Abstract
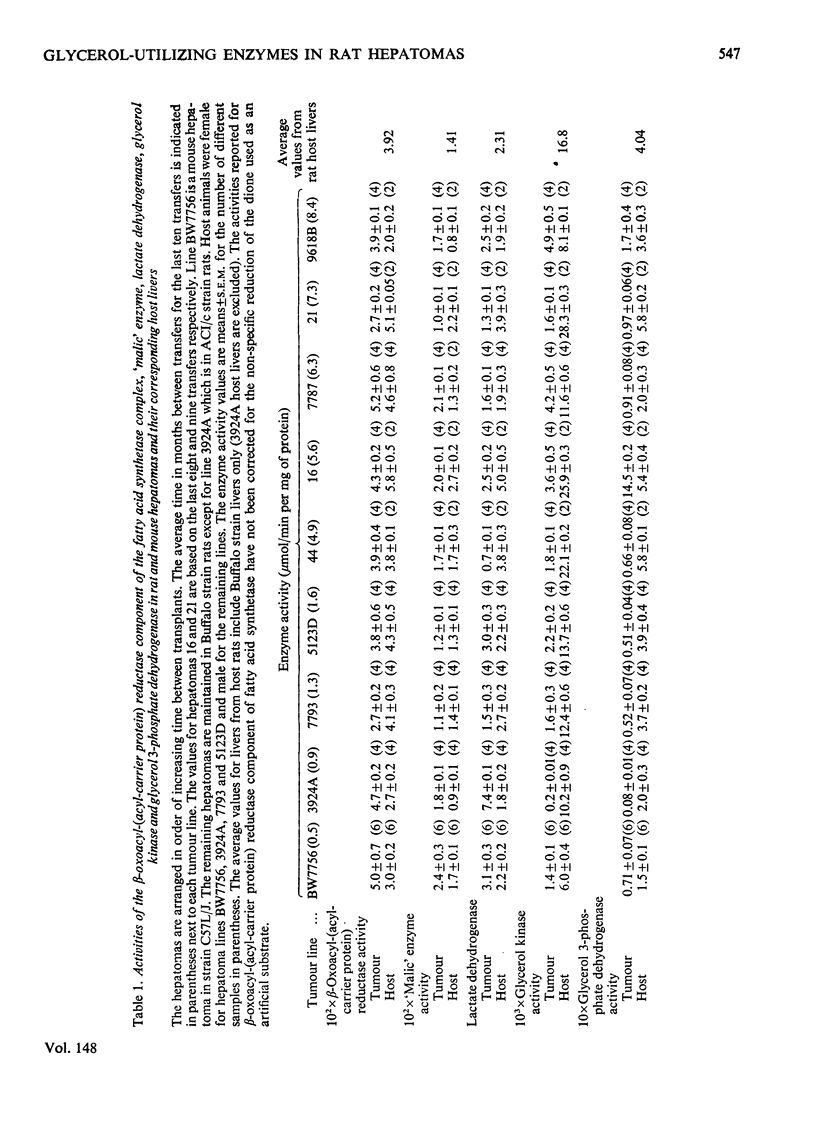
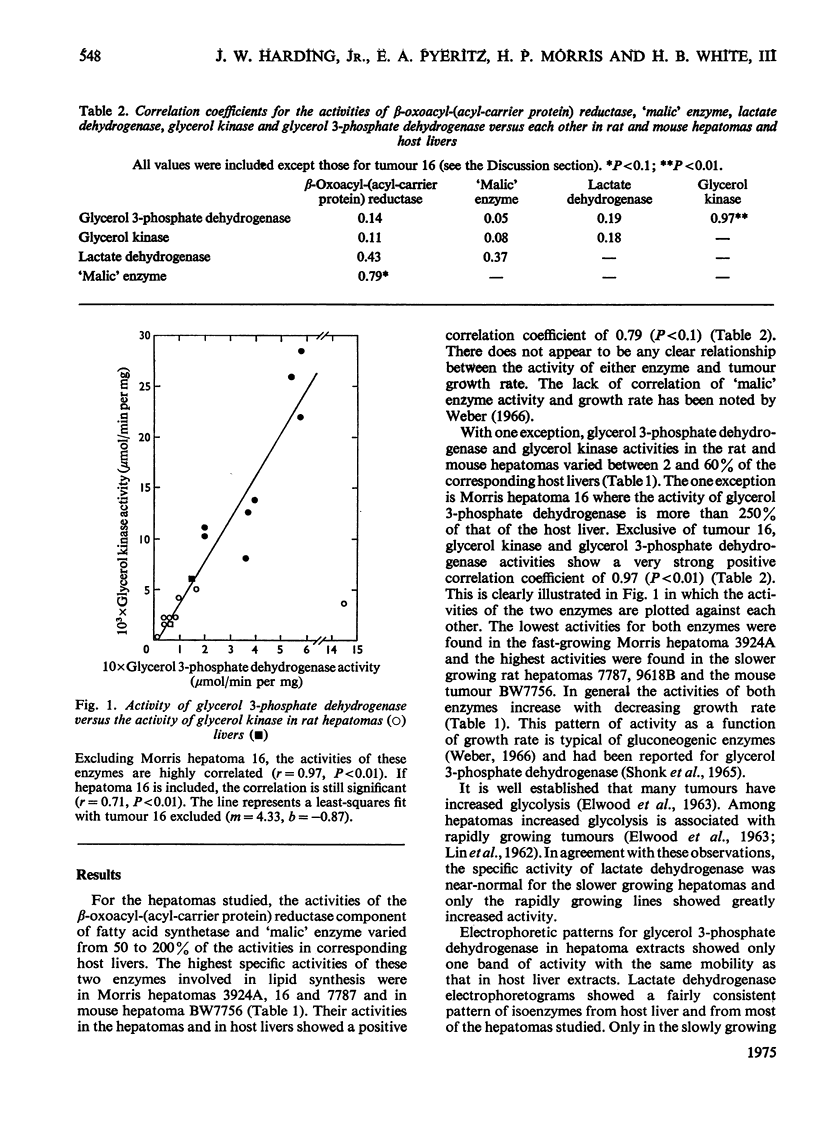
The activities of glycerol 3-phosphate dehydrogenase (EC 1.1.1.8), glycerol kinase (EC 2.7.1.30), lactate dehydrogenase (EC 1.1.1.27), "malic' enzyme (L-malate-NADP+ oxidoreductase; EC 1.1.1.40) and the beta-oxoacyl-(acyl-carrier protein) reductase component of the fatty acid synthetase complex were measured in nine hepatoma lines (8 in rats, 1 in mouse) and in the livers of host animals. With the single exception of Morris hepatoma 16, which had unusually high glycerol 3-phosphate dehydrogenase activity, the activities of glycerol 3-phosphate dehydrogenase and glycerol kinase were highly correlated in normal livers and hepatomas (r = 0.97; P less than 0.01). The activities of these two enzymes were not strongly correlated with the activities of any of the other three enzymes. The primary function of hepatic glycerol 3-phosphate dehydrogenase appears to be in gluconeogenesis from glycerol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann R. H., Bässler K. H., Wagner K. The relation between the blood-level of a substrate and enzyme kinetics studied with glycerol in the rat. Hoppe Seylers Z Physiol Chem. 1974 May;355(5):576–582. doi: 10.1515/bchm2.1974.355.1.576. [DOI] [PubMed] [Google Scholar]
- BLOCH-FRANKENTHAL L., LANGAN J., MORRIS H. P., WEINHOUSE S. FATTY ACID OXIDATION AND KETOGENESIS IN TRANSPLANTABLE LIVER TUMORS. Cancer Res. 1965 Jun;25:732–736. [PubMed] [Google Scholar]
- BOXER G. E., SHONK C. E. Low levels of soluble DPN-linked alpha-glycerophosphate dehydrogenase in tumors. Cancer Res. 1960 Jan;20:85–91. [PubMed] [Google Scholar]
- Burch H. B., Lowry O. H., Delaney L. M. Enzymes of glycerol metabolism in developing rat liver and kidney. Enzyme. 1974;17(3):168–178. doi: 10.1159/000459326. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Cornbleet P. J., Vorbeck M. L., Lucas F. V., Esterly J. A., Morris H. P., Martin A. P. Differences in distribution pattern of marker enzymes among subcellular fractions from Morris hepatoma 16. Cancer Res. 1974 Feb;34(2):439–446. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dutler H., Coon M. J., Kull A., Vogel H., Waldvogel G., Prelog V. Fatty acid synthetase from pig liver. 1. Isolation of the enzyme complex and characterization of the component with oxidoreductase activity for alicyclic ketones. Eur J Biochem. 1971 Sep 24;22(2):203–212. doi: 10.1111/j.1432-1033.1971.tb01533.x. [DOI] [PubMed] [Google Scholar]
