Abstract
Total hip arthroplasty (THA) has significantly improved the lives of patients with degenerative hip disorders. The direct anterior approach (DAA) is favored for its minimally invasive nature, leading to less postoperative pain and a faster recovery. The bikini incision (BI) approach was developed to enhance aesthetic outcomes while maintaining the clinical and functional benefits of the DAA. Despite its advantages, the BI technique presents challenges, controversies persist regarding its efficacy and safety, and there is no consensus within the medical community about its overall benefits. Incisions aligned with Langer’s lines, like the BI, promote better healing and minimal scarring. Studies indicate that BI patients report higher satisfaction with scar appearance and texture compared to traditional DAA patients. However, the BI carries a higher risk of lateral femoral cutaneous nerve (LFCN) injury, although most symptoms resolve within 6 months. For obese patients, the BI is associated with fewer complications, such as infections and delayed healing, compared to the conventional DAA, making it a safe and effective option. BI patients also experience better aesthetic outcomes and functional recovery, with reduced pain and itching. The BI technique in THA represents a significant advancement, offering improved aesthetic and wound-healing outcomes. The shift from the traditional DAA to the BI aligns with patient preferences for scars that are less visible and conspicuous. Despite the steep learning curve and risks, careful patient selection and refined surgical techniques can enhance the BI’s benefits. Future research should focus on long-term outcomes and comparative studies to further establish the BI’s efficacy and safety. As patient demand for aesthetically favorable surgeries grows, the BI is likely to become a preferred approach in THA.
Keywords: Total hip arthroplasty, Minimally invasive, Bikini incision approach, Direct anterior approach, Hip, Lateral femoral cutaneous nerve
Introduction
Total hip arthroplasty (THA) is a transformative procedure that has greatly reduced pain and improved the lives of those with hip joint disorders leading to degeneration [1, 2]. Among the various surgical techniques for THA, the direct anterior approach (DAA) has gained attention because it is a muscle-sparing method, working through a plane between the sartorius and tensor fascia latae muscles [3]. This minimally invasive approach is praised for reducing postoperative pain, promoting a quicker recovery, and lowering dislocation rates [4–7]. The DAA has a long history. It was first described by Carl Hueter in 1881 for treating hip infections and injuries, later refined by Smith-Petersen in 1917 for orthopedic use [8, 9], and subsequently pioneered for hip arthroplasty by Judet [10]. The modern DAA gained further popularity, especially in the US, after Matta highlighted its advantages in 2005 [3].
As THA techniques have evolved, there has been an increasing focus on the use of minimally invasive approaches to reduce complications and enhance cosmetic outcomes [11]. This has led to the development of the minimally invasive bikini incision (BI) approach, introduced by Leunig in 2013 as a refinement of the DAA. The BI approach offers both aesthetic and functional improvements over traditional methods [12]. It has shown excellent clinical and aesthetic outcomes [13–15], though concerns about its learning curve and complexity remain [16–18]. Additionally, some have raised concerns about higher postoperative complication rates associated with the BI technique [19–21].
Our aim is to provide a balanced analysis of the BI approach, exploring its technical aspects, benefits, challenges, and impact on patient outcomes. We discuss incision methods, aesthetic results, the learning curve, and patient selection criteria to offer a comprehensive view of this technique.
Langer’s lines
Since Karl Langer first identified the predominant directions of skin tension—known as Langer’s lines—in 1861, they have become an essential guide for surgical incisions. Initially seen as static and unrelated to scar healing [22], recent studies in plastic, craniomaxillofacial, and orthopedic surgery have renewed interest in their clinical importance. It is now understood that Langer’s lines are dynamic: they adjust their orientation during the healing process [23]. Incisions made along these lines, which align with the skin’s natural tension and collagen fibers, reduce tension at wound edges, minimize the risk of wound dehiscence, and promote better healing with minimal scarring [24, 25] (Fig. 1).
Fig. 1.
Images illustrating the different stages of surgical wound healing: at 7 days post-surgery (a), at 1 month post-surgery (b), and at 9 months post-surgery (c)
Research from vascular surgery supports the benefits of horizontal incisions in reducing wound complications and infections compared to vertical ones. A randomized controlled trial by Swinnen et al. [26] involving 88 patients (116 groins) found that vertical incisions had a rate of complications of 47.5%, compared to just 12.7% for horizontal incisions (p < 0.001). Additionally, fewer infections occurred with horizontal incisions (n = 3) compared to vertical ones (n = 10). This improved healing was linked to the alignment of horizontal incisions with Langer’s lines [26]. Building on the principle of respecting natural skin tension and Langer’s lines, Leunig introduced the BI as a modification of the DAA for hip replacement surgery [12]. This oblique incision, following the groin’s natural skin crease, was designed to enhance both subjective and objective aesthetic outcomes while preserving the precision of component placement and ensuring the overall safety of the procedure.
Surgical technique
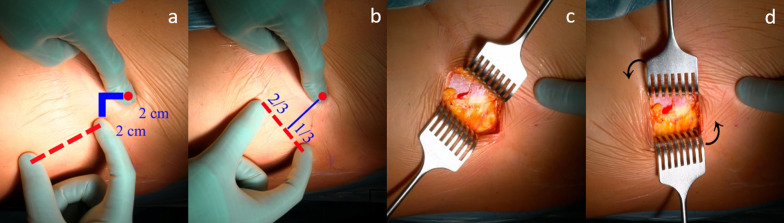
When performing the surgical technique for BI THA, patients are positioned supine [3, 27] on a standard radiolucent table or with the foot of the operated leg secured by a boot on a dedicated traction table managed by a non-scrubbed assistant, which allows control of the traction, rotation, and adduction or abduction. The patient’s lower limb is positioned with the leg at 20° of flexion, with neutral abduction and rotation. In the standard longitudinal DAA, the surgical incision is started 2 cm distally and 2 cm laterally from the anterior superior iliac spine and averages 7 cm [28]. In the BI, a 6- to 8-cm incision is made along Langer’s line at the groin fold, guided by an imaginary line perpendicular to the anterior superior iliac spine (ASIS) that crosses the inguinal fold [12]: the incision lies two-thirds lateral and one-third medial to this line, representing the projection of the standard DDA incision to the groin fold (Fig. 2).
