Abstract
Hemolytic-uremic syndrome (HUS) is a serious disease in children, attributable in the majority of cases to infection with Shiga toxin (Stx)-producing Escherichia coli. Using gnotobiotic piglets orally infected with E. coli O157:H7, which develop Stx-related cerebellar lesions and fatal neurological symptoms, we show that administration of Stx2-specific antiserum well after challenge protected, in a dose-response fashion, against these symptoms for at least 24 h after bacterial challenge. Twenty-six of 30 piglets given Stx2 antiserum survived the challenge, compared to only 4 of 16 animals given control serum or saline. Given our observations in piglets, Stx antibody of human origin may likewise prevent HUS in children.
Hemolytic-uremic syndrome (HUS) is the leading cause of acute renal failure in young children (13–16). Strong epidemiological evidence links the majority of HUS cases in children to infection with Shiga toxin (Stx)-producing Escherichia coli (STEC) (1, 7, 12, 21). STEC strains liberate one or both of two toxins, known as Stx1 and Stx2 (16, 17), of which Stx2 appears to be the one most frequently linked with HUS (15). Stx-related HUS tends to occur either sporadically (14) or in outbreaks that can be traced to a common source of bacterial contamination (10). Incidents are more frequent in certain geographic locations and at certain times of the year. Although there are many STEC serotypes (14, 15, 21), O157:H7 is the one most frequently linked to HUS in children and the very elderly in the United States. Watery, mostly bloody diarrhea is the predominant symptom. Following a prodromal period of several days, HUS and other systemic complications may develop in certain individuals. HUS is marked by microangiopathic hemolytic anemia, thrombocytopenia, renal dysfunction, and on rare occasions neurological complications (9, 25).
Currently there is no effective treatment or prophylaxis for HUS. Stx appears to induce little serum antibody even in recently confirmed cases of HUS (3, 15), and the use of human immunoglobulins in children at risk has had little impact on the clinical outcome (3). In general, passively administered specific antibodies have been much more effective in preventing toxin-mediated diseases than in protecting against microbial agents. Antibodies against tetanus represent a good example. Therefore, we believe that exogenously produced and administered neutralizing antibodies will have a greater impact on the outcome of HUS if administered early in the course of the infection. The prodromal period between onset of diarrhea and development of HUS provides a window for early intervention which may improve the clinical outcome. Administration of antitoxin antibody will likely prevent HUS in contact cases. The purpose of this study was to determine whether Stx2 antibody administration could prevent systemic complications associated with Stx2 absorption from the gut as in children with HUS. Gnotobiotic (GB) piglets have been shown to be highly susceptible to infections with enterohemorrhagic E. coli. When challenged orally, GB piglets exhibit profound diarrhea due to severe mucosal damage associated with bacterial attachment and effacement of colonocytes (22, 23). More than 85% of GB piglets infected with STEC strains develop toxin-mediated neurological lesions manifested clinically by ataxia, head-pressing, recumbency, and death (24). Like humans, GB piglets develop complications when the infecting STEC strain produces Stx2; the only difference is that humans develop HUS and piglets develop neurological complications. In this study we used the GB piglet model to determine whether exogenous, Stx2-specific neutralizing antibody, administered at intervals following oral challenge with a Stx2-producing strain, could protect piglets against neurological complications.
Bacterial strains.
E. coli O157:H7 strain 86-24, which produces Stx2 only, was used in these experiments (9). Strain TUV86-2, a Stx2 deletion mutant (11), was included to illustrate the direct link between Stx2 and central nervous system (CNS) involvement in piglets. Bacteria were grown in LB broth MacConkey (Difco Laboratories, Detroit, Mich.). Bacteria from infected pig tissue were cultured at 37°C on MacConkey (Difco) and blood agar plates (Becton Dickinson Microbiology Systems, Cockeysville, Md.). Antiserum was produced by immunizing two piglets six times over 6 weeks with intramuscular injections of 200 μg of affinity-purified Stx2 (5), suspended in 1 ml of phosphate-buffered saline (PBS), and emulsified with an equal volume of Freund’s incomplete adjuvant. Stx2 toxoid (formalin inactivated) was used for the first two injections; these were followed by four injections of active Stx2 toxin.
