Abstract
Background
Biomarkers for sarcopenia are lacking. We examined the diagnostic power of serum creatinine to cystatin C ratio for identifying low magnetic resonance imaging-muscle volume and low grip strength in a large observational study of UK Biobank older adults.
Methods
Serum creatinine and cystatin C were measured via immunoassays (Beckman Coulter AU5800 and Siemens Advia 1800, respectively) and grip strength by hydraulic hand dynamometer at baseline visit (2008–2010). magnetic resonance imaging-thigh fat-free muscle volume and DXA-derived appendicular lean mass were measured at imaging visit (2014–2018). Extreme outliers were removed, and covariates (demographic, lifestyle, and clinical factors, as well as time elapsed between baseline-imaging visit) were adjusted for in statistical models.
Results
12 873 older adults (mean age: 63.5 ± 2.7 years, 44.2% women) were included for fat-free muscle volume and appendicular lean mass/body mass index; 149 707 older adults (mean age: 64.0 ± 2.9 years, 50.5% women) for grip strength. Despite significant associations (p < .05), in fully adjusted models, creatinine to cystatin C showed poor to acceptable diagnostic power for identifying low fat-free muscle volume when using cutpoints of 20th percentile (area under the curve: 0.577 men; 0.622 women) and T scores of −2 (area under the curve: 0.596 men; 0.659 women) and −2.5 (area under the curve: 0.609 men; 0.722 women). In fully adjusted model, creatinine to cystatin C showed poor diagnostic power (area under the curves: <0.70) for identifying low appendicular lean mass/body mass index or low grip strength, irrespective of the cutpoint used.
Conclusions
Creatinine to cystatin C may not be a suitable biomarker for identifying low muscle volume or low strength in older adults. This finding, drawn from a large sample size and the use of advanced medical imaging, marks an important contribution to the sarcopenia field.
Keywords: Biomarkers, Medical Imaging, Sarcopenia, Skeletal Muscle
In the European Consensus Revision, sarcopenia is described as a progressive and generalized skeletal muscle disorder that may present as low muscle mass, strength, and/or function (1). These age-related changes in the structure and function of skeletal muscle may be accelerated by poor lifestyle (ie, physical inactivity, low nutritional status) and/or disease-related (immunological changes with acute/chronic diseases) factors (2,3). Socioeconomic consequences of this muscle disorder are profound, with older adults at high risk of poor health-related quality of life (4), falls (5), and fractures (5).
Since sarcopenia was first described as the “age-related loss of lean body mass” by Dr Irwin Rosenberg in 1989, there has been a progressive increase in research efforts to understand the pathophysiology of this disorder in addition to optimal diagnostic and prognostic markers. In terms of the latter, identifying a blood biomarker that holds high diagnostic capacity for low muscle mass has proven elusive. One biomarker in the muscle field that has gathered attention is the ratio of serum creatinine to cystatin C (Cr:Cyc), as recently highlighted in the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases working report relating to biochemical markers on sarcopenia treatment (6).
Creatinine and cystatin C are both commonly used in the assessment of kidney disease (7). Around 98% of creatine is stored in skeletal muscle, and following metabolism, its product creatinine is filtered by the kidneys and excreted in urine. Both muscle metabolism and kidney function can influence serum creatinine levels in a bidirectional manner, making it difficult to interpret this standalone biomarker (8). The nephrology field has sought to counteract this limitation by developing reference equations including Cystatin C, a protein found in similar quantities in human tissues and also filtered by the kidneys (6,8).
Emerging studies have looked at the relationship between Cr:Cyc and metrics of lean mass or muscle mass in older adults. Moderate associations were found between Cr:Cyc and CT-muscle cross-sectional area in one retrospective study of 226 ICU patients (9). Another study (10) showed poor sensitivity and specificity of Cr:Cyc for identifying low appendicular muscle mass/height2 in 371 community-dwelling older adults, although this study used bioelectrical impedance analysis to estimate appendicular lean mass, which is not the gold standard of measuring muscle mass (11,12). Contrary to these findings, a cross-sectional study showed positive relationships between Cr:Cyc and appendicular lean mass (by bioelectrical impedance analysis) in 908 community-dwelling older adults in Japan (13). A very recent study (14) showed positive associations between Cr:Cyc and DXA-appendicular lean mass (adjusted for height squared or body mass index [BMI]) obtained 5 years later in 1 118 community-dwelling older women. Neither of these studies directly examined the diagnostic capability of Cr:Cyc for identifying low lean mass using an Receiver Operating Characteristic (ROC) analysis or related tests.
