Abstract
A Candida albicans efg1 cph1 double mutant is nonfilamentous under standard laboratory conditions and avirulent in mice. However, this mutant produced filaments in the tongues of immunosuppressed gnotobiotic piglets and when embedded in agar, demonstrating that an Efg1p- and Cph1p-independent pathway for promotion of filamentous growth exists.
The fungal pathogen Candida albicans is an important nosocomial pathogen that causes diseases ranging from superficial mucosal infections to life-threatening systemic disease. Like primary fungal pathogens, C. albicans is dimorphic and is capable of growth in a yeast form or in filamentous forms. Filamentous growth is believed to be an important step in the invasion-and-pathogenesis process (4, 10).
A variety of stimuli promote filamentous growth in culture. In particular, growth at 37°C or higher temperatures in media containing serum or a combination of amino acids promotes hyphal growth (for a review, see reference 6). Several gene products are involved in the regulation of filamentous growth, including the products of the CPH1 (9), EFG1 (12), CZF1 (3), TUP1 (2), and RBF1 (7) genes.
A mutant strain lacking the transcription factors Efg1p and Cph1p is extremely defective in filamentous growth at 37°C (10). In this study, laboratory growth conditions that differ from standard conditions for the stimulation of hyphal growth were used to promote filamentous growth and the efg1 cph1 double null mutant was observed to form filaments. In addition, an alternative animal model, the immunosuppressed gnotobiotic (IGB) newborn piglet, was used to study the virulence of this mutant. IGB animals were highly susceptible to wild-type C. albicans, and mucosal invasion and dissemination were consistently observed (1). The efg1 cph1 double null mutant produced lesions containing filamentous forms in the tongues of IGB piglets. Therefore, these results demonstrate that an Efg1p- and Cph1p-independent pathway for promotion of filamentous growth exists in C. albicans and that this pathway can be utilized during in vivo infection.
Filamentous growth of an efg1 cph1 double null mutant during experimental infection.
Oral inoculation of newborn germfree piglets (11) with wild-type C. albicans SC5314 (5), followed by immunosuppression with cyclosporine and methylprednisolone (8), resulted in colonization of the gastrointestinal tract by C. albicans, dissemination to internal organs, and development of oral thrush, conjunctivitis, and corneal lesions (1). Tongues of animals inoculated with SC5314 were completely covered with fungal material, and culture of tongue homogenates yielded a mean of 9 × 106 CFU/g (wet weight) of tissue. To study filamentous growth during this experimental infection, strain CKY138 (CAI-4 efg1::hisG/efg1::hisG cph1::hisG/cph1::hisG ura3/ura3 ade2::pDBI52), derived from SC5314, was inoculated orally into germfree piglets. CKY138 was constructed by transformation of strain can36 (efg1/efg1 cph1/cph1 ura3/ura3; kind gift of G. Fink) with vector pDBI52 (Ura+) (3). Despite the reduced virulence of strains lacking Efg1p and Cph1p in mice (10) and IGB piglets (1), oral inoculation of IGB piglets led to extensive colonization of the gastrointestinal tract and development of sparse but clinically apparent, mild thrush lesions and superficial lesions of the eye. Consistent with the reduced virulence of the mutant strain, these oral lesions required a much longer time to develop than did lesions caused by wild-type C. albicans. Homogenates of tongue tissue containing lesions yielded a mean of 2 × 105 CFU/g (wet weight) of tissue.
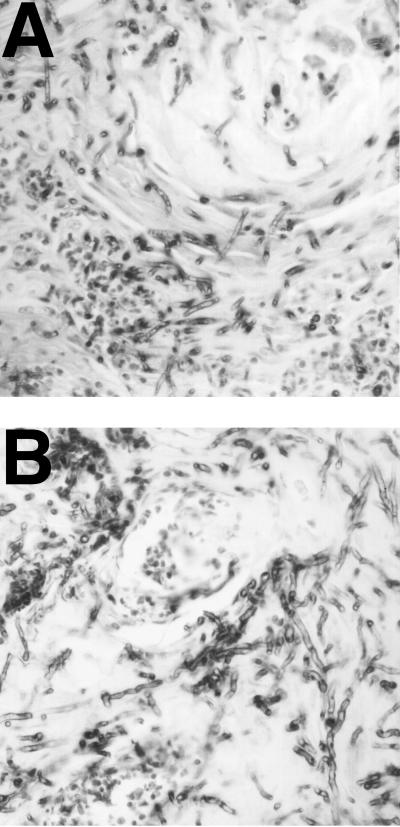
To determine the morphology of the organisms in tongue lesions, infected tissue was fixed in 10% formalin and sectioned. Immunohistological tests using antibodies produced by repeated intramuscular injection of formalin-killed C. albicans were performed. The wild-type organism (SC5314) produced locally invasive lesions with extensive filamentous growth throughout the keratin and epithelial layers (Fig. 1A). In some lesions, organisms were observed in subepithelial layers invading muscle tissue. The mutant CKY138 (efg1/efg1 cph1/cph1) produced superficial lesions limited to the epithelial layer, but filamentous growth was clearly observed (Fig. 1B), despite the fact that this strain has been reported to be nonfilamentous in culture (10).
