Abstract
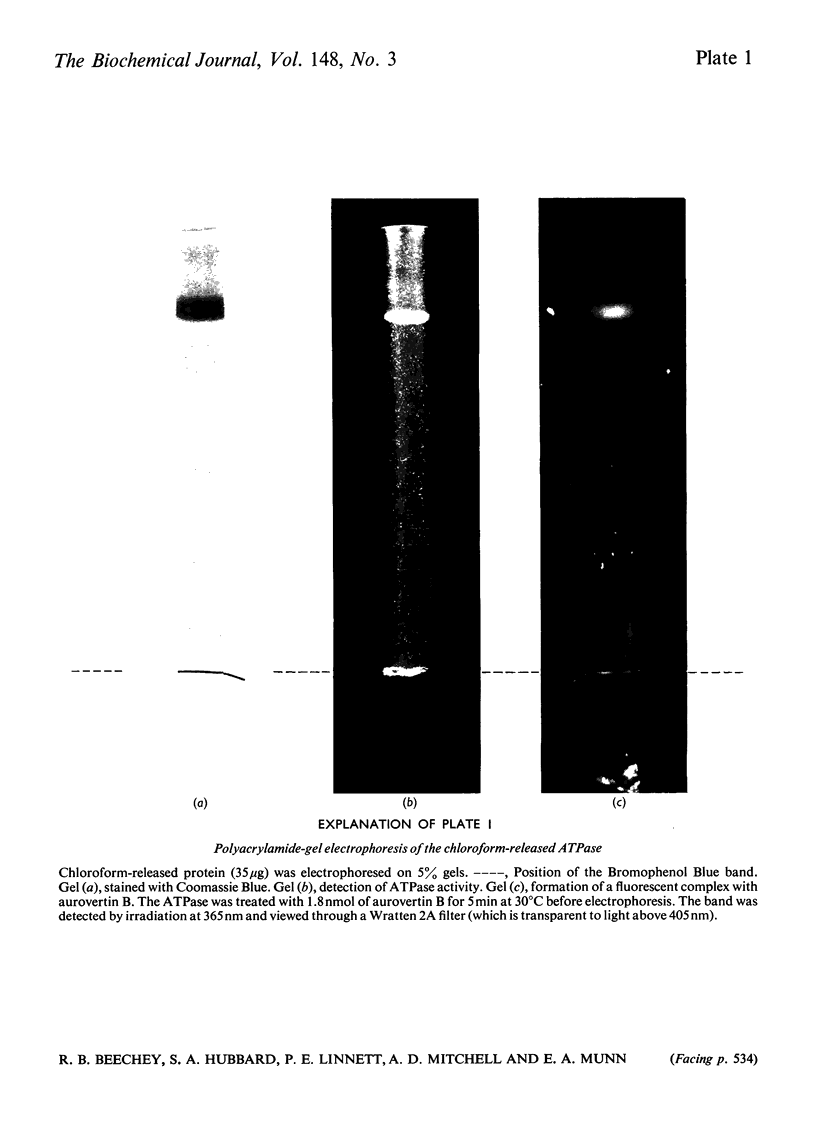
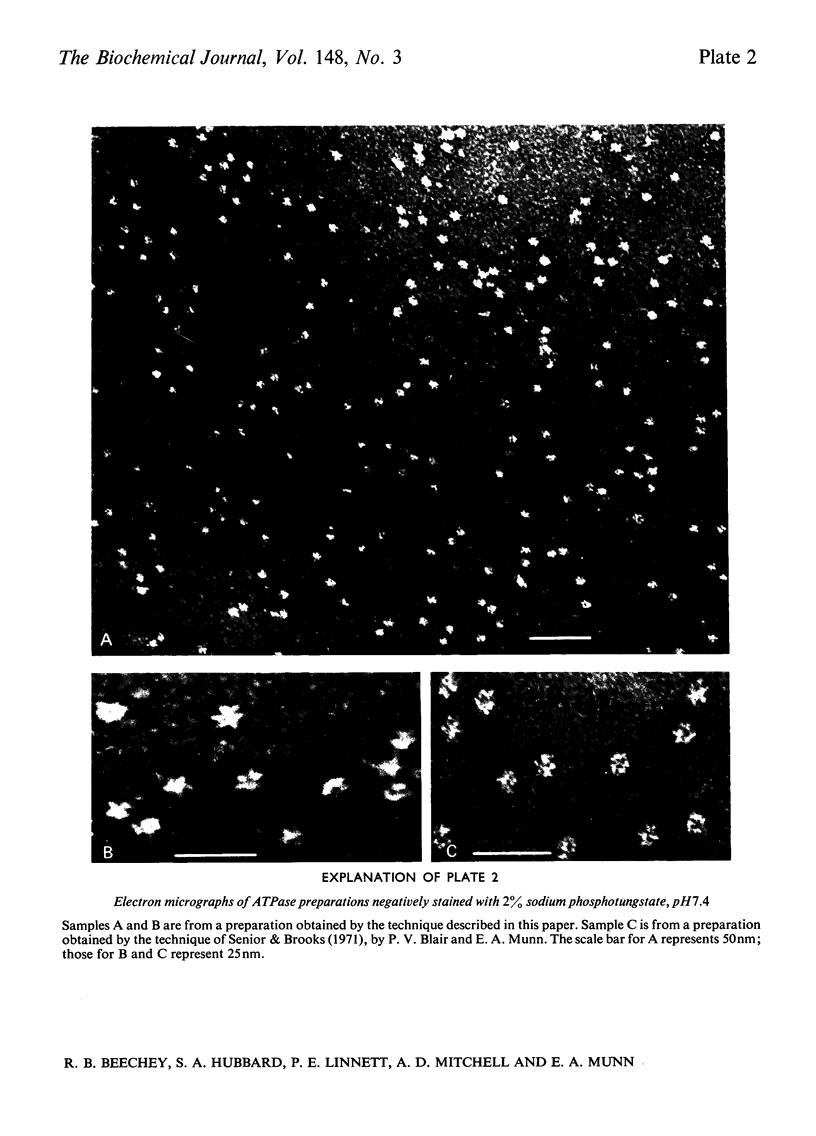
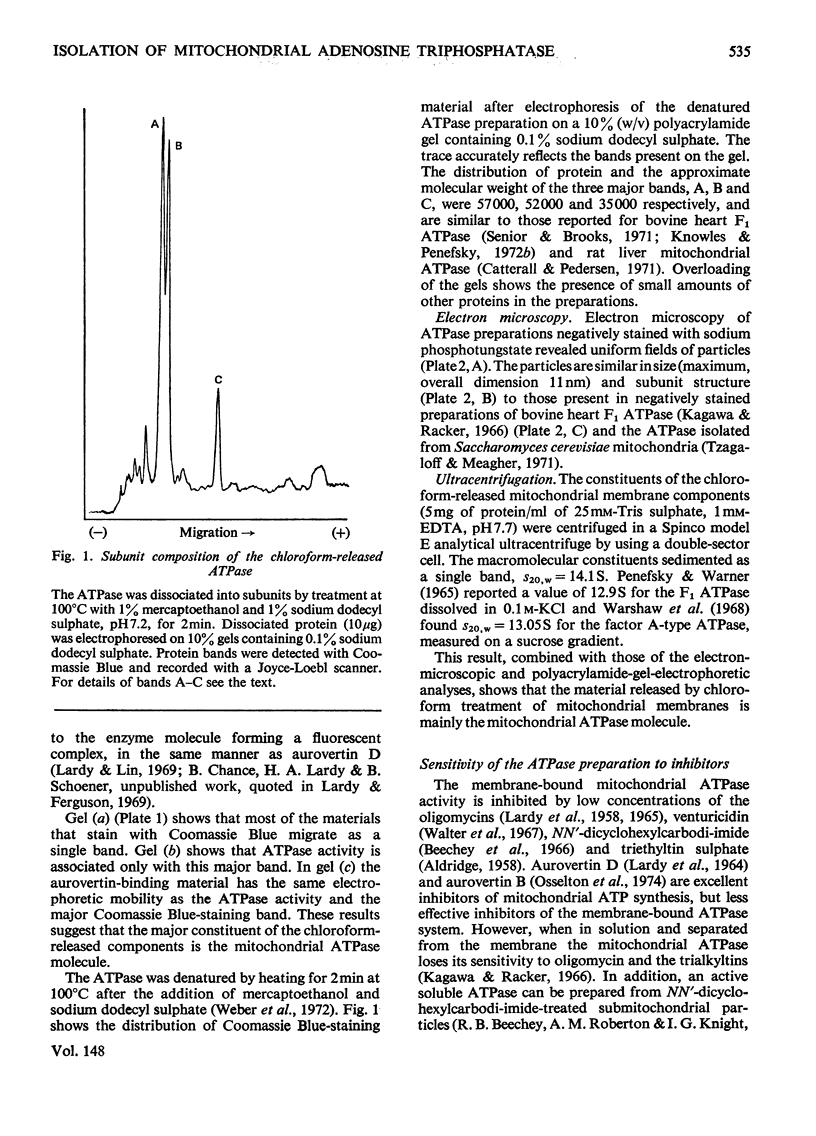
An almost pure form of the bovine heart mitochondrial adenosine triphosphatase (ATPase) is released from the membrane by shaking submitochondrial particles with chloroform. Analyses on polyacrylamide gels and by electron microscopy, and also sensitivity to inhibitors, show that the chloroform-released enzyme is similar to other ATPase preparations from bovine heart mitochondria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. The biochemistry of organotin compounds: trialkyltins and oxidative phosphorylation. Biochem J. 1958 Jul;69(3):367–376. doi: 10.1042/bj0690367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDREOLI T. E., LAM K. W., SANADI D. R. STUDIES ON OXIDATIVE PHOSPHORYLATION. X. A COUPLING ENZYME WHICH ACTIVATES REVERSED ELECTRON TRANSFER. J Biol Chem. 1965 Jun;240:2644–2653. [PubMed] [Google Scholar]
- Beechey R. B., Holloway C. T., Knight I. G., Roberton A. M. Dicyclohexylcarbodiimide--an inhibitor of oxidative phosphorylation. Biochem Biophys Res Commun. 1966 Apr 6;23(1):75–80. doi: 10.1016/0006-291x(66)90271-3. [DOI] [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. I. Purification, homogeneity, and physical properties. J Biol Chem. 1971 Aug 25;246(16):4987–4994. [PubMed] [Google Scholar]