As seen by the earlier studies, there is some inconsistency in findings, which may partly be explained by differences in study designs, sample sizes, and most importantly, techniques used to evaluate muscle mass or lean mass. In regard to medical imaging techniques for quantifying muscle size, none of the previously mentioned studies have used accurate assessments of muscle volume by magnetic resonance imaging (MRI) (11).
Thus, we sought to advance knowledge on this topic by examining the diagnostic power of Cr:Cyc for identifying low MRI-muscle volume obtained 4 years later in a very large population of older men and women in the UK Biobank. In this cohort, lower MRI-muscle volume has been shown to be strongly associated with lower grip strength, a lower frequency of stair climbing, and a slow walking pace in 9 615 men and women when using a cross-sectional analysis (15). Consequently, we also included DXA-derived lean mass and grip strength as outcomes, as these muscle metrics are frequently used in the sarcopenia field (11).
Method
Study Design
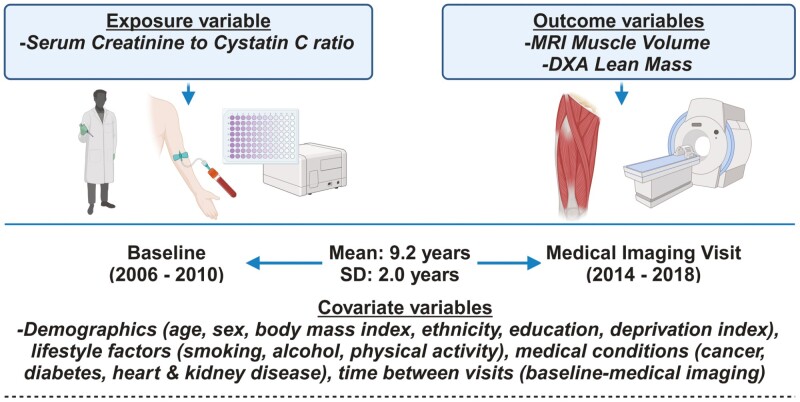
This analysis was based on participants from the UK Biobank study. This study made use of the UK Biobank resource, application ID 92647. The Northwest Multicenter Research Ethics Committee in the United Kingdom approved the study, and prior to the start of the investigation, written informed consent was obtained (REC reference: 11/NW/03/820). The recruitment process and measurements used to collect data for this study can be found on the UK Biobank website: https://www.ukbiobank.ac.uk. Figure 1 shows the study design for the primary outcome of interest.
Figure 1.
Shows the study design for the primary sample of interest.
Participants
UK Biobank participants who remained active and attended the first imaging visit were included (2014–2019). Participants younger than 60 years were excluded as well as those with missing or extreme creatinine or cystatin C obtained in 2006–2010 (considered extreme if greater than Q3 + 1.5 × (Q3–Q1) or less than Q1 – 1.5 × (Q3–Q1), where Q1 and Q3 represent the first and third quartiles). We used Tukey’s method to define outliers (16). Additionally, participants lacking MRI-muscle volume and DXA-lean mass, or any covariate data were excluded. The resulting cohort, termed the imaging cohort, consisted of 12 873 samples (Supplementary Figure 1). For specific outcomes, the sample sizes were 9 731 for fat-free muscle volume (FFMV), 10 260 for BMI-adjusted appendicular lean mass (ALM), and 10 484 for height-adjusted ALM. Participants attending baseline visits underwent similar inclusion and exclusion criteria for the outcome of grip strength at baseline (n = 149 707), forming the baseline cohort (Supplementary Figure 2).
Exposure: Creatinine to Cystatin C Ratio
Serum cystatin C levels were measured using a latex-enhanced immuno-turbidimetric assay conducted by Siemens (Erlangen, Germany) on the Siemens Advia 1800. Serum creatinine levels were assessed with an enzyme-based assay conducted by Beckman Coulter (High Wycombe, United Kingdom) on the Beckman Coulter AU5800. The coefficient of variation for both cystatin C and creatinine was controlled at 3% or less, as determined by internal quality control samples with known high, medium, and low concentrations. For technical details and quality control information, please refer to the reports from the UK Biobank: https://biobank.ctsu.ox.ac.uk/showcase/refer.cgi?id=5636.