FIG. 1.
Filamentous growth of C. albicans during experimental infection. Newborn, germfree piglets were inoculated orally with either SC5314 (wild type) or CKY138 (efg1/efg1 cph1/cph1). Sections of fixed tongue tissue were examined by immunohistochemical analysis using anti-C. albicans antibody and photographed at a magnification of ×40. Panels A, tongue section from an animal inoculated with SC5314; B, tongue section from an animal inoculated with the CKY138 mutant.
To demonstrate that the reduced virulence observed with strain CKY138 was due to the efg1 cph1 mutations, strain HLC84 (efg1::hisG/efg1::hisG cph1::hisG/cph1::hisG ura3/ura3 leu2::EFG1+) was constructed by transformation of a wild-type copy of the EFG1 gene from plasmid HLB134 (10) into strain can36. This strain exhibited nearly normal virulence in the IGB piglet model and produced lesions in the tongue that were very similar to wild-type lesions (1). Homogenates of tongue tissue yielded a mean of 6 × 106 CFU/g (wet weight) of tissue. Therefore, deletion of EFG1 and CPH1 resulted in a mutant strain with reduced virulence but the loss of these two transcription factors was not sufficient to eliminate filamentous growth during infection or the development of invasive lesions in the tongue.
In the cornea, both SC5314 (wild type) and CKY138 (efg1/efg1 cph1/cph1) produced lesions as well. In lesions produced by SC5314, extensive filamentous growth was observed while lesions produced by CKY138 contained predominantly yeast cells (data not shown). In addition, an efg1 cph1 double null strain failed to become filamentous when engulfed by macrophages (10). Therefore, filamentous growth of mutant CKY138 was not observed in all tissues, indicating that different sites within the host may provide different signals for initiation of filamentous growth.
Laboratory conditions that promote filamentous growth of mutant CKY138.
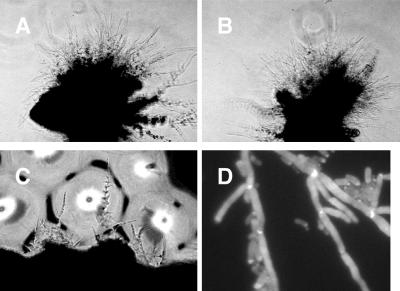
Previous results demonstrated that physical environmental cues due to growth within a matrix promoted filamentous growth of C. albicans in the laboratory (see Fig. 3A) (3). Plating of cells on a macroscopically rough agar surface was found to have a similar effect. A rough agar surface was produced by shaking molten YPS agar (1% yeast extract, 2% Bacto Peptone, 2% sucrose, 1% agar) to introduce bubbles and allowing the agar to solidify. Cells were then plated on the surface. After incubation at 25°C, colonies that grew on the smooth parts of the plate developed into colonies having sharp edges composed of yeast cells (Fig. 2A and B), while colonies that grew on the rough parts of the plate developed into flat, ragged colonies that produced filaments after several days (Fig. 2C and D).
FIG. 3.