- Horstman L. L., Racker E. Partial resolution of the enzyme catalyzing oxidative phosphorylation. XXII. Interaction between mitochondrial adenosine triphosphatase inhibitor and mitochondrial adenosine triphosphatase. J Biol Chem. 1970 Mar 25;245(6):1336–1344. [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2461–2466. [PubMed] [Google Scholar]
- Knowles A. F., Penefsky H. S. The subunit structure of beef heart mitochondrial adenosine triphosphatase. Isolation procedures. J Biol Chem. 1972 Oct 25;247(20):6617–6623. [PubMed] [Google Scholar]
- Knowles A. F., Penefsky H. S. The subunit structure of beef heart mitochondrial adenosine triphosphatase. Physical and chemical properties of isolated subunits. J Biol Chem. 1972 Oct 25;247(20):6624–6630. [PubMed] [Google Scholar]
- Kozlov I. A., Mikelsaar H. N. On the subunit structure of soluble mitochondrial ATPase. FEBS Lett. 1974 Jul 15;43(2):212–214. doi: 10.1016/0014-5793(74)81002-1. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., CONNELLY J. L., JOHNSON D. ANTIBIOTIC STUDIES. II. INHIBITION OF PHOSPHORYL TRANSFER IN MITOCHONDRIA BY OLIGOMYCIN AND AUROVERTIN. Biochemistry. 1964 Dec;3:1961–1968. doi: 10.1021/bi00900a030. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., WITONSKY P., JOHNSON D. ANTIBIOTICS AS TOOLS FOR METABOLIC STUDIES. IV. COMPARATIVE EFFECTIVENESS OF OLIGOMYCINS A, B, C, AND RUTAMYCIN AS INHIBITORS OF PHOSPHORYL TRANSFER REACTIONS IN MITOCHONDRIA. Biochemistry. 1965 Mar;4:552–554. doi: 10.1021/bi00879a027. [DOI] [PubMed] [Google Scholar]