Outcomes: MRI-Muscle Volume, DXA-Lean Mass & Grip Strength
MRI scans to quantify total thigh FFMV were conducted using a Siemens Aera 1.5 T scanner (Syngo MR D13) (Siemens, Erlangen, Germany) and AMRA Researcher (AMRA Medical AB, Linköping, Sweden) image analysis software (17). The FFMV was defined as the total volume of all voxels with a fat fraction <50% (considered “viable muscle tissue”) in the left and right anterior and posterior thighs (15,18). A dual-energy X-ray absorptiometry machine (GE-Lunar iDXA, Madison, WI) was used to quantify appendicular lean mass (sum of lean mass from arms and legs). Daily quality control was carried out for both MRI and DXA scans; see: https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=100003. Grip strength was measured using a Jamar J00105 hydraulic hand dynamometer with participants sitting upright in a chair and forearms resting on armrests (elbows bent at a 90-degree angle). Participants exerted their maximal force during a 3 second effort on the right and left sides. Verbal encouragement was provided. The maximal force from either side was used in the analysis.
To assess the discriminative power of Cr:Cyc, we dichotomized baseline grip strength and 3 imaging outcomes (FFMV, ALM/BMI, and ALM/Height2) using sex-specific cutpoints set at 2 or 2.5 standard deviations (SDs) below an age-and-sex-specific mean, that is, T score −2 or −2.5, in a reference population based on previous studies using grip strength (19), FFMV (20), ALM/Height2 (1), and ALM/BMI (21). Sensitivity analyses were conducted using sex-specific 20th percentiles in the data.
Baseline Covariates
Covariates were preselected based on the potential association with the exposure (Cr:Cyc) (7,22) or outcomes related to musculoskeletal health (2,14,23). Baseline covariates included self-reported demographics, that is, chronological age in years at recruitment, sex (Men or Women), ethnicity (categorized as White, Black, Asian, or Other), and educational qualification (college or university degree, A levels/AS levels or equivalent, O levels/GCSEs or equivalent, CSEs or equivalent, NVQ/HND/HNC or equivalent, other professional qualifications, eg, nursing, teaching, or none of the above). Additionally, socioeconomic status was assessed by an area-based material deprivation index, the Townsend deprivation index, where higher values indicate higher levels of material deprivation. Physiological or lifestyle factors were also considered, such as BMI, smoking status (never, current, or previous), alcohol intake frequency (from never to daily or almost daily), and physical activity group based on the short International Physical Activity Questionnaire (IPAQ) (24). Other covariates included the disease states of myocardial infarction, stroke, heart failure, cancer, chronic kidney disease, and diabetes, as well as the time difference between baseline and first imaging visits for imaging outcomes only. Disease states were determined using the first occurrence data derived by UK Biobank, which included multisource data (primary care data, hospital inpatient data, death register records, and self-reported medical conditions) based on 3-character ICD-10 codes to find the first diagnoses. We also calculated eGFR (based on the 2021 CKD-EPI creatinine–cystatin C equation (7)) and used it as a potential covariate. A list of data fields, including ICD-10 codes and ICD-9 codes if used for the disease covariates, and their field IDs for data extraction is provided in Supplementary Table 1.
Statistical Methods
A descriptive analysis was conducted to summarize variables using proper statistics in the baseline and imaging cohorts, separately (Table 1). The outcomes of interest were further summarized for each individual quantile group of Cr:Cyc. Four quantile groups were compared for each outcome using a Kruskal–Wallis test (Table 2).
Table 1.