The antiserum was collected and stored at −70°C until use. Control serum was collected from an unimmunized sow. Sera were tested for neutralizing activity against Stx2 (concentration of 100 pg) in HeLa cell culture (6). The anti-Stx2 titer present in the control serum and antiserum was determined by enzyme-linked immunosorbent assay. Microtiter plates (Costar no. 9018; Corning Costar Corp., Corning, N.Y.) were coated (50 μl/well) with Stx2 (1 μg/ml in PBS). Antiserum was serially diluted in triplicate on plates. Plates were incubated and then washed and incubated with antiporcine immunoglobulin (IgM)- and IgG-alkaline phosphatase labeled antibody (Bethyl Laboratories, Montgomery, Tex.). The assay was developed with p-nitrophenylphosphate (1 mg/ml; Sigma, St. Louis, Mo.), and the A405 was determined. The titer was defined as the highest dilution which gave an optical density value greater than twice the background. Control serum contained IgM and IgG titers of 1:3,200 and 1:1,600, respectively. The antiserum contained IgM and IgG titers of 1:400 and 1:51,200, respectively. Thus, the anti-Stx2 IgG titer of the antiserum was 32 times greater than that of the control serum. Although the control serum contained Stx2-reactive antibody, at a 1:10 dilution, no toxin neutralizing activity was observed in vitro, whereas at a 1:64,000 dilution of antiserum, greater than 90% toxin neutralization was observed. Likely, the Stx2-reactive antibody in the control serum was due to a cross-reactive antigen, such as Stx2v, which is antigenically related to enterohemorrhagic E. coli Stx2 and produced by E. coli strains that naturally infect swine (16).
Animals and experimental procedure.
Fifty-nine GB piglets, derived by cesarean section from five litters, were randomized into five groups (Table 1) and maintained within sterile isolators for the duration of the experiment. Within 24 h of birth, piglets were challenged orally with 1010 E. coli O157:H7 strain 86-24. This high inoculum usually induces, within 48 to 96 h of challenge, neurological signs and brain lesions associated with Stx2 in >85% of piglets. Thirty piglets were treated with a single intraperitoneal (i.p.) injection of 5 ml (∼4 ml/kg of body weight) of swine Stx2 antiserum, given at 6 (8 animals), 12 (11 animals), or 24 (11 animals) h after bacterial challenge. Sixteen control piglets were given a single i.p. injection of PBS (6 animals) or 5 ml (∼4 ml/kg) of control serum (10 animals) 6 and 12 h after bacterial challenge, respectively. Two additional control groups were also included; a group of five untreated animals was challenged with the Stx2 deletion mutant strain TUV86-2, and a second group of four was neither challenged nor treated (Table 1). After euthanasia, piglets were examined for gross abnormalities. Liver, kidney, small and large intestine, and brain tissue were formalin fixed for histopathology. Blood and small and large intestinal mucosal scrapings were streaked on blood and MacConkey agar plates for bacterial identification and quantitation.
TABLE 1.
Impact of i.p. administration of Stx2 swine immune serum (4 ml/kg) given at three different intervals, on development of neurological complications in piglets challenged with 1010 organisms of strain 86-24
| Treatment group | Time (h) after challenge | No. of pigs | No. with:
|
||
|---|---|---|---|---|---|
| Colonic lesions | CNS
|
||||
| Signsa | Lesions | ||||
| Placebo | 6 | 6 | 6 | 5 | 5 |
| Swine control serum | 12 | 10 | 9 | 8b | 8 |
| Stx2 swine immune serum | 6 | 8 | 8 | 0 | 0 |
| 12 | 11 | 11 | 1 | 1d | |
| 24 | 11 | 11 | 3 | 1 | |
| Control 1 | NAe | 5 | 5 | 0 | 0 |
| Control 2 | NA | 4 | 0 | 0 | 0 |
Two groups were given the same dose of either swine control serum from an unimmunized animal or PBS (placebo). An untreated control group (control 1) challenged with the Stx2 deletion mutant strain TUV86-2 (12) was included to illustrate the role of Stx2 in CNS involvement. A second control group (control 2) received neither challenge nor treatment.
All piglets except those in the swine control serum were euthanized when recumbent before death.
Mild symptoms of ataxia but no neurological lesions were observed in one piglet.
Mild characteristic neurological lesions were observed in one of the asymptomatic animals.
NA, not applicable.
Clinical observations.