Fig. 2.
Starting from the ASIS, the surgeon moves 2 cm distally and laterally; this serves as the proximal reference point for the DAA incision (a). The imaginary line is then adjusted to run parallel to the iliac crest, positioning the starting point of the DAA incision two-thirds lateral and one-third medial to this line (b). The index finger is on the ASIS; the soft subcutaneous tissue is mobilized by the deep tissue, creating a “mobile window” (c). Moving the retractors vertically simultaneously, the mobile window is created in the direction of the muscle fibers, recreating the classical space for the standard DAA (d)
After careful and smooth subcutis dissection, a plane is developed between the subcutis and the fascial plane; using two blunt retractors, the skin is rotated by 90° (replicating traditional DAA conditions), identifying the fascia over the tensor fascia latae and sartorius muscles. The rest of the surgery follows the standard DAA approach to the hip joint [27]. The fascia is incised over the tensor fascia latae muscle to minimize the risk of injuring the lateral femoral cutaneous nerve. The intermuscular and interneural space is then bluntly dissected: the tensor fascia latae is retracted laterally, while the sartorius and rectus femoris are retracted medially. Branches of the lateral circumflex artery are ligated or coagulated. The capsule is fully exposed and carefully opened, creating a thick flap that is sutured at the end of the surgery. The femoral neck osteotomy is performed in situ with an oscillating saw, and the head is removed using a corkscrew, with the leg in slight traction and external rotation.
Acetabular and femoral bones are prepared for implant positioning using dedicated instrumentation with curved and offset handles to ease bone preparation and avoid soft-tissue impingement. The iliofemoral and pubofemoral ligaments are incised to improve proximal femur exposure. Accurate posterior–medial capsular release at the proximal femur is performed before femoral broaching, with the patient’s leg in external rotation, extension, and adduction. After positioning the cup and femur implants, the reduction maneuver is performed, followed by suturing.
Risks of lateral femorocutaneous nerve injury
The DAA is often criticized for its risk of neural damage, particularly to the lateral femoral cutaneous nerve (LFCN), which can result in temporary or permanent anesthesia, paresthesia, or dysesthesia in the anterolateral thigh [20, 29]. The most common neuropathic symptom following DAA total hip arthroplasty (THA) is numbness, reported in 15–37% of patients, though it is usually temporary [30–32]. The LFCN is a purely sensory nerve originating from the second and third lumbar nerves [33], and its variable anatomy can influence the likelihood of injury during surgery.
Anatomical variability of the LFCN
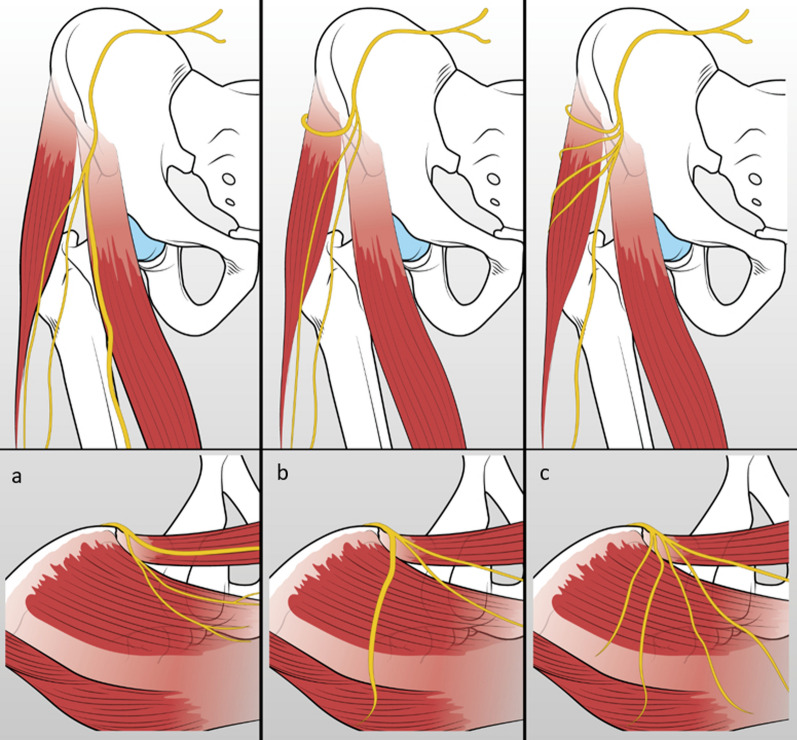
The LFCN’s course varies significantly, crossing the inguinal ligament approximately 1.4 cm medial to the ASIS and showing different branching patterns [34]. Rudin et al. [35] identified three main types of LFCN branching in cadaver studies. The sartorius type was observed in 36% of cases and features a dominant anterior branch that runs along the lateral border of the sartorius muscle. The posterior type occurs in 32% of cases and is characterized by a prominent posterior branch that crosses over the TFL muscle. The fan type, also present in 32% of cases, consists of multiple branches spreading across the anterolateral thigh and intersecting with both the TFL and sartorius muscles (Fig. 3).
Fig. 3.