- ELWOOD J. C., LIN Y. C., CRISTOFALO V. J., WEINHOUSE S., MORRIS H. P. GLUCOSE UTILIZATION IN HOMOGENATES OF THE MORRIS HEPATOMA 5123 AND RELATED TUMORS. Cancer Res. 1963 Jul;23:906–913. [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- Fondy T. P., Solomon J., Ross C. R. Comparison of cytoplasmic glycerol-3-phosphate dehydrogenase from rat liver and muscle. Arch Biochem Biophys. 1971 Aug;145(2):604–611. doi: 10.1016/s0003-9861(71)80020-6. [DOI] [PubMed] [Google Scholar]
- Goodridge A. G. The effect of starvation and starvation followed by feeding on enzyme activity and the metabolism of [U-14C]glucose in liver from growing chicks. Biochem J. 1968 Jul;108(4):667–673. doi: 10.1042/bj1080667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Reduction of palmitoyl dihydroxyacetone phosphate by mitochondria. J Biol Chem. 1968 Jun 25;243(12):3542–3543. [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of phosphatidic acid from dihydroxyacetone phosphate. Biochem Biophys Res Commun. 1968 Dec 30;33(6):929–935. doi: 10.1016/0006-291x(68)90401-4. [DOI] [PubMed] [Google Scholar]
- Harding J. W., Jr, Pyeritz E. A., Copeland E. S., White H. B., 3rd Role of glycerol 3-phosphate dehydrogenase in glyceride metabolism. Effect of diet on enzyme activities in chicken liver. Biochem J. 1975 Jan;146(1):223–229. doi: 10.1042/bj1460223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINGENBERG M., BUECHER T. Biological oxidations. Annu Rev Biochem. 1960;29:669–708. doi: 10.1146/annurev.bi.29.070160.003321. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., WOODFORD M. FRUCTOSE 1, 6-DIPHOSPHATASE IN STRIATED MUSCLE. Biochem J. 1965 Feb;94:436–445. doi: 10.1042/bj0940436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelovich L., Sabine J. R. Control of lipid metabolism in hepatomas: effects of fasting and dietary fat on the activities of several glycolytic and Krebs-cycle enzymes in mouse liver and hepatoma BW 7756. Biochim Biophys Acta. 1970 Mar 10;202(2):269–276. doi: 10.1016/0005-2760(70)90188-8. [DOI] [PubMed] [Google Scholar]