Two piglets that succumbed within 48 h after bacterial challenge from severe diarrhea, before neurological symptoms were apparent, and two that died overnight and therefore could not be assessed clinically or histologically were excluded from the study. The remaining 51 challenged piglets developed various degrees of diarrhea and wasting which did not appear to have been influenced by the treatment. Of the 30 animals that received Stx2 antiserum, 4 developed neurological abnormalities (Table 1), including ataxia, incoordination, head pressing, and finally recumbency and leg paddling. Progression of symptoms was rapid and occurred within 4 to 8 h of onset. No symptoms or lesions were observed in eight animals treated 6 h after bacterial challenge. Of the 11 animals treated 12 h after bacterial challenge, one developed mild nonfatal neurological symptoms. Of the 11 animals treated 24 h after challenge, 3 developed symptoms (Table 1). Five of the six placebo-treated piglets developed neurological symptoms, while 8 of the 10 treated with swine control serum developed symptoms. The results show a clear time-related response. Four age-matched animals, which were neither treated nor challenged with bacteria, remained healthy throughout the experiment. None of the five piglets challenged with Stx2 deletion mutant strain TUV86-2 showed neurologic symptoms or lesions, but all had diarrhea.
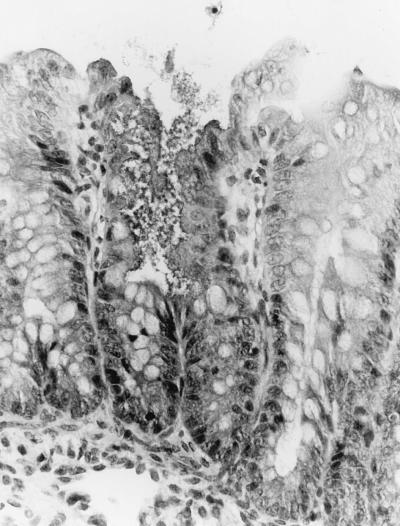
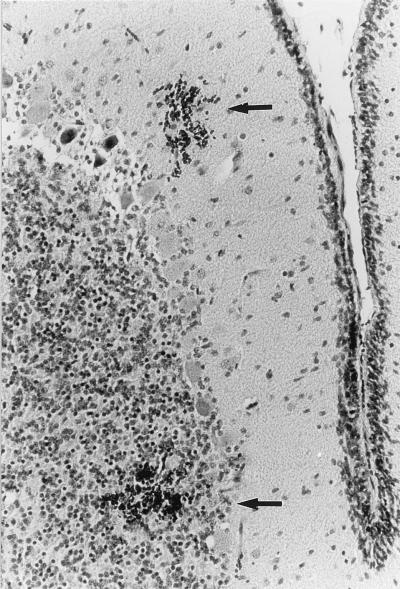
Colonic and ileal lesions of bacterial attachment and effacement among the treatment groups challenged with strain 86-24 were qualitatively similar to one another and to those previously reported for animals colonized with these organisms (22–24). Variable numbers of short bacterial rods were attached to the apical surfaces of superficial epithelial cells in the ileum and colon, and many of those cells exhibited loss of apical cytoplasm, lending a scalloped appearance to the mucosal surface (Fig. 1). Severe ileal lesions, characterized by necrosis of superficial epithelial cells, large numbers of bacteria, and infarcts in the lamina propria of villi, and shortened villi, were present in some animals. Microscopic changes in the CNS were most prominent in the cerebellar grey matter but were also occasionally apparent in the cerebellar white matter and the cerebral hemispheres. Lesions consisted of scattered, multiple foci of hemorrhage and necrosis within the granular and molecular cerebellar layers. Hemorrhagic zones were variably sized and often adjacent to focal aggregates of necrotic granular layer of neurons, suggestive of infarction (Fig. 2). In addition, mesenteric vascular plexi contained small vessels accentuated by surrounding aggregates of lymphocytes. Inflammatory cells were present within vessel walls, and there was focal discontinuity and loss of mural detail.
FIG. 1.
Section through the large intestine of a piglet challenged orally with E. coli O157:H7 strain 86-24 4 days earlier. A disrupted colonic surface damaged by bacterial attachment and effacement seen in the middle, with no evidence of inflammation. The mucosa on either side of the lesion looks still intact (hematoxylin and eosin; original magnification, ×400).
FIG. 2.
Section through the cerebellum of a piglet challenged orally with E. coli O157:H7 strain 86-24, which produces Stx2, showing focal hemorrhages (arrows) in the molecular and granular layers (hematoxylin and eosin; original magnification, ×400).
These experiments show that piglets can be protected from the systemic effect of Stx2 with exogenous specific neutralizing antibody, even when given after bacterial challenge. There was a clear correlation between time of administration of Stx2 antiserum and protection. Animals were protected fully when treated within 6 h and slightly less at 12 h after challenge. As expected, administration of antiserum 24 h after bacterial challenge was less protective than administration at earlier times. Nevertheless, protection was still considerable at 24 h; 7 of 11 were free of neurological symptoms or brain lesions, compared with 3 of 16 control animals. In piglets, in which neurological symptoms begin approximately 48 h after challenge (much sooner than in humans), these results are significant and suggest that children too, could likewise be protected against development of renal failure and other systemic complications, if treated early with neutralizing Stx-specific antibody. This time point is likely to be at the onset of bloody diarrhea or when infections with Stx-producing bacteria are confirmed. An early administration of antibody to a sibling of affected individuals, to other contact children, or in the wake of an outbreak in a day-care center, for instance, will, in our view, reduce the incidence of HUS and improve the clinical outcome of infection.
The GB piglet was selected to demonstrate the efficacy of antibody against Stx2 because it can be challenged orally with bacteria without preconditioning, is susceptible to all Stx2-producing STEC, and the liberated toxin is absorbed through the gut as in humans. The dynamics of antibody neutralization of toxins taken up gradually from the gut lumen is quite different from toxin given systemically and mimics more closely the situation in HUS. We have also used immune pig serum in the pig model, to maximize the effector function of the Stx antibody. A streptomycin-treated mouse model that can be challenged orally with STEC has also been described (26); however, the relative lethal dose of Stx2 is considerably higher than that required for piglets and presumably for children since in both species the disease occurs naturally.
No HUS-specific treatment or prevention methods are currently available. The most commonly used supportive treatments include plasma exchange and infusions of fresh-frozen plasma, corticosteroids, intravenous immunoglobulin, and antibiotics, of which plasma exchange appears to be somewhat beneficial (4, 18, 19). Human immunoglobulins used therapeutically or prophylactically have some benefit in conditions in which the etiology of the systemic disease is either unknown, as in Kawasaki syndrome, or ill defined, as in thrombotic thrombocytopenic purpurea, or when technically it is not possible to produce specific antibodies, as for some of the viral hepatitis infections. The failure to demonstrate any benefit for human immunoglobulin in HUS may be due to the absence of significant amounts of neutralizing Stx antibody, particularly against Stx2, in sera of healthy or even convalescent individuals (8). The lack of antibodies in convalescent sera in confirmed cases of Stx-mediated HUS is curious, since both toxins are highly antigenic.
Systemic administration of Stx antibody did not protect piglets against mucosal damage and diarrhea, which in piglets at least are attributed largely to intimin-mediated bacterial attachment and effacement to ilial enterocytes and colonocytes. A similar outcome would be expected to occur in children who are given Stx antibodies systemically to prevent development of HUS.
These experiments confirm our hypothesis that early administration of highly specific neutralizing antibodies, even when given after bacterial exposure, is protective against systemic complications associated with Stx. We have also shown that while the protection was time dependent, it was still protective in the majority of piglets 24 h after bacterial challenge. These observations strongly suggest that protection of children at risk of HUS is more than likely with an early administration of human or humanized neutralizing Stx-specific antibody. Since the half-life of exogenous immunoglobulin in humans is reported to range between 6 and 14 days (2, 20), probably a single effective dose would be sufficient.
Acknowledgments
This work was supported by Public Health Service grant 5RO1AI41326-02 from the National Institutes of Health.
The technical assistance of Melissa Paris, Sue Chapman, and Jessica Brisban is greatly appreciated.