Participant Characteristics of the Imaging or Baseline Cohort
| Imaging (n = 12 873) |
Baseline (n = 149 707) |
|
|---|---|---|
| Predictor of interest | ||
| Creatinine to cystatin C ratio (Cr/Cys) | 0.92 ± 0.15 | 0.88 ± 0.15 |
| Creatinine (mg/dL) | 0.83 ± 0.15 | 0.82 ± 0.15 |
| Cystatin C (mg/L) | 0.91 ± 0.11 | 0.94 ± 0.13 |
| Baseline covariates | ||
| Age at baseline (years) | 63.48 ± 2.73 | 64.04 ± 2.84 |
| Time between 2 visits (years) | 9.2 ± 2.04 | |
| BMI (kg/m2) | 26.62 ± 3.87 | 27.34 ± 4.34 |
| Sex (=women) | 5 686 (44.17%) | 75 633 (50.52%) |
| Ethnicity | ||
| White | 12 658 (98.33%) | 145 791 (97.38%) |
| Black | 27 (0.21%) | 974 (0.65%) |
| Asian | 27 (0.21%) | 974 (0.65%) |
| Other | 86 (0.67%) | 1 100 (0.73%) |
| Education | ||
| College or University degree | 1 383 (10.74%) | 14 103 (9.42%) |
| A levels/AS levels or equivalent | 5 580 (43.35%) | 43 240 (28.88%) |
| O levels/GCSEs or equivalent | 211 (1.64%) | 3 272 (2.19%) |
| CSEs or equivalent | 870 (6.76%) | 11 008 (7.35%) |
| NVQ/HND/HNC or equivalent | 2 562 (19.90%) | 31 865 (21.28%) |
| Other prof. qualifications | 891 (6.92%) | 10 608 (7.09%) |
| None of the above | 1 376 (10.69%) | 35 611 (23.79%) |
| Townsend deprivation index | −2.13 ± 2.58 | −1.67 ± 2.89 |
| Smoking status | ||
| Never | 6 916 (53.72%) | 75 042 (50.13%) |
| Previous | 5 353 (41.58%) | 63 123 (42.16%) |
| Current | 604 (4.69%) | 11 542 (7.71%) |
| IPAQ | ||
| High | 5 427 (42.16%) | 62 019 (41.43%) |
| Moderate | 5 481 (42.58%) | 62 983 (42.07%) |
| Low | 1 965 (15.26%) | 24 705 (16.50%) |
| Alcohol | ||
| Never | 571 (4.44%) | 11 548 (7.71%) |
| Special occasions only | 948 (7.36%) | 16 451 (10.99%) |
| 1–3 times a month | 1 142 (8.87%) | 14 342 (9.58%) |
| Once or twice a week | 2 840 (22.06%) | 35 281 (23.57%) |
| 3 or 4 times a week | 3 584 (27.84%) | 34 568 (23.09%) |
| Daily or almost daily | 3 788 (29.43%) | 37 517 (25.06%) |
| Disease diagnosis | ||
| Myocardial infarction (=yes) | 545 (4.23%) | 9 211 (6.15%) |
| Stroke (=yes) | 187 (1.45%) | 3 106 (2.07%) |
| Heart failure (=yes) | 33 (0.26%) | 986 (0.66%) |
| Cancer (=yes) | 716 (5.56%) | 11 721 (7.83%) |
| Chronic kidney disease (=yes) | 134 (1.04%) | 1 910 (1.28%) |
| Diabetes (=yes) | 552 (4.29%) | 9 660 (6.45%) |
Notes: BMI = body mass index; IPAQ = International Physical Activity Questionnaire.
Table 2.