- Lardy H. A., Ferguson S. M. Oxidative phosphorylation in mitochondria. Annu Rev Biochem. 1969;38:991–1034. doi: 10.1146/annurev.bi.38.070169.005015. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., MONROY G. C. A NATURALLY OCCURRING INHIBITOR OF MITOCHONDRIAL ADENOSINE TRIPHOSPHATASE. J Biol Chem. 1963 Nov;238:3762–3769. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Penefsky H. S. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XVI. Chemical modification of mitochondrial adenosine triphosphatase. J Biol Chem. 1967 Dec 25;242(24):5789–5795. [PubMed] [Google Scholar]
- Penefsky H. S., Warner R. C. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VI. Studies on the mechanism of cold inactivation of mitochondrial adenosine triphosphatase. J Biol Chem. 1965 Dec;240(12):4694–4702. [PubMed] [Google Scholar]
- Roberton A. M., Beechey R. B., Holloway C. T., Knight I. G. The effect of aurovertin on a soluble mitochondrial adenosine triphosphatase. Biochem J. 1967 Sep;104(3):54C–55C. doi: 10.1042/bj1040054c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANADI D. R., FLUHARTY A. L. ON THE MECHANISM OF OXIDATIVE PHOSPHORYLATION. VII. THE ENERGY-REQUIRING REDUCTION OF PYRIDINE NUCLEOTIDE BY SUCCINATE AND THE ENERGY-YIELDING OXIDATION OF REDUCED PYRIDINE NUCLEOTIDE BY FUMARATE. Biochemistry. 1963 May-Jun;2:523–528. doi: 10.1021/bi00903a023. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Brooks J. C. Studies on the mitochondrial oligomycin-insensitivt ATPase. I. An improved method of purification and the behavior of the enzyme in solutions of various depolymerizing agents. Arch Biochem Biophys. 1970 Sep;140(1):257–266. doi: 10.1016/0003-9861(70)90030-5. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Brooks J. C. The subunit composition of the mitochondrial oligomycin-insensitive ATPase. FEBS Lett. 1971 Oct 1;17(2):327–329. doi: 10.1016/0014-5793(71)80178-3. [DOI] [PubMed] [Google Scholar]
- Senior A. E. The structure of mitochondrial ATPase. Biochim Biophys Acta. 1973 Dec 31;301(3):249–277. doi: 10.1016/0304-4173(73)90006-2. [DOI] [PubMed] [Google Scholar]
- Toson G., Contessa A. R., Bruni A. Solubilization of mitochondrial ATPase by phospholipids. Biochem Biophys Res Commun. 1972 Jul 25;48(2):341–347. doi: 10.1016/s0006-291x(72)80056-1. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- Walter P., Lardy H. A., Johnson D. Antibiotics as tools for metabolic studies. X. Inhibition of phosphoryl transfer reactions in mitochondria by peliomycin, ossamycin, and venturicidin. J Biol Chem. 1967 Nov 10;242(21):5014–5018. [PubMed] [Google Scholar]
- Warshaw J. B., Lam K. W., Nagy B., Sanadi D. R. Studies on oxidative phosphorylation. XV. Latent adenosine 5'-triphosphatase activity of factor A. Arch Biochem Biophys. 1968 Feb;123(2):385–396. doi: 10.1016/0003-9861(68)90149-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]