The different branching patterns of the lateral femoral cutaneous nerve within the thigh. a The sartorius type (36%, characterized by a dominant anterior branch running along the lateral side of the sartorius muscle. b The posterior type (32%), featuring a thick posterior branch running perpendicular to the belly of the tensor fasciae latae muscle. c The fan type (32%), characterized by multiple nerve branches spreading out with equal thickness
LFCN injury in BI vs. DAA THA: comparing study outcomes
The risk of LFCN injury has been examined in multiple studies comparing the BI with the standard longitudinal DAA. Sang et al. [19] conducted a randomized study involving 99 BI THA patients and 96 DAA patients, finding a higher incidence of LFCN injury in the BI group at 1.5 months post-surgery (36.4% vs. 21.9%, p < 0.05). Notably, while the initial rate of injury was higher for the BI, the majority of patients in both groups recovered over time, and by 6 months, there was no significant difference in persistent symptoms (7.1% for the BI vs. 4.2% for the DAA). This suggests that while the BI may pose a higher short-term risk, long-term outcomes are similar to the DAA. Sang et al. suggested that positioning the BI incision more laterally might reduce the risk of LFCN injury, particularly as more medial incisions or blunt dissection can increase vulnerability [19]. Supporting this, Thaler et al. [36] analyzed the relationship between various anterior approaches and LFCN patterns, noting that all types of skin incisions could affect the nerve, but the fan-like distribution of the LFCN branches in particular raised the risk for the BI. This finding reinforces the importance of careful incision placement to minimize nerve damage.
However, not all studies agree on the higher risk of LFCN injury with the BI. In a larger study of nearly 1,000 patients, Leunig et al. [13] found no long-term increase in hypoesthesia with the BI compared to the DAA. Over a 2- to 4-year follow-up, they noted similar rates of sensory disturbance between the two approaches, with approximately 15% of DAA patients and 8% of BI patients reporting hypoesthesia. Furthermore, less than 10% of these patients described the numbness as bothersome, suggesting that it did not significantly affect quality of life even when present. Adding to the mixed evidence, Butler et al. [37] conducted a systematic review focusing on LFCN injuries in the BI versus the DAA. Of the five relevant studies they analyzed, two reported no statistically significant difference between the approaches [12, 38], while two others found a higher incidence of LFCN injuries in BI patients, though without statistical significance [19, 39]. Leunig’s 2018 study, as previously mentioned, found significantly lower rates of hypoesthesia in BI patients (p < 0.001) [13]. These contrasting results indicate a lack of consensus in the literature regarding the frequency and severity of LFCN injuries in BI versus DAA THA.
Risk factors and preventive strategies
While LFCN injury can affect patient recovery and quality of life, the symptoms often improve over time without functional limitations. Studies with an 8-year follow-up showed that the prevalence of numbness decreased to about 10% [30, 31]. The main causes of LFCN damage are often linked to prolonged compression by retractors rather than direct nerve injury. As such, surgical time and technique are crucial factors, with experienced surgeons being less likely to cause nerve compromise. Additionally, mechanical entrapment of the LFCN during fascia closure can lead to severe postoperative pain or long-term numbness [40]. To minimize the risk of LFCN injury, two key precautions are recommended: first, when the LFCN is visible, care should be taken to avoid it during suture placement; and second, if the nerve is not visible, sutures should be placed as laterally as possible to reduce the likelihood of entrapment.
Treatment of LFCN injuries
For patients experiencing iatrogenic meralgia paresthetica, conservative treatment options like local anesthetic injections with or without steroids have shown good results. Ultrasound-guided injections allow for precise targeting of the nerve entrapment site, which can effectively alleviate symptoms [41]. If conservative treatment fails, surgical options like neurolysis or neurectomy may offer significant and lasting symptom relief [42].
Periprosthetic fractures and the BI
Periprosthetic fractures are a well-recognized complication of THA performed via the DAA [43]. They present both functional challenges for patients and a significant socioeconomic burden due to increased hospitalization costs [44, 45]. A comparative study by Leunig et al. involving 964 patients found no significant difference in periprosthetic fracture rates between the BI and DAA groups (one fracture in the BI vs. two in the DAA group). These results are consistent with findings by Alva et al. [46], who reported similar frequencies of periprosthetic fractures in both approaches, with three trochanteric fractures (0.35%) and four femoral shaft fractures (0.47%) in BI patients.
Intraoperative femoral fractures may require immediate reduction, which can be challenging with a BI. Some authors recommend extending the transverse skin incision longitudinally (into a T-shape) to allow better access for cerclage wiring and subsequent fracture stabilization [46–48]. Alternatively, a second small incision over the fracture site can be made to minimize trauma, allowing the fracture to be anatomically reduced and stabilized.
Obesity and bikini incision
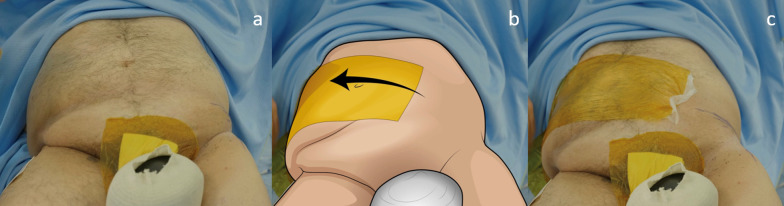
Obesity is a growing concern in orthopedic surgery, as it is strongly associated with the increased incidence of end-stage osteoarthritis [49, 50]. This comorbidity poses challenges for THA, especially when using the BI. The pannus of fat present in obese patients can obstruct the operative field, making the BI more complex. As Corten et al. [47] suggest, it is crucial to assess the extent of skin covered by the pannus in the inguinal and anterior thigh regions, as these areas should be avoided during surgery to prevent complications like infections and wound dehiscence. Therefore, incisions should be placed away from the pannus without compromising access to the acetabulum and proximal femur. Intraoperative techniques, such as using a sterile drape to retract the pannus towards the contralateral side of the abdomen, can help maintain a clear surgical field (Fig. 4).
Fig. 4.
Images from left to right show the various stages of using a sterile drape to reduce the bulk of abdominal fat and enhance the visibility of the surgical field. a Abdominal fat covers the hypothetical surgical incision. b The drape is initially positioned on the ipsilateral side of the abdomen relative to the surgical site, and then it is drawn contralaterally to clear the operative field. c The surgical site appears free from adipose tissue thanks to the positioning of the sterile drape
Despite these challenges, studies suggest that the outcomes of the BI in obese patients are comparable to those in non-obese patients. Manrique et al. [38], in a retrospective case–control study, found that obese patients (BMI > 30) who underwent the BI had significantly fewer wound healing complications (16.6% for the DAA vs. 0% for the BI, p < 0.05) and dysesthesia (6.3% vs. 0% for the BI) than those who had the conventional DAA. Additionally, obese patients in the BI group experienced shorter surgery durations and hospital stays, with no significant differences in estimated blood loss, intraoperative complications, or infection rates compared to non-obese patients.
These findings are consistent with those of Nizam et al. [51] and Verhaegen et al. [52]. Nizam et al. [51] concluded that the BI technique is both safe and effective for obese patients, with no significant differences in complication rates between obese and non-obese groups. Verhaegen et al. [52] similarly reported a reduction in wound complications among obese patients undergoing the BI, further supporting the clinical efficacy of this approach.
Unsuitable patients
Certain patient profiles are less suitable for the BI approach due to specific anatomical or physiological challenges. Young, muscular patients, who tend to have hypertrophic muscles, present particular difficulties. The increased muscle bulk can make surgical exposure more challenging, leading to potential complications during the procedure [18, 53]. Additionally, patients with severe hip dysplasia or sequelae from conditions like Perthes disease pose unique anatomical challenges. These conditions often involve an altered acetabular and femoral anatomy, such as abnormal bony coverage, valgus or varus deformities, and changes in femoral anteversion. These anatomical differences may necessitate a wider surgical exposure, requiring the surgeon to extend the incision longitudinally, which is not compatible with the BI approach, particularly if additional procedures are needed for the acetabulum or femur [54, 55]. Patients requiring revision arthroplasty are also generally unsuitable for the BI. Revision surgeries often involve substantial scar tissue, altered bone anatomy from previous surgeries, and the need for extensive dissection to address implant failures or bone loss. In these cases, a more extended surgical approach is required, which the BI does not provide. While it is possible to convert the BI into an ilioinguinal approach for better exposure, this modification still limits the surgeon’s access to the proximal femur, complicating revision procedures. Similarly, patients with previous trauma treated with internal fixation devices like cephalomedullary nails or plate and screw systems are not good candidates for BI THA. The presence of pre-existing longitudinal scars often necessitates a more extensive incision, making the BI unsuitable for these cases.
Learning curve
The concept of a learning curve in surgery refers to the stages a surgeon experiences as they acquire proficiency in a new technique. These stages typically include: (1) a rapid improvement phase during early training, (2) a period of slower progress as experience increases, (3) a plateau where additional experience has little effect on outcomes, and (4) a late decline due to factors like age-related reductions in manual dexterity or eyesight [56, 57].
For the DAA in THA, numerous studies confirm its safety and effectiveness, largely due to its muscle-sparing nature, which leads to less postoperative pain and quicker rehabilitation [58, 59]. However, the DAA is technically demanding, especially for surgeons with limited experience, and the learning curve is steeper than for other approaches. Nairn et al. [60] conducted a systematic review revealing that the steepest improvement occurs in the first 30 surgeries, and a plateau is reached at around 100 procedures. Operative time also decreased significantly during these first 30 cases, with times approaching those of more traditional approaches after about 50 surgeries [60]. Similarly, Masonis et al. [61] confirmed a reduction in surgical time after 100 cases, an important factor in reducing infection rates [62–64]. Complication rates are also linked to the surgeon’s experience. In Nairn et al.’s review, early DAA patients experienced an average complication rate of 20.8%, which dropped to 7.6% as surgeons became more proficient [60]. Revision rates and implant survival also improved with experience. Surgeons who had performed over 100 DAA cases achieved outcomes comparable to those seen with other approaches, with a revision rate of 1.1% [60]. De Steiger et al. [65], found that surgeons who had performed between 51 and 100 DAAs had a cumulative revision rate of 3% at 4 years, while those with more than 100 procedures had a rate of 2% (p = 0.33). Peters et al. [66], in a study of 15,875 THAs, observed that patients treated by surgeons who had performed no more than 50 DAAs had a 64% higher risk of revision. However, the complication rate began to stabilize and decrease after 100 surgeries, suggesting that the learning curve for DAA THA is approximately 100 cases [66, 67].
Although no specific studies have addressed the learning curve for the BI, it is reasonable to assume that it mirrors the DAA learning curve. Surgeons must first master the DAA, and once they achieve proficiency after about 100 cases, they can transition to the BI to ensure optimal outcomes and maintain patient safety.
Aesthetic outcomes
Minimally invasive approaches and improved scar aesthetics are increasingly emphasized in orthopedic surgery. The BI offers favorable aesthetic outcomes compared to traditional longitudinal incisions, as horizontal incisions tend to promote better wound healing and reduce scarring [38, 39].
This has been demonstrated in multiple studies. For instance, Leunig et al. [12] observed superior cosmetic outcomes with the BI after 6 months, and their 2018 study further confirmed these findings, with patients reporting lower average scores on the University of North Carolina’s “4P” scar scale for itching, pain, paresthesia, and scar texture (0.2 BI vs. 0.4 DAA; p = 0.01) [13]. Wang et al. corroborated these results, finding that BI patients had significantly better SCAR scores at 6 months postoperatively compared to DAA patients (7.4 ± 1.8 vs 9.3 ± 2.0, respectively; p < 0.001) [39]. Di Martino et al. also reported superior scar quality in BI patients who took the POSAS questionnaire, as they gave significantly lower scores than those observed in both DAA and posterolateral approach (PL) groups [14.1 ± 3.9 (p = 0.021) and 14.5 ± 3 (p = 0.0032), respectively] [68]. Additionally, Jin et al. [14] found that BI patients had shorter incision lengths (9.7 ± 1.6 mm vs. 10.8 ± 2.0 mm; p < 0.01) and higher postoperative satisfaction compared to those who underwent the PL for bilateral THA.