Fully Adjusted Associations (Predicted Values and Their 95% Confidence Intervals) for Outcomes Across Quartile Groups of Cr:Cyc
| Group | Q1 | Q2 | Q3 | Q4 | Non-Linearity p-Value |
Association p-Value |
|
|---|---|---|---|---|---|---|---|
| All | 24.291 (24.217, 24.368) |
28.952 (28.861, 29.052) |
34.101 (33.992, 34.194) |
38.278 (38.185, 38.38) |
<1e−323 | 2.38E−37 | |
| Grip strength | Women | 21.958 (21.881, 22.031) |
23.059 (23, 23.122) |
23.744 (23.68, 23.809) |
24.528 (24.449, 24.604) |
<1e−323 | 6.44E−43 |
| Men | 37.555 (37.445, 37.671) |
39.421 (39.336, 39.504) |
40.361 (40.269, 40.447) |
41.299 (41.194, 41.413) |
<1e−323 | 6.74E−50 | |
| All | 8.255 (8.185, 8.319) |
9.519 (9.418, 9.618) |
10.657 (10.574, 10.745) |
11.386 (11.312, 11.466) |
1.73E−80 | 3.58E−13 | |
| FFMV | Women | 7.592 (7.528, 7.656) |
7.765 (7.714, 7.811) |
7.857 (7.805, 7.906) |
8.01 (7.952, 8.069) |
4.46E−49 | 9.19E−09 |
| Men | 11.345 (11.269, 11.421) |
11.606 (11.542, 11.671) |
11.757 (11.692, 11.829) |
11.893 (11.815, 11.974) |
8.08E−39 | 2.99E−09 | |
| All | 0.678 (0.673, 0.682) |
0.778 (0.771, 0.784) |
0.852 (0.847, 0.858) |
0.906 (0.901, 0.911) |
2.36E−37 | 0.002 | |
| ALM/BMI | Women | 0.628 (0.623, 0.633) |
0.652 (0.648, 0.656) |
0.666 (0.662, 0.67) |
0.681 (0.676, 0.687) |
1.05E−21 | 0.007 |
| Men | 0.891 (0.885, 0.897) |
0.915 (0.911, 0.92) |
0.926 (0.921, 0.93) |
0.94 (0.935, 0.945) |
2.84E−18 | 0.002 | |
| All | 6.587 (6.550, 6.628) |
7.017 (6.972, 7.063) |
7.399 (7.361, 7.438) |
7.719 (7.684, 7.758) |
2.03E−76 | 3.75E−05 | |
| ALM/Height2 | Women | 6.364 (6.312, 6.416) |
6.346 (6.307, 6.391) |
6.342 (6.298, 6.378) |
6.339 (6.303, 6.381) |
6.38E−29 | 5.39E−04 |
| Men | 7.758 (7.713, 7.806) |
7.806 (7.766, 7.841) |
7.851 (7.816, 7.896) |
7.933 (7.888, 7.972) |
6.53E−47 | 2.42E−04 |
Notes: ALM = appendicular lean mass; BMI = body mass index; Cr:Cyc = creatinine to cystatin C; FFMV = fat-free muscle volume; IPAQ = International Physical Activity Questionnaire.
The nonlinearity of the relationship between Cr:Cyc and each continuous outcome was visualized by sex and for the overall sample by means and their confidence intervals for individual quartile groups of Cr:Cyc (Supplementary Figure 3), and it was formally tested by a chi-square test comparing the goodness of fit between a cubic spline linear regression model with the knots at the 25th, 50th, and 75th percentiles of Cr:Cyc and a linear regression model. Given that most nonlinearity test results were statistically significant, except for ALM/Height2 in women, the cubic spline linear regression models were used for subsequent association analyses to link Cr:Cyc with continuous outcomes.
The association between Cr:Cyc and an outcome was tested by a chi-square test comparing the goodness of fit between the cubic spline linear regression model and its reduced model without the nonlinear function of Cr:Cyc. Additionally, a bootstrap method was implemented to repeatedly simulate individual data from the observed data with replacement to create 1 000 replicates of the data to fit the same association model. The fitted values were collected across replicates to calculate the mean predicted values for individual quartile groups of Cr:Cyc and their 95% confidence intervals. Both unadjusted and adjusted models were fitted, adjusting for baseline covariates for all outcomes (age, sex, BMI, ethnicity, education, Townsend deprivation index, smoking status, alcohol intake frequency, IPAQ activity group, myocardial infarction, stroke, heart failure, cancer, chronic kidney disease, and diabetes), and time difference between baseline and imaging visits additionally for imaging outcomes.
The discriminative power of Cr:Cyc for a dichotomized outcome was evaluated in a cubic spline logistic regression model, with the knots at the 25th, 50th, and 75th percentiles of Cr:Cyc. The results were presented with a ROC curve including the area under the curve (AUC). Sensitivity analyses were performed in sex-specific groups and/or using different outcome cutpoints. Additionally, we reported the ROCs and AUCs of covariates-adjusted Cr:Cys (Cr:Cys after regressing out the effects of covariates using a linear regression model). Given some studies (25) have used the biomarker difference (creatinine minus cystatin C) instead of the ratio (creatinine/cystatin C) in predicting muscle-outcomes, we repeated the analysis using the biomarker difference to see if this influenced results. All AUCs were interpreted as acceptable (0.7–0.8), excellent (0.8–0.9), or outstanding (>0.9) following recommendations in clinical research (26). All hypothesis tests were 2-sided. p-Values below 5% were deemed to be statistically significant. The statistical analyses were performed in R version 4.2.2, using the packages including “spline,” “pROC,” and “boot.”
