202-8.
References
- 1.Passamonti F, Mora B. Myelofibrosis. Blood. 2023;141:1954–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tefferi A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98:801–21. [DOI] [PubMed] [Google Scholar]
- 3.Xu Z, Gale RP, Zhang Y, Qin T, Chen H, Zhang P, et al. Unique features of primary myelofibrosis in Chinese. Blood. 2012;119:2469–73. [DOI] [PubMed] [Google Scholar]
- 4.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–90. [DOI] [PubMed] [Google Scholar]
- 5.Gerds AT, Verstovsek S, Vannucchi AM, Al-Ali HK, Lavie D, Kuykendall AT, et al. Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis previously treated with a JAK inhibitor (MOMENTUM): an updated analysis of an international, double-blind, randomised phase 3 study. Lancet Haematol. 2023. 10.1016/S2352-3026(23)00174-6. [DOI] [PubMed]
- 6.Gangat N, Begna KH, Al-Kali A, Hogan W, Litzow M, Pardanani A, et al. Predictors of anemia response to momelotinib therapy in myelofibrosis and impact on survival. Am J Hematol. 2023;98:282–9. 10.1002/ajh.26778. [DOI] [PubMed] [Google Scholar]
- 7.Jin J, Du X, Zhou D, Li J, Li J, Hou M, et al. Efficacy and safety of JAK inhibitor ruxolitinib in Chinese patients with myelofibrosis: results of a 1-year follow-up of A2202. Zhonghua Xue Ye Xue Za Zhi. 2016;37:858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du X, Zhou D. Efficacy and safety of JAK inhibitor INC424 in patients with primary and post-polycythemia vera or post-essential thrombocythemia myelofibrosis in the Chinese population. Front Med. 2016;10:437–43. [DOI] [PubMed] [Google Scholar]
- 9.Jung CW, Shih LY, Xiao Z, Jie J, Hou HA, Du X, et al. Efficacy and safety of ruxolitinib in Asian patients with myelofibrosis. Leuk Lymphoma. 2015;56:2067–74. [DOI] [PubMed] [Google Scholar]
- 10.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98. [DOI] [PubMed] [Google Scholar]
- 12.Tremblay D, Mascarenhas J. Next generation therapeutics for the treatment of myelofibrosis. Cells. 2021. 10.3390/cells10051034. [DOI] [PMC free article] [PubMed]
- 13.de Melo Campos P. Primary myelofibrosis: current therapeutic options. Rev Bras Hematol Hemoter. 2016;38:257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Association L. Chinese guideline on the diagnosis and treatment of primary myelofibrosis (2019). Zhonghua Xue Ye Xue Za Zhi. 2019;40:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-Trillos A, Gaya A, Maffioli M, Arellano-Rodrigo E, Calvo X, Díaz-Beyá M, et al. Efficacy and tolerability of hydroxyurea in the treatment of the hyperproliferative manifestations of myelofibrosis: results in 40 patients. Ann Hematol. 2010;89:1233–7. [DOI] [PubMed] [Google Scholar]
- 16.Mesa RA, Vannucchi AM, Mead A, Egyed M, Szoke A, Suvorov A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4:e225–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asshoff M, Petzer V, Warr MR, Haschka D, Tymoszuk P, Demetz E, et al. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood. 2017;129:1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhou H, Jiang Z, Wu D, Zhuang J, Li W, et al. Safety and efficacy of jaktinib in the treatment of Janus kinase inhibitor-naïve patients with myelofibrosis: results of a phase II trial. Am J Hematol. 2022;97:1510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhou H, Duan M, Gao S, He G, Jing H, et al. Safety and efficacy of jaktinib (a novel JAK inhibitor) in patients with myelofibrosis who are intolerant to ruxolitinib: a single-arm, open-label, phase 2, multicenter study. Am J Hematol. 2023;98:1588–97. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhang Q, Liu Q, Dang H, Gao S, Wang W, et al. Safety and efficacy of jaktinib (a novel JAK inhibitor) in patients with myelofibrosis who are relapsed or refractory to ruxolitinib: a single-arm, open-label, phase 2, multicenter study. Am J Hematol. 2023;98:1579–87. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhou H, Zhuang J, He A, Li Y, Yang L, et al. A randomized, double-blind, phase 3 study of jaktinib versus hydroxyurea (HU) in patients (pts) with intermediate-2 or high-risk myelofibrosis (MF). J Clin Oncol. 2023;41:7015. [Google Scholar]
- 22.Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascarenhas J, Hoffman R, Talpaz M, Gerds AT, Stein B, Gupta V, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4:652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-Boluda JC, Pereira A, Correa JG, Alvarez-Larran A, Ferrer-Marin F, Raya JM, et al. Prognostic risk models for transplant decision-making in myelofibrosis. Ann Hematol. 2018;97:813–20. [DOI] [PubMed] [Google Scholar]
- 25.Tefferi A. Jaktinib and momelotinib for the treatment of myelofibrosis-Birds of a feather? Am J Hematol. 2023. 10.1002/ajh.27036. [DOI] [PubMed]
- 26.Patel AA, Odenike O. The next generation of JAK inhibitors: an update on fedratinib, momelotonib, and pacritinib. Curr Hematol Malig Rep. 2020;15:409–18. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhou H, Jiang Z, Wu D, Zhuang J, Li W, et al. Efficacy, safety, and survival findings after long-term follow-up of ZGJAK002: a phase 2 study comparing jaktinib at 100 mg twice daily (BID) and 200 mg once daily (QD) in patients with myelofibrosis. Am J Hematol. 2024;99:774–9. [DOI] [PubMed] [Google Scholar]
- 28.Yarilina A, Xu K, Chan C, Ivashkiv LB. Regulation of inflammatory responses in tumor necrosis factor–activated and rheumatoid arthritis synovial macrophages by JAK inhibitors. Arthritis Rheum. 2012;64:3856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There was no data-sharing plan set out at the start of this study. Specific requests for non-identifiable data for valid academic reasons as judged by the trial management group will be granted, with appropriate data sharing agreement, and should be addressed to the chief investigator.