- LIN Y. C., ELWOOD J. C., ROSADO A., MORRIS H. P., WEINHOUSE S. Glucose metabolism in a low-glycolysing tumour, the Morris Hepatoma 5123. Nature. 1962 Jul 14;195:153–155. doi: 10.1038/195153a0. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN J. M. Reductions and oxidations in mammalian biosyntheses. J Theor Biol. 1961 Jan;1:98–103. doi: 10.1016/0022-5193(61)90029-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb R. G., Fallon H. J. Glycerolipid formation from sn-glycerol-3-phosphate by rat liver cell fractions. The role of phosphatidate phosphohydrolase. Biochim Biophys Acta. 1974 Apr 26;348(1):166–178. doi: 10.1016/0005-2760(74)90103-9. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Robinson J., Taylor K. A radiochemical enzymatic activity assay for glycerol kinase and hexokinase. Biochim Biophys Acta. 1967 Mar 15;132(2):338–346. doi: 10.1016/0005-2744(67)90153-2. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- PESCE A., MCKAY R. H., STOLZENBACH F., CAHN R. D., KAPLAN N. O. THE COMPARATIVE ENZYMOLOGY OF LACTIC DEHYDROGENASES. I. PROPERTIES OF THE CRYSTALLINE BEEF AND CHICKEN ENZYMES. J Biol Chem. 1964 Jun;239:1753–1761. [PubMed] [Google Scholar]
- Pearce J. The effect of dietary fat on lipogenic enzymes in the liver of the domestic fowl. Biochem J. 1968 Oct;109(4):702–704. doi: 10.1042/bj1090702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G. A., Sorrels M. F., Reiser R. Alternate triose phosphate pathways for glyceride biosynthesis in rat liver. Biochem Biophys Res Commun. 1968 Apr 19;31(2):252–256. doi: 10.1016/0006-291x(68)90738-9. [DOI] [PubMed] [Google Scholar]
- Robinson J., Newsholme E. A. Some properties of hepatic glycerol kinase and their relation to the control of glycerol utilization. Biochem J. 1969 May;112(4):455–464. doi: 10.1042/bj1120455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Clark D. G., Katz J. Pathways of glyceride glycerol synthesis. Biochem J. 1974 May;140(2):249–251. doi: 10.1042/bj1400249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. R., Curry S., Schwartz A. W., Fondy T. P. Multiple molecular forms of cytoplasmic glycerol-3-phosphate dehydrogenase in rat liver. Arch Biochem Biophys. 1971 Aug;145(2):591–603. doi: 10.1016/s0003-9861(71)80019-x. [DOI] [PubMed] [Google Scholar]
- SACKTOR B., DICK A. R. Alpha-glycerophosphate and lactic dehydrogenases of hematopoietic cells from leukemic mice. Cancer Res. 1960 Oct;20:1408–1412. [PubMed] [Google Scholar]
- SHONK C. E., ARISON R. N., KOVEN B. J., MAJIMA H., BOXER G. E. ENZYME PATTERNS IN HUMAN TISSUES. 3. GLYCOLYTIC ENZYMES IN NORMAL AND MALIGNANT TISSUES OF THE COLON AND RECTUM. Cancer Res. 1965 Feb;25:206–213. [PubMed] [Google Scholar]
- WEBER G., BANERJEE G., MORRIS H. P. Comparative biochemistry of hepatomas. I. Carbohydrate enzymes in Morris hepatoma 5123. Cancer Res. 1961 Aug;21:933–937. [PubMed] [Google Scholar]
- WEBER G. BEHAVIOR AND REGULATION OF ENZYME SYSTEMS IN NORMAL LIVER AND IN HEPATOMAS OF DIFFERENT GROWTH RATES. Adv Enzyme Regul. 1963;1:321–340. doi: 10.1016/0065-2571(63)90028-1. [DOI] [PubMed] [Google Scholar]
- WEBER G., CANTERO A. Glucose-6-phosphate utilization in hepatoma, regenerating and newborn rat liver, and in the liver of fed and fasted normal rats. Cancer Res. 1957 Nov;17(10):995–1005. [PubMed] [Google Scholar]
- WEBER G., MORRIS H. P. COMPARATIVE BIOCHEMISTRY OF HEPATOMAS. III. CARBOHYDRATE ENZYMES IN LIVER TUMORS OF DIFFERENT GROWTH RATES. Cancer Res. 1963 Aug;23:987–994. [PubMed] [Google Scholar]
- Warkentin D. L., Fondy T. P. Isolation and characterization of cytoplasmic L-glycerol-3-phosphate dehydrogenase from rabbit-renal-adipose tissue and its comparison with the skeletal-muscle enzyme. Eur J Biochem. 1973 Jul 2;36(1):97–109. doi: 10.1111/j.1432-1033.1973.tb02889.x. [DOI] [PubMed] [Google Scholar]
- Weber G., Henry M. C., Wagle S. R., Wagle D. S. Correlation of enzyme activities and metabolic pathways with growth rate of hepatomas. Adv Enzyme Regul. 1964;2:335–346. doi: 10.1016/s0065-2571(64)80024-8. [DOI] [PubMed] [Google Scholar]
- White H. B., 3rd, Kaplan N. O. Purification and properties of two types of diphosphopyridine nucleotide-linked glycerol 3-phosphate dehydrogenases from chicken breast muscle and chicken liver. J Biol Chem. 1969 Nov 10;244(21):6031–6039. [PubMed] [Google Scholar]
- White H. B., 3rd, Kaplan N. O. Separate physiological roles for two isozymes of pyridine nucleotide-linked glycerol-3-phosphate dehydrogenase in chickens. J Mol Evol. 1971;1(1):158–172. [PubMed] [Google Scholar]