Results
Population Characteristics
Table 1 shows the participant characteristics of the imaging and baseline cohorts. A total of 12 873 older adults (mean age: 63.5 ± 2.7 years, 44.2% women) were included in the imaging cohort for MRI/DXA outcomes (FFMV, ALM/BMI & ALM/Height2), and 149 707 older adults (mean age: 64.0 ± 2.9 years, 50.5% women) in the baseline cohort for grip strength outcome.
When using a T score of −2 as the cutpoint, the prevalence of low FFMV in our population was 4.7% (200) in women and 7.8% (426) in men. Supplementary Table 2 shows the full prevalence of low FFMV, low ALM/BMI, low ALM/height2, and low grip strength using age- and sex-specific normative values (20th percentile and T scores of −2SD and −2.5SDs).
Associations Between Cr:Cys and Outcomes
Lower Cr:Cys was significantly associated with lower grip strength, FFMV, ALM/BMI, and ALM/Height2 overall and by sex after adjusting for covariates (p < .05), except for ALM/Height2 in men and in women (Table 2). The corresponding unadjusted analysis showed similar results (absolute differences ≤0.091, data not shown).
Sensitivity analysis showed negligible effect of interchanging CKD (from linked health records) with eGFR as a covariate on the association between Cr:Cys and outcomes in fully adjusted models (data not shown). We also refitted models by excluding non-White participants for the same outcomes (data not shown). Again, associations were not materially altered in fully adjusted models.
Interchanging the biomarker ratio for the biomarker difference resulted in significant associations with outcomes but smaller effect sizes or smaller mean differences between quartile groups (Supplementary Table 4).
Diagnostic Power of Cr:Cys for Outcomes
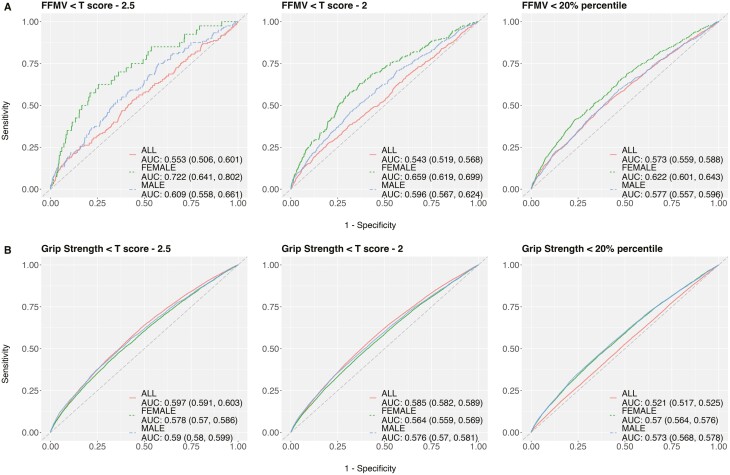
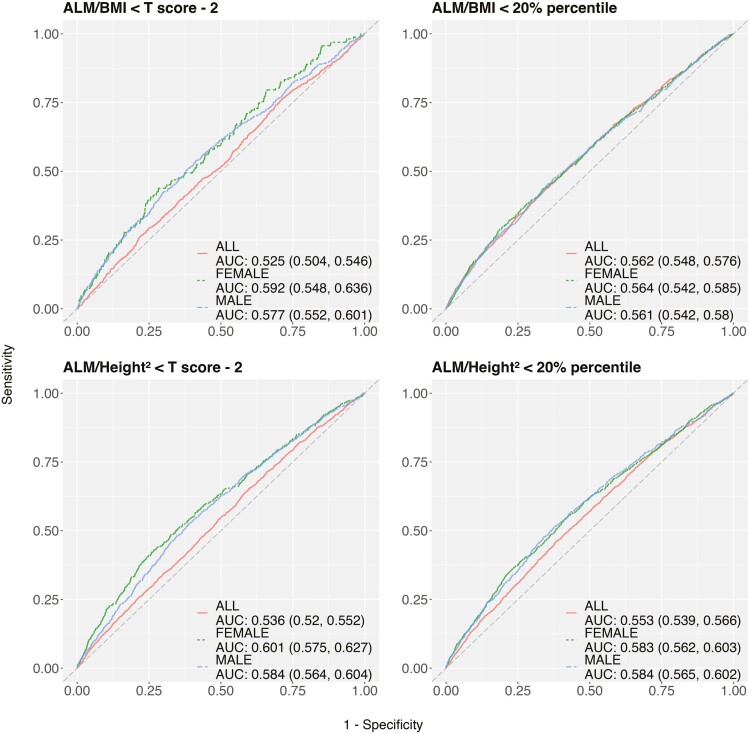
The sex-specific models showed better AUCs than a model using all samples across outcomes, irrespective of covariates (Figures 2 and 3 fully adjusted; Supplementary Figures 4–6 unadjusted). For sex-specific models, pushing the threshold to the extreme improved the AUC but it came with greater uncertainty due to a small number of cases, as reflected in the 95% confidence interval (Figures 2 and 3 fully adjusted; Supplementary Figures 4–6 unadjusted). The AUC was generally low regardless of outcomes, cutpoints, and subgroups. In the fully adjusted models, Cr:Cyc showed poor to acceptable diagnostic power for identifying low FFMV in older adults when using cutpoints of the 20th percentile (AUC: 0.577 vs 0.589 using age only in men; 0.622 vs 0.531 using age only in women) and T scores of −2 (AUC: 0.596 vs 0.59 using age only in men; 0.659 vs 0.543 using age only in women) and −2.5 (AUC: 0.609 vs 0.593 using age only in men; 0.722 vs 0.561 using age only in women). In the fully adjusted model, Cr:Cyc showed poor diagnostic power (AUCs: <0.70) for identifying low ALM/BMI or low grip strength, irrespective of the cutpoint used.