Conclusions
The BI represents a significant evolution in surgical technique for primary total hip arthroplasty (THA), offering both aesthetic and clinical benefits. It not only enhances the appearance of surgical scars but also promotes better wound healing by aligning the incision with the body’s natural contours and Langer’s lines. This shift from traditional longitudinal DAA incisions to the BI reflects a broader surgical philosophy aimed at maximizing both functional recovery and patient satisfaction, particularly in terms of cosmetic outcomes.
Studies consistently report that the BI yields favorable results regarding scar appearance, with patients experiencing higher satisfaction and fewer scar-related complications compared to traditional DAA incisions. The approach minimizes tissue trauma, respecting the skin’s elastic properties, and it supports a more physiological healing process. As societal preferences evolve, particularly regarding aesthetics, it is likely that more young, fit individuals as well as older patients will increasingly request the BI for THA, following trends observed in other surgical specialties where scar appearance influences patient choice [69, 70].
However, important challenges remain. The learning curve for the BI is still undetermined, though research suggests that 100 DAA procedures are necessary for surgeons to achieve proficiency and reduce complication rates [60, 65, 66]. As the BI requires similar expertise, ongoing training and education are essential to ensure successful outcomes. Surgeons must also be mindful of specific risks, such as LFCN injury and diaphyseal fractures, which demand careful management and surgical precision.
Future research should focus on overcoming these challenges, paying particular attention to long-term functional outcomes and complication rates associated with the BI in THA. Comparative studies with traditional approaches could provide valuable insights into its efficacy and safety.
In conclusion, the BI is a promising advancement in anterior THA, offering a cosmetically superior option without compromising early functional recovery. Although further research and clinical experience are needed, the demand for the BI in THA is expected to grow, and surgeons proficient in the DAA should prepare for more complex cases. Clear communication with patients regarding expectations and potential risks, such as the need for additional incisions in the event of complications, is essential. Ultimately, careful patient selection, taking into account individual risks and benefits, will enhance the potential of the BI to become a preferred approach in THA.
Acknowledgements
Not applicable.
Abbreviations
- THA
Total hip arthroplasty
- DAA
Direct anterior approach
- BI
Bikini incision
- LFCN
Lateral femoral cutaneous nerve
- ASIS
Anterior superior iliac spine
- PL
Posterolateral approach
Author contributions
C.F. and A.D.M. designed the study; C.D., F.T., and F.P. performed the search of the literature and screened all potentially relevant articles; M.B. and M.M.G. wrote the manuscript.
Funding
The authors received no financial support for the publication of this article.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Learmonth ID, Young C, Rorabeck C (2007) The operation of the century: total hip replacement. Lancet 370:1508–1519. 10.1016/S0140-6736(07)60457-7 [DOI] [PubMed] [Google Scholar]
- 2.Agarwal N, To K, Khan W (2021) Cost effectiveness analyses of total hip arthroplasty for hip osteoarthritis: a PRISMA systematic review. Int J Clin Pract 75:e13806. 10.1111/ijcp.13806 [DOI] [PubMed] [Google Scholar]
- 3.Matta JM, Shahrdar C, Ferguson T (2005) Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res 441:115–124. 10.1097/01.blo.0000194309.70518.cb [DOI] [PubMed] [Google Scholar]
- 4.Rivera F, Comba LC, Bardelli A (2022) Direct anterior approach hip arthroplasty: how to reduce complications—a 10-years single center experience and literature review. World J Orthop 13:388–399. 10.5312/wjo.v13.i4.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins BT, Barlow DR, Heagerty NE, Lin TJ (2015) Anterior vs. posterior approach for total hip arthroplasty, a systematic review and meta-analysis. J Arthroplasty 30:419–434. 10.1016/j.arth.2014.10.020 [DOI] [PubMed] [Google Scholar]
- 6.Graves SC, Dropkin BM, Keeney BJ, Lurie JD, Tomek IM (2016) Does surgical approach affect patient-reported function after primary THA? Clin Orthop Relat Res 474:971–981. 10.1007/s11999-015-4639-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amlie E, Havelin LI, Furnes O, Baste V, Nordsletten L, Hovik O, Dimmen S (2014) Worse patient-reported outcome after lateral approach than after anterior and posterolateral approach in primary hip arthroplasty. Acta Orthop 85:463–469. 10.3109/17453674.2014.934183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rachbauer F, Kain MSH, Leunig M (2009) The history of the anterior approach to the hip. Orthop Clin North Am 40:311–320. 10.1016/j.ocl.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 9.Smith-Petersen MN (1917) A new supra-articular subperiosteal approach to the hip joint. JBJS s2–15:592
- 10.Judet J, Judet R (1950) The use of an artificial femoral head for arthroplasty of the hip joint. J Bone Joint Surg Br 32-B:166–173. 10.1302/0301-620X.32B2.166 [DOI] [PubMed] [Google Scholar]
- 11.Ponziani L, Di Caprio F, Tentoni F, Grana S, Di Meo A, Gigli M (2021) Anterior and antero-lateral mini-invasive approaches for primary total hip replacement. Acta Biomed 92:e2021014. 10.23750/abm.v92iS3.11704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leunig M, Faas M, von Knoch F, Naal FD (2013) Skin crease ‘bikini’ incision for anterior approach total hip arthroplasty: surgical technique and preliminary results. Clin Orthopaed Related Res 471:2245. 10.1007/s11999-013-2806-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leunig M, Hutmacher JE, Ricciardi BF, Impellizzeri FM, Rüdiger HA, Naal FD (2018) Skin crease “bikini” incision for the direct anterior approach in total hip arthroplasty: a two- to four-year comparative study in 964 patients. Bone Joint J 100-B:853–861. 10.1302/0301-620X.100B7.BJJ-2017-1200.R2 [DOI] [PubMed] [Google Scholar]
- 14.Jin X, Chen G, Chen M, Riaz MN, Wang J, Yang S, Xu W (2023) Comparison of postoperative outcomes between bikini-incision via direct anterior approach and posterolateral approach in simultaneous bilateral total hip arthroplasty: a randomized controlled trial. Sci Rep 13:7023. 10.1038/s41598-023-29146-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alessio-Mazzola M, Colombo P, Barducci N, Ghezzi E, Zagra L, Caldora P, Ometti M, Placella G, Salini V (2024) Direct anterior approach with conventional instruments versus robotic posterolateral approach in elective total hip replacement for primary osteoarthritis: a case–control study. J Orthop Traumatol 25:9. 10.1186/s10195-024-00753-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnham RRJ, Kiernan H, Ortega LF, Wesolowski M, Tauchen A, Russo M, Gerscovich D, Brown NM (2022) Defining the learning curve of anterior total hip arthroplasty after fellowship-specific training. JAAOS J Am Acad Orthopaed Surgeons 30:e131. 10.5435/JAAOS-D-21-00232 [DOI] [PubMed] [Google Scholar]
- 17.Zappley N, Fraval A, Hozack WJ, Brown SA (2024) Vertical or horizontal (bikini) incision for direct anterior total hip arthroplasty: outcomes of early (< 90 day) revision. J Arthroplasty S0883–5403(24):00550–00553. 10.1016/j.arth.2024.05.078 [DOI] [PubMed] [Google Scholar]
- 18.Hallert O, Li Y, Brismar H, Lindgren U (2012) The direct anterior approach: initial experience of a minimally invasive technique for total hip arthroplasty. J Orthop Surg Res 7:17. 10.1186/1749-799X-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sang W, Xue S, Xu Y, Liu Y, Zhu L, Ma J (2021) Bikini incision increases the incidence of lateral femoral cutaneous nerve injury in direct anterior approach hip arthroplasty: a prospective ultrasonic, electrophysiological, and clinical study. J Arthroplasty 36:3463–3470. 10.1016/j.arth.2021.05.012 [DOI] [PubMed] [Google Scholar]
- 20.Banasiak S, Hartel M, Frosch K-H, Berger-Groch J (2024) Postoperative lymphedema after primary total hip arthroplasty: prospective analysis of bikini incision-type direct anterior approach versus established standard approaches. J Orthop Surg Res 19:54. 10.1186/s13018-023-04525-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagano S, Plate JF, Kappenschneider T, Reinhard J, Scharf M, Maderbacher G (2024) Polyethylene liner dissociation in total hip arthroplasty: a retrospective case–control study on a single implant design. J Orthop Traumatol 25:38. 10.1186/s10195-024-00785-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edlich RF, Carl BA (1998) Predicting scar formation: from ritual practice (Langer’s lines) to scientific discipline (static and dynamic skin tensions). J Emerg Med 16:759–760. 10.1016/s0736-4679(98)00070-5 [DOI] [PubMed] [Google Scholar]
- 23.Bush J, Ferguson MWJ, Mason T, McGrouther G (2007) The dynamic rotation of Langer’s lines on facial expression. J Plast Reconstr Aesthet Surg 60:393–399. 10.1016/j.bjps.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 24.Hwang K (2018) Revisiting the skin lines on the forehead and glabellar area. J Craniofac Surg 29:209–211. 10.1097/SCS.0000000000004073 [DOI] [PubMed] [Google Scholar]
- 25.Atkinson J-AM, McKenna KT, Barnett AG, McGrath DJ, Rudd M (2005) A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer’s skin tension lines. Plast Reconstr Surg 116:1648–1656. 10.1097/01.prs.0000187147.73963.a5. (discussion 1657-1658) [DOI] [PubMed] [Google Scholar]
- 26.Swinnen J, Chao A, Tiwari A, Crozier J, Vicaretti M, Fletcher J (2010) Vertical or transverse incisions for access to the femoral artery: a randomized control study. Ann Vasc Surg 24:336–341. 10.1016/j.avsg.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 27.Faldini C, Rossomando V, Brunello M, D’Agostino C, Ruta F, Pilla F, Traina F, Di Martino A (2024) Anterior minimally invasive approach (AMIS) for total hip arthroplasty: analysis of the first 1000 consecutive patients operated at a high volume center. J Clin Med 13:2617. 10.3390/jcm13092617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faldini C, Tassinari L, Pederiva D, Rossomando V, Brunello M, Pilla F, Geraci G, Traina F, Di Martino A (2024) Direct anterior approach in total hip arthroplasty for severe Crowe IV dysplasia: retrospective clinical and radiological study. Medicina (Kaunas) 60:114. 10.3390/medicina60010114 [DOI] [PMC free article] [PubMed]
- 29.Goulding K, Beaulé PE, Kim PR, Fazekas A (2010) Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res 468:2397–2404. 10.1007/s11999-010-1406-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patton RS, Runner RP, Lyons RJ, Bradbury TL (2018) Clinical outcomes of patients with lateral femoral cutaneous nerve injury after direct anterior total hip arthroplasty. J Arthroplasty 33:2919-2926.e1. 10.1016/j.arth.2018.04.032 [DOI] [PubMed] [Google Scholar]
- 31.Gala L, Kim PR, Beaulé PE (2019) Natural history of lateral femoral cutaneous nerve neuropraxia after anterior approach total hip arthroplasty. Hip Int 29:161–165. 10.1177/1120700019827201 [DOI] [PubMed] [Google Scholar]
- 32.De Geest T, Fennema P, Lenaerts G, De Loore G (2015) Adverse effects associated with the direct anterior approach for total hip arthroplasty: a Bayesian meta-analysis. Arch Orthop Trauma Surg 135:1183–1192. 10.1007/s00402-015-2258-y [DOI] [PubMed] [Google Scholar]
- 33.Homma Y, Baba T, Sano K, Ochi H, Matsumoto M, Kobayashi H, Yuasa T, Maruyama Y, Kaneko K (2016) Lateral femoral cutaneous nerve injury with the direct anterior approach for total hip arthroplasty. Int Orthopaed SICOT 40:1587–1593. 10.1007/s00264-015-2942-0 [DOI] [PubMed] [Google Scholar]
- 34.Majkrzak A, Johnston J, Kacey D, Zeller J (2010) Variability of the lateral femoral cutaneous nerve: an anatomic basis for planning safe surgical approaches. Clin Anat 23:304–311. 10.1002/ca.20943 [DOI] [PubMed] [Google Scholar]
- 35.Rudin D, Manestar M, Ullrich O, Erhardt J, Grob K (2016) The anatomical course of the lateral femoral cutaneous nerve with special attention to the anterior approach to the hip joint. J Bone Joint Surg Am 98:561–567. 10.2106/JBJS.15.01022 [DOI] [PubMed] [Google Scholar]
- 36.Thaler M, Dammerer D, Hechenberger F, Hörmann R, Van Beeck A, Stofferin H (2021) The anatomical course of the lateral femoral cutaneous nerve in relation to various skin incisions used for primary and revision total hip arthroplasty with the direct anterior approach. J Arthroplasty 36:368–373. 10.1016/j.arth.2020.07.052 [DOI] [PubMed] [Google Scholar]
- 37.Butler J, Singleton A, Miller R, Morse B, Naylor B, DeCook C (2022) Bikini incision vs longitudinal incision for anterior total hip arthroplasty: a systematic review. Arthroplasty Today 17:1. 10.1016/j.artd.2022.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manrique J, Paskey T, Tarabichi M, Restrepo C, Foltz C, Hozack WJ (2019) Total hip arthroplasty through the direct anterior approach using a bikini incision can be safely performed in obese patients. J Arthroplasty 34:1723–1730. 10.1016/j.arth.2019.03.060 [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Yue Y, Yang Z, Chen L, Li Q, Kang P (2021) Comparison of postoperative outcomes between traditional longitudinal incision and bikini incision in total hip arthroplasty via direct anterior approach: a randomized controlled trial. J Arthroplasty 36:222–230. 10.1016/j.arth.2020.07.047 [DOI] [PubMed] [Google Scholar]
- 40.Hasija R, Kelly JJ, Shah NV, Newman JM, Chan JJ, Robinson J, Maheshwari AV (2018) Nerve injuries associated with total hip arthroplasty. J Clin Orthop Trauma 9:81–86. 10.1016/j.jcot.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palamar D, Terlemez R, Misirlioglu TO, Aydın FY, Akgun K (2023) Ultrasound-guided treatment of meralgia paresthetica: with or without corticosteroid? A double-blinded, randomized controlled study. Ann Indian Acad Neurol 26:67–72. 10.4103/aian.aian_883_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dengler NF (2023) Treatment of idiopathic meralgia paresthetica—is there reliable evidence yet? Neurol Res 45:429–434. 10.1080/01616412.2022.2151115 [DOI] [PubMed] [Google Scholar]
- 43.Crawford DA, Berend KR (2021) Reduction of periprosthetic proximal femur fracture in direct anterior total hip according to stem design. Orthop Clin North Am 52:297–304. 10.1016/j.ocl.2021.05.002 [DOI] [PubMed] [Google Scholar]
- 44.González-Martín D, Pais-Brito JL, González-Casamayor S, Guerra-Ferraz A, González-Pérez JM, Jiménez-Sosa A, Herrera-Pérez M (2022) Economic impact of periprosthetic hip fractures. Rev Esp Cir Ortop Traumatol 66:477–484. 10.1016/j.recot.2022.01.008 [DOI] [PubMed]
- 45.Axenhus M, Mukka S, Magnéli M, Sköldenberg O (2024) Comparative outcomes of uncemented and cemented stem revision in managing periprosthetic femoral fractures: a retrospective cohort study. J Orthop Traumatol 25:35. 10.1186/s10195-024-00777-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alva A, Nizam I, Gogos S (2021) Minimizing complications in bikini incision direct anterior approach total hip arthroplasty: a single surgeon series of 865 cases. J Exp Orthop 8:1. 10.1186/s40634-020-00318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corten K, Holzapfel BM (2021) Direct anterior approach for total hip arthroplasty using the “bikini incision.” Oper Orthop Traumatol 33:318–330. 10.1007/s00064-021-00721-y [DOI] [PubMed] [Google Scholar]
- 48.Cohen EM, Vaughn JJ, Ritterman SA, Eisenson DL, Rubin LE (2017) Intraoperative femur fracture risk during primary direct anterior approach cementless total hip arthroplasty with and without a fracture table. J Arthroplasty 32:2847–2851. 10.1016/j.arth.2017.04.020 [DOI] [PubMed] [Google Scholar]
- 49.Guillemin F, Rat AC, Mazieres B, Pouchot J, Fautrel B, Euller-Ziegler L, Fardellone P, Morvan J, Roux CH, Verrouil E, Saraux A, Coste J, 3000 Osteoarthritis Group (2011) Prevalence of symptomatic hip and knee osteoarthritis: a two-phase population-based survey. Osteoarthritis Cartilage 19:1314–1322. 10.1016/j.joca.2011.08.004 [DOI] [PubMed]