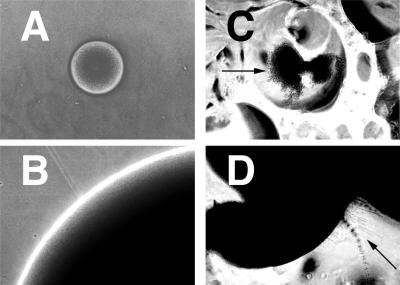
Filamentous growth of strain CKY138. Exponentially growing cells of CKY101 (wild type) (A) and CKY138 (efg1/efg1 cph1/cph1) (B) grown in YPD medium (1% yeast extract, 2% Bacto Peptone, 2% glucose) at 30°C were mixed with molten YPS medium and plated. After 3 days of incubation at 25°C, colonies were photographed at a magnification of ×10. Panel C shows exponentially growing cells of strain CKY138 plated on a rough YPS agar surface, incubated at 25°C for 5 days, and photographed at a magnification of ×10. Panel D shows CKY138 cells extracted from a colony grown on rough YPS agar, stained with Calcofluor white, and photographed at a magnification of ×100 by using a standard 4′,6-diamidino-2-phenylindole (DAPI) filter set.
FIG. 2.
Growth of wild-type cells on the surface of rough or smooth agar. Cells of SC5314 (EFG1+/EFG1+ CPH1+/CPH1+) were plated on YPS plates containing rough and smooth areas and incubated at 25°C (see text). At various times, colonies were observed and photographed with a Nikon TM300 inverted-phase microscope with a 10× objective. The arrow in panel C indicates the edge of the colony. The arrow in panel D indicates filaments. Panels A and B show colonies grown on a smooth surface (A, 1 day; B, 3 days). Panels C and D show colonies grown on a rough surface (C, 1 day; D, 3 days).
Strain CKY138 (efg1/efg1 cph1/cph1) does not produce hyphae at 37°C in serum-containing media (10). This result is striking because this condition strongly stimulates hyphal growth of wild-type cells. However, CKY138 exhibited filamentous growth in response to plating in a matrix (Fig. 3B) or on a macroscopically rough surface (Fig. 3C). Wild-type C. albicans is capable of forming both hyphae, elongated cells lacking constrictions at the septa, and pseudohyphae, variably elongated cells in chains with constrictions at the septa. To determine whether the filaments produced by CKY138 were hyphae or pseudohyphae, Calcofluor white staining of cells extracted from colonies was performed (3). Constrictions were observed at septa separating the cells in filaments, indicating that the filaments were pseudohyphae (Fig. 3D).
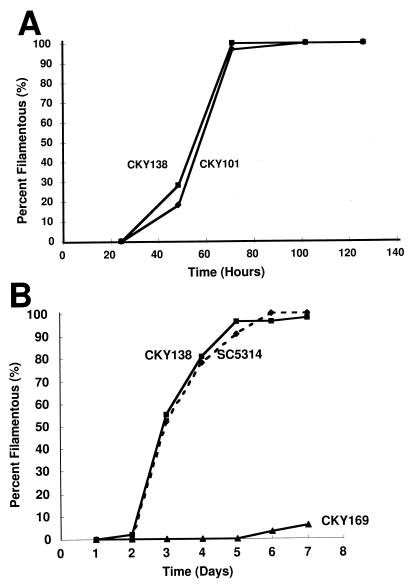
To compare the responses of wild-type and CKY138 cells to growth conditions, cells were grown as previously described (3) and embedded or plated on a rough surface. In some experiments, wild-type strain CKY101, constructed by transformation of CAI-4 (ura3/ura3) with vector pDBI52 (3), was used. Either 200 embedded colonies or 50 to 100 colonies growing within rough areas were observed at various times. A colony that contained at least 20 filaments protruding from the periphery of the colony was arbitrarily defined as a filamentous colony. The kinetics of filamentous growth of wild-type strains and the CKY138 mutant were similar under both conditions (Fig. 4A and B). In contrast, cells growing on the surfaces of smooth plates did not produce filaments until 6 or 7 days of incubation (data not shown) (3).
FIG. 4.
Kinetics of filamentous growth of strain CKY138 and wild-type C. albicans. Cells of strains CKY101 (wild type), CKY138 (efg1/efg1 cph1/cph1 ade2::Ura+), SC5314 (wild type), and CKY169 (czf1/czf1 cph1/cph1 ade2::Ura+) were embedded in YPS agar (A) or plated on rough YPS agar surfaces and incubated at 25°C. The percentage of filamentous colonies is plotted as a function of time. Panels: A, CKY101 and CKY138 embedded; B, SC5314, CKY138, and CKY169 plated on rough YPS agar.