Figure 2.
Receiver operator characteristic curves shows the diagnostic power of Cr:Cyc for identifying low FFMV (A) and low grip strength (B) using different cutpoints. Models are fully adjusted for covariates. Notes: Cr:Cyc = creatinine to cystatin C; FFMV = fat-free muscle volume.
Figure 3.
Receiver operator characteristic curves shows the diagnostic power of Cr:Cyc for identifying low ALM/BMI or ALM/Height2 using different cutpoints. Models are fully adjusted for covariates. Notes: ALM = appendicular lean mass; BMI = body mass index; Cr:Cyc = creatinine to cystatin C.
Interchanging the biomarker ratio for the biomarker difference resulted in negligible differences in diagnostic power, with overlapped 95% confidence intervals of AUC (Supplementary Figures 7–10).
Discussion
We sought to examine the diagnostic power of Cr:Cyc for identifying low MRI-muscle volume measured 4 years later in a large observational study of UK Biobank adults. Findings showed that Cr:Cyc was associated with FFMV (and most muscle metrics, including ALM/BMI and grip strength) in the fully adjusted analyses. However, this biomarker offered poor to acceptable (at best) diagnostic power for identifying low FFMV in older men and women. Findings were poor when interchanging FFMV for ALM/BMI or grip strength. This finding was not materially altered by the inclusion of relevant covariates. Together, our findings suggest that Cr:Cyc may not be a suitable biomarker for identifying low muscle volume or low grip strength in older adults.
Previous studies have investigated the association between Cr:Cyc and muscle metrics in the context of aging and acute/chronic diseases. Similar to our findings, positive associations have been observed between Cr:Cyc and CT-muscle area in ICU patients (9) as well as between this biomarker and ALM/BMI or grip strength in various studies, including older community-dwelling Australian women (14) and Japanese men (13). While these studies did not include an AUC-ROC discriminatory analysis, one such study (10) found that Cr:Cyc offered poor sensitivity and specificity for identifying low appendicular muscle mass/height2 in 371 community-dwelling older adults. Our study corroborates these findings and makes an important contribution to this research topic by including an accurate measure of muscle volume via MRI (11). We also included a full complement of adjustments for demographics, lifestyle factors, and chronic diseases in men and women. Our sample size is at least 10-fold greater than previous studies on this topic, and we were able to evaluate the diagnostic performance of Cr:Cyc across important sex-specific muscle-outcomes relevant to the field of sarcopenia. We did not utