Abstract
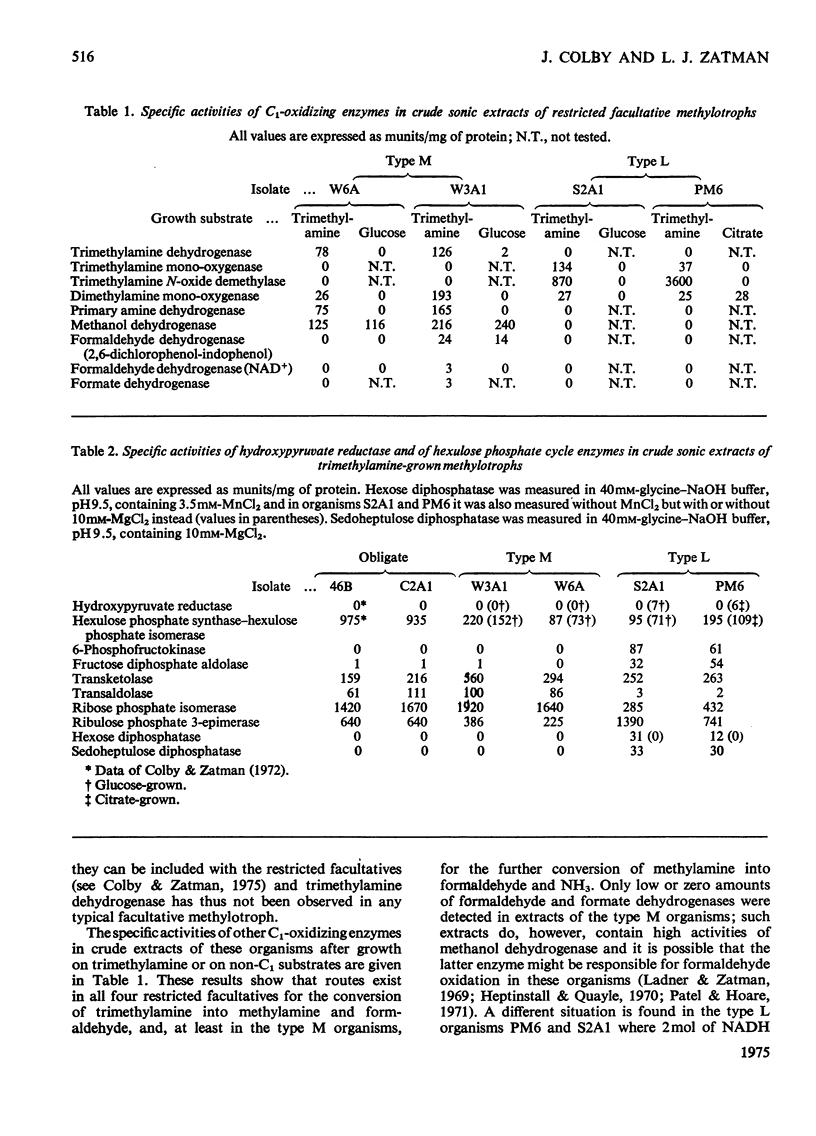
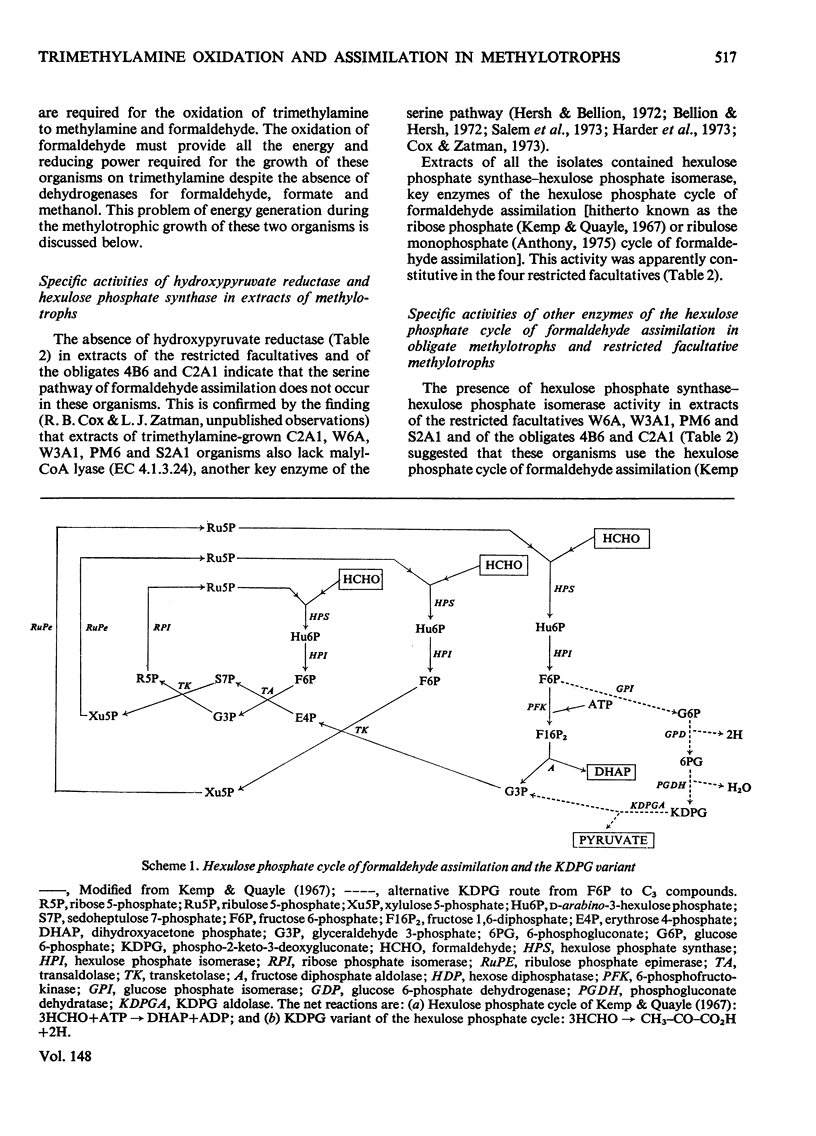
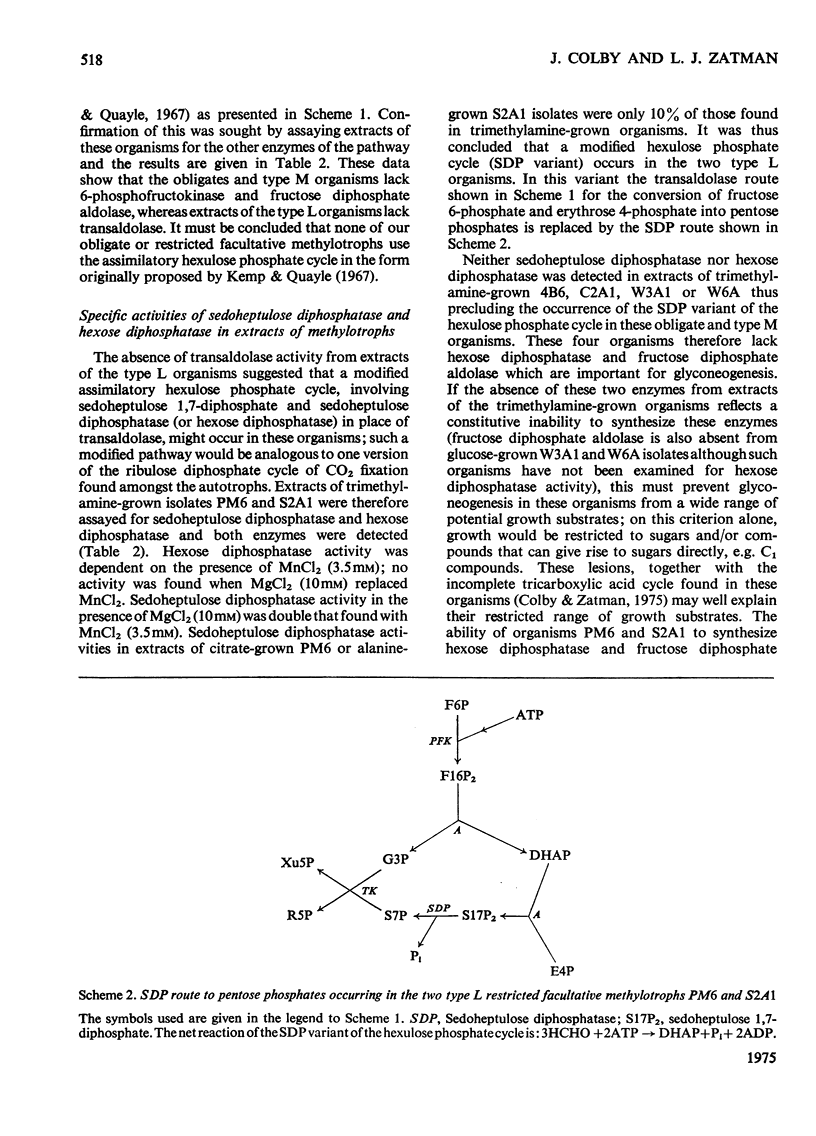
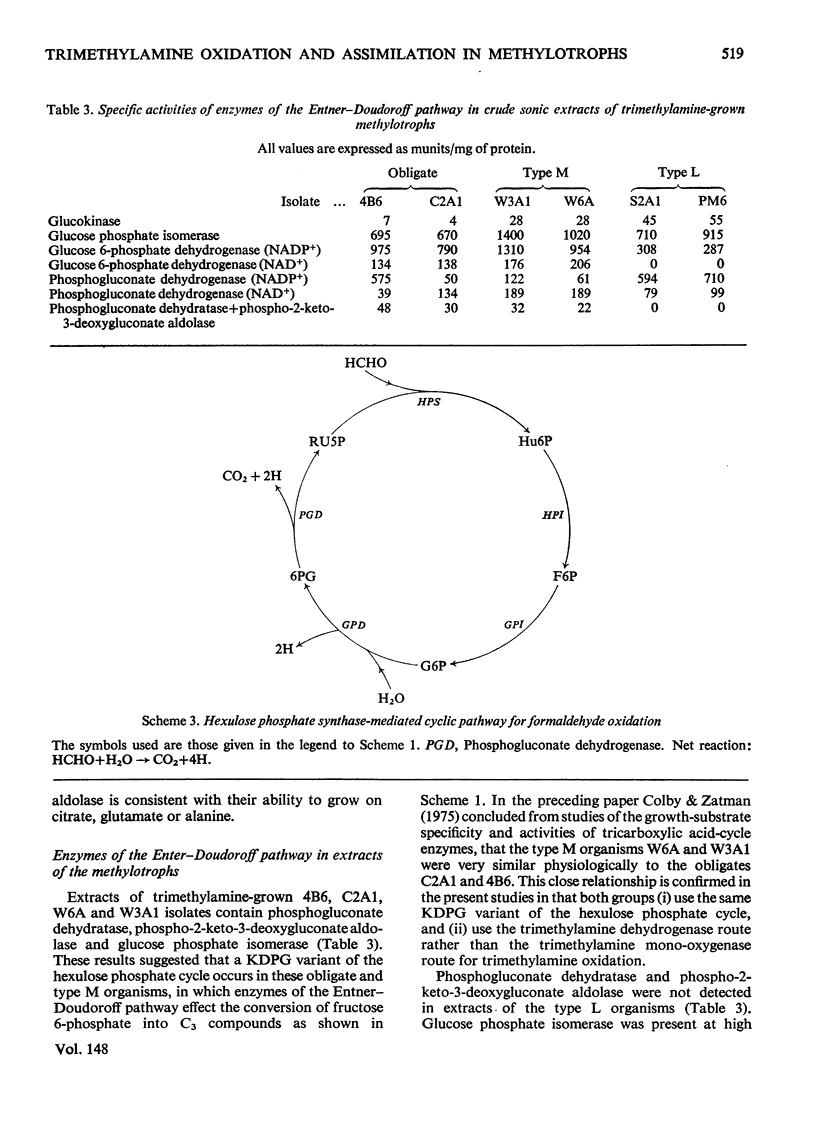
Extracts of trimethylamine-grown W6A and W3A1 (type M restricted facultative methylotrophs) contain trimethylamine dehydrogenase whereas similar extracts of Bacillus PM6 and Bacillus S2A1 (type L restricted facultative methylotrophs) contain trimethylamine mono-oxygenase and trimethylamine N-oxide demethylase but no trimethylamine dehydrogenase. Extracts of the restricted facultatives and of the obligate methylotroph C2A1 contain hexulose phosphate synthase-hexulose phosphate isomerase activity; hydroxypyruvate reductase was not detected. Neither the restricted facultatives nor the obligates 4B6 and C2A1 contain all the enzymes of the hexulose phosphate cycle of formaldehyde assimilation as originally proposed by Kemp & Quayle (1967). Organisms PM6 and S2A1 lack transaldolase and use a modified cycle involving sedoheptulose 1,7-diphosphate and sedoheptulose diphosphatase. The obligates 4B6 and C2A1, and the type M organisms W6A and W3A1, use a different modification of the assimilatory hexulose phosphate cycle involving the Entner-Doudoroff-pathway enzymes phosphogluconate dehydratase and phospho-2-keto-3-deoxygluconate aldolase. The lack of fructose diphosphate aldolase and hexose diphosphatase in these organisms may be a partial explanation of their restricted growth-substrate range. Enzymological evidence suggests that all the obligates and the restricted facultatives use a dissimilatory hexulose phosphate cycle to accomplish the complete oxidation of formaldehyde to CO2 and water.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., JANG R. Purification and properties of phosphoribo-isomerase from alfalfa. J Biol Chem. 1954 Aug;209(2):847–855. [PubMed] [Google Scholar]
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. M., Lang C. A. Nicotinamide-adenine dinucleotide pyrophosphatase in the growing and aging mosquito. Biochem J. 1966 Nov;101(2):392–396. doi: 10.1042/bj1010392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C. The biochemistry of methylotrophic micro-organisms. Sci Prog. 1975 Summer;62(246):167–206. [PubMed] [Google Scholar]
- Attwood M. M., Harder W. A rapid and specific enrichment procedure for Hyphomicrobium spp. Antonie Van Leeuwenhoek. 1972;38(3):369–377. doi: 10.1007/BF02328108. [DOI] [PubMed] [Google Scholar]
- Bellion E., Hersh L. B. Methylamine metabolism in a pseudomonas species. Arch Biochem Biophys. 1972 Nov;153(1):368–374. doi: 10.1016/0003-9861(72)90457-2. [DOI] [PubMed] [Google Scholar]
- Boulton C. A., Crabbe M. J., Large P. J. Microbial oxidation of amines. Partial purification of a trimethylamine mono-oxygenase from Pseudomonas aminovorans and its role in growth on trimethylamine. Biochem J. 1974 May;140(2):253–263. doi: 10.1042/bj1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Hexose phosphate synthese and tricarboxylic acid-cycle enzymes in bacterium 4B6, an obligate methylotroph. Biochem J. 1972 Aug;128(5):1373–1376. doi: 10.1042/bj1281373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Purification and properties of the trimethylamine dehydrogenase of bacterium 4B6. Biochem J. 1974 Dec;143(3):555–567. doi: 10.1042/bj1430555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Tricarboxylic acid-cycle and related enzymes in restricted facultative methylotrophs. Biochem J. 1975 Jun;148(3):505–511. doi: 10.1042/bj1480505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Trimethylamine metabolism in obligate and facultative methylotrophs. Biochem J. 1973 Jan;132(1):101–112. doi: 10.1042/bj1320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. B., Zatman L. J. Hexose phosphate synthase in trimethylamine-grown bacterium 2B2, a facultative methylotroph. Biochem J. 1974 Aug;141(2):605–608. doi: 10.1042/bj1410605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. S., Mehta R. J., Hoare D. S. New obligate methylotroph. J Bacteriol. 1972 Feb;109(2):916–921. doi: 10.1128/jb.109.2.916-921.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall J., Quayle J. R. Pathways leading to and from serine during growth of Pseudomonas AM1 on C1 compounds or succinate. Biochem J. 1970 Apr;117(3):563–572. doi: 10.1042/bj1170563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh L. B., Bellion E. Malate cleavage reaction in Pseudomonas species, (Shaw strain MA). Biochem Biophys Res Commun. 