Not all C. albicans strains become filamentous on rough agar. Mutant strain CKY169 (CAI-4 czf1::hisG/czf1::hisG cph1::hisG/cph1::hisG ade2::pDBI52), lacking the putative transcription factors Czf1p and Cph1p, was defective in producing hyphae under these conditions (Fig. 4B) and was also delayed in producing filaments in response to matrix embedding (3). Therefore, the responses to matrix embedding and to growth on a rough surface were affected by mutations in the same genes, indicating that the two processes were regulated similarly.
These experiments and previous studies demonstrate that cues from the physical environment regulate hyphal growth in C. albicans. In these experiments, the rich medium used and the low temperature did not promote rapid filamentous growth of C. albicans when cells were growing on the surface of a smooth layer of agar (3) or in a liquid culture (data not shown). Since an individual plate contained some rough parts and some smooth parts, the chemical environments of colonies growing on rough and smooth parts of the plate would be expected to be the same, yet these colonies developed very differently. Therefore, C. albicans responds to the nature of the physical environment and, in some physical environments, produces hyphae. The existence of another well-studied response to the physical environment, thigmotropism (6), a tropism caused by contact with a solid surface, demonstrates that C. albicans must possess mechanisms for sensing the physical environment and responding to that environment. The question of whether responses to growth on a rough surface, embedding and thigmotropism, use common sensing mechanisms awaits further study.
Filamentous growth in the tongues of IGB piglets of a mutant strain, CKY138 (efg1/efg1 cph1/cph1), that is deficient in response to standard chemical and temperature cues for induction of hyphal growth demonstrates that there is a pathway for regulation of filamentous growth that is independent of Efg1p and Cph1p and that this pathway can function during experimental infection. Despite its defect in forming hyphae in response to a temperature shift and serum, CKY138 responded to growth on a rough surface or to matrix embedding and produced pseudohyphae. Since important pathways for stimulation of hyphal growth are defective in this strain, this mutant may have allowed detection of the effects of a physical environment sensing mechanism that stimulates filamentous growth of C. albicans in tissue. Further genetic study of the physical environment sensing pathway may reveal genes that are important for tissue invasion during oral infection.
Acknowledgments
We thank Ralph Isberg and Andrew Camilli for helpful discussions and comments on the text. We are also grateful to Gerry Fink for strains and plasmids and to Angela Giusani for technical assistance. The use of resources and facilities of the Division of Infectious Diseases at the Tufts University School of Veterinary Medicine is greatly appreciated.
This work was supported in part by National Institute of Allergy and Infectious Diseases grants R01 AI 38591 (to C.A.K.) and K08 AI01407 (to K.A.A.).
REFERENCES
- 1.Andrutis, K. A., P. J. Riggle, C. A. Kumamoto, and S. R. Tzipori. Experimental mucocutaneous and disseminated candidiasis in immunosuppressed gnotobiotic piglets. Submitted for publication.
- 2.Braun B R, Johnson A D. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 3.Brown, D. H., Jr., A. Giusani, X. Chen, and C. A. Kumamoto. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Submitted for publication. [DOI] [PubMed]
- 4.Corner B E, Magee P T. Candida pathogenesis: unravelling the threads of infection. Curr Biol. 1997;7:R691–R694. doi: 10.1016/s0960-9822(06)00357-5. [DOI] [PubMed] [Google Scholar]
- 5.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gow N A R. Germ tube growth of Candida albicans. Curr Top Med Mycol. 1997;8:43–55. [PubMed] [Google Scholar]
- 7.Ishii N, Yamamoto M, Yoshihara F, Arisawa M, Aoki Y. Biochemical and genetic characterization of Rbf1p, a putative transcription factor of Candida albicans. Microbiology. 1997;143:429–435. doi: 10.1099/00221287-143-2-429. [DOI] [PubMed] [Google Scholar]
- 8.Kondova I, Mansfield K, Buckholt M A, Stein B, Widmer G, Carville A, Lackner A, Tzipori S. Transmission and serial propagation of Enterocytozoon bieneusi from humans and rhesus macaques in gnotobiotic piglets. Infect Immun. 1998;66:5515–5519. doi: 10.1128/iai.66.11.5515-5519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Köhler J, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 10.Lo H-J, Köhler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 11.Makin T J, Tzipori S. Inexpensive technique for the production and maintenance of gnotobiotic piglets, calves and lambs. Aust Vet J. 1980;56:353–358. doi: 10.1111/j.1751-0813.1980.tb09558.x. [DOI] [PubMed] [Google Scholar]
- 12.Stoldt V R, Sonnenborn A, Leuker C E, Ernst J F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]