- 50.Berry PA, Jones SW, Cicuttini FM, Wluka AE, Maciewicz RA (2011) Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis Rheum 63:700–707. 10.1002/art.30182 [DOI] [PubMed] [Google Scholar]
- 51.Nizam I, Dabirrahmani D, Alva A, Choudary D (2022) Bikini anterior hip replacements in obese patients are not associated with an increased risk of complication. Arch Orthop Trauma Surg 142:2919–2926. 10.1007/s00402-021-04143-0 [DOI] [PubMed] [Google Scholar]
- 52.Verhaegen JCF, Wei R, Kim P, Beaulé PE, Corten K, Grammatopoulos G (2023) The safety and efficacy of the anterior approach total hip arthroplasty as per body mass index. J Arthroplasty 38:314-322.e1. 10.1016/j.arth.2022.08.021 [DOI] [PubMed] [Google Scholar]
- 53.Di Martino A, Keating C, Butsick MJ, Platano D, Berti L, Hunter LN, Faldini C (2024) Enhancing recovery: surgical techniques and rehabilitation strategies after direct anterior hip arthroplasty. J Orthop Traumatol 25:45. 10.1186/s10195-024-00786-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faldini C, Brunello M, Pilla F, Geraci G, Stefanini N, Tassinari L, Di Martino A (2023) Femoral head autograft to manage acetabular bone loss defects in THA for Crowe III hips by DAA: retrospective study and surgical technique. J Clin Med 12:751. 10.3390/jcm12030751 [DOI] [PMC free article] [PubMed]
- 55.Lu Z, Chen Q, Lan Y, Xie S, Lin F, Feng E (2024) Subtrochanteric osteotomy in direct anterior approach total hip arthroplasty for Crowe IV dysplasia—surgical technique and literature review. Orthop Surg 16:766–774. 10.1111/os.13996 [DOI] [PMC free article] [PubMed]
- 56.Hopper AN, Jamison MH, Lewis WG (2007) Learning curves in surgical practice. Postgrad Med J 83:777–779. 10.1136/pgmj.2007.057190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Shen X, Zhang R, Chen M, Ma R, Zhang Z, Zhang H, Yang B, Zhu C (2024) Radiographic evaluation of robot-assisted versus manual total hip arthroplasty: a multicenter randomized controlled trial. J Orthop Traumatol 25:33. 10.1186/s10195-024-00773-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faldini C, Perna F, Mazzotti A, Stefanini N, Panciera A, Geraci G, Mora P, Traina F (2017) Direct anterior approach versus posterolateral approach in total hip arthroplasty: effects on early post-operative rehabilitation period. J Biol Regul Homeost Agents 31:75–81 [PubMed] [Google Scholar]
- 59.Meermans G, Fawley D, Zagra L, ten Broeke RHM, Johnson K, Bernard T, Thomason HC (2024) Accuracy of cup placement compared with preoperative surgeon targets in primary total hip arthroplasty using standard instrumentation and techniques: a global, multicenter study. J Orthop Traumatol 25:25. 10.1186/s10195-024-00766-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nairn L, Gyemi L, Gouveia K, Ekhtiari S, Khanna V (2021) The learning curve for the direct anterior total hip arthroplasty: a systematic review. Int Orthop 45:1971–1982. 10.1007/s00264-021-04986-7 [DOI] [PubMed] [Google Scholar]
- 61.Masonis J, Thompson C, Odum S (2008) Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics 31. (12 Suppl 2) [PubMed]
- 62.Wang Q, Goswami K, Shohat N, Aalirezaie A, Manrique J, Parvizi J (2019) Longer operative time results in a higher rate of subsequent periprosthetic joint infection in patients undergoing primary joint arthroplasty. J Arthroplasty 34:947–953. 10.1016/j.arth.2019.01.027 [DOI] [PubMed] [Google Scholar]
- 63.Scigliano NM, Carender CN, Glass NA, Deberg J, Bedard NA (2022) Operative time and risk of surgical site infection and periprosthetic joint infection: a systematic review and meta-analysis. Iowa Orthop J 42:155–161 [PMC free article] [PubMed] [Google Scholar]
- 64.Namba RS, Inacio MCS, Paxton EW (2013) Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am 95:775–782. 10.2106/JBJS.L.00211 [DOI] [PubMed] [Google Scholar]
- 65.de Steiger RN, Lorimer M, Solomon M (2015) What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res 473:3860–3866. 10.1007/s11999-015-4565-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters RM, Ten Have BLEF, Rykov K, Van Steenbergen L, Putter H, Rutgers M, Vos S, Van Steijnen B, Poolman RW, Vehmeijer SBW, Zijlstra WP (2022) The learning curve of the direct anterior approach is 100 cases: an analysis based on 15,875 total hip arthroplasties in the Dutch Arthroplasty Register. Acta Orthop 93:775–782. 10.2340/17453674.2022.4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H (2009) A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty 24:698–704. 10.1016/j.arth.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 68.Di Martino A, Brunello M, Rossomando V, Pederiva D, Schilardi F, Stefanini N, Geraci G, Faldini C (2023) Aesthetic results, functional outcome and radiographic analysis in THA by direct anterior, bikini and postero-lateral approach: is it worth the hassle? J Clin Med 12:1072. 10.3390/jcm12031072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.AbelleyraLastoria DA, Benny CK, Hing CB (2023) Subjective scar assessment scales in orthopaedic surgery and determinants of patient satisfaction: a systematic review of the literature. Chin J Traumatol 26:276–283. 10.1016/j.cjtee.2023.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rafiq MS, Khan MM (2016) Scar pain, cosmesis and patient satisfaction in laparoscopic and open cholecystectomy. J Coll Physicians Surg Pak 26:216–219 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.