1972 Aug 7;48(3):712–719. doi: 10.1016/0006-291x(72)90407-x. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Gluconate catabolism in Rhizobium japonicum. J Bacteriol. 1970 Mar;101(3):698–704. doi: 10.1128/jb.101.3.698-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. B., Quayle J. R. Microbial growth on C1 compounds. Uptake of [14C]formaldehyde and [14C]formate by methane-grown Pseudomonas methanica and determination of the hexose labelling pattern after brief incubation with [14C]methanol. Biochem J. 1967 Jan;102(1):94–102. doi: 10.1042/bj1020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner A., Zatman L. J. Formaldehyde oxidation by the methanol dehydrogenase of seudomonas PP. J Gen Microbiol. 1969 Mar;55(3):xvi–xvi. [PubMed] [Google Scholar]
- Patel R. N., Hoare D. S. Physiological studies of methane and methanol-oxidizing bacteria: oxidation of C-1 compounds by Methylococcus capsulatus. J Bacteriol. 1971 Jul;107(1):187–192. doi: 10.1128/jb.107.1.187-192.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem A. R., Hacking A. J., Quayle J. R. Cleavage of malyl-Coenzyme A into acetyl-Coenzyme A and glyoxylate by Pseudomonas AM1 and other C1-unit-utilizing bacteria. Biochem J. 1973 Sep;136(1):89–96. doi: 10.1042/bj1360089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. IV. Pasteur effect and Crabtree effect in ascites tumor cells. J Biol Chem. 1959 May;234(5):1036–1041. [PubMed] [Google Scholar]