REFERENCES
- 1.Amorosi E L, Ultmann J E. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine. 1966;45:139–159. [Google Scholar]
- 2.Ballow M. Mechanisms of action of intravenous immune serum globulin therapy. J Pediatr Infect Dis. 1994;13:806. doi: 10.1097/00006454-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Bitzan M, Klemt M, Steffans T, Muller-Wiefel D I. Differences in verotoxin neutralizing activity of therapeutic immunoglobulins and sera from healthy controls. Infection. 1993;21:140–145. doi: 10.1007/BF01710530. [DOI] [PubMed] [Google Scholar]
- 4.Brichard B, Vermylen C, Heremans M T, Ninane G, Cornu G. Plasma infusion as treatment for 33 children with haemolytic uraemic syndrome: a good therapy? Acta Clin Belg. 1993;48:156–163. doi: 10.1080/17843286.1993.11718303. [DOI] [PubMed] [Google Scholar]
- 5.Donohue-Rolfe A, Acheson D W K, Kane A V, Keusch G T. Purification of Shiga toxin and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect Immun. 1989;56:3888–3893. doi: 10.1128/iai.57.12.3888-3893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donohue-Rolfe A, Keusch G T, Edson C, Thorley-Lawson D, Jacewicz M. Pathogenesis of Shigella diarrhea. IX. Simplified high yield purification of shigella toxin and characterization of subunit composition and function by the use of subunit specific monoclonal and polyclonal antibodies. J Exp Med. 1984;160:1767–1781. doi: 10.1084/jem.160.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gransden W R, Damm S, Anderson J D, et al. Further evidence associating hemolytic uremic syndrome with infection by verotoxin-producing Escherichia coli O157:H7. J Infect Dis. 1986;154:522–524. doi: 10.1093/infdis/154.3.522. [DOI] [PubMed] [Google Scholar]
- 8.Greatorex J S, Thorne G M. Humoral immune response to Shiga-like toxins and Escherichia coli O157 lipopolysaccharide in hemolytic-uremic syndrome patients and healthy subjects. J Clin Microbiol. 1994;32:1172–1178. doi: 10.1128/jcm.32.5.1172-1178.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin P M, Ostroff S M, Tauxe R V, Greene K D, Wells J G, Lewis J H, Blake P A. Illnesses associated with Escherichia coli O157:H7 infections; abroad clinical spectrum. Ann Intern Med. 1988;109:705–712. doi: 10.7326/0003-4819-109-9-705. [DOI] [PubMed] [Google Scholar]
- 10.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 11.Gunzer F, Bohn U, Fuchs S, Muhldorfer I, Hacker J, Tzipori S, Donohue-Rolfe A. Construction and characterization of an isogenic slt-ii deletion mutant of enterohemorrhagic Escherichia coli. Infect Immun. 1998;66:2337–2341. doi: 10.1128/iai.66.5.2337-2341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karmali M A, Petric M, Lim C, et al. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 13.Karmali M A. Infection with verotoxin Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karmali M A, Steele B T, Petric M, Lim C. Sporadic cases of hemolytic uremic syndrome associates with fecal cytotoxin and cytotoxin-producing Escherichia coli. Lancet. 1983;i:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 15.Kleanthous H, Smith H R, Scotland S M, Gross R J, Rowe B, Tayler C M, Milford D V. Haemolytic uraemic syndrome in the British Isles 1985-9: association with verotoxin producing Escherichia coli. Part 2. Microbiological aspects. Arch Dis Child. 1990;65:722–727. doi: 10.1136/adc.65.7.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien A D, Holmes R K. Shiga and Shiga-like toxins. Microbiol Rev. 1987;51:206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira A, Mazzara R, Monteagudo J, Sanz C, Puig L, Martinez A, Ordinas A, Castillo R. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome: a multivariate analysis of factors predicting the response to plasma exchange. Ann Hematol. 1995;70:319–323. doi: 10.1007/BF01696619. [DOI] [PubMed] [Google Scholar]
- 19.Roberts A W, Gillett E A, Fleming S J. Hemolytic uremic syndrome/thrombotic thrombocytopenic purpura: outcome with plasma exchange. J Clin Apheresis. 1991;6:150–154. doi: 10.1002/jca.2920060305. [DOI] [PubMed] [Google Scholar]
- 20.Schiff R I. Intravenous gammaglobulin: pharmacology, clinical uses and mechanisms of action. Pediatr Allergy Immunol. 1994;5:63. doi: 10.1111/j.1399-3038.1994.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 21.Spika J S, Parsons J E, Nordenberg D, et al. Hemolytic uremic syndrome and diarrhea associated with Escherichia coli O157:H7 in a day care center. J Pediatr. 1986;109:287–291. doi: 10.1016/s0022-3476(86)80386-9. [DOI] [PubMed] [Google Scholar]
- 22.Tzipori S, Wachsmuth I K, Smithers J, Jackson C. Studies in gnotobiotic piglets on non-O157:H7 Escherichia coli serotypes isolated from patients with hemorrhagic colitis. Gastroenterology. 1988;94:590–597. doi: 10.1016/0016-5085(88)90228-4. [DOI] [PubMed] [Google Scholar]
- 23.Tzipori S, Wachsmuth I K, Chapman C, et al. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis. 1986;154:712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- 24.Tzipori S, Gunzer F, Donnenberg M S, DeMontigny L, Kaper J B, Donohue-Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun. 1995;63:3621–3627. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzipori S, Chow C W, Powell H R. Cerebral involvement associated with Escherichia coli O157:H7 infection: observations in humans and gnotobiotic piglets. J Clin Pathol. 1988;41:1099–1103. doi: 10.1136/jcp.41.10.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadolkowski E A, Sung L M, Burris J A, Samuel J E, O’Brien A D. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun. 1990;58:3959–3965. doi: 10.1128/iai.58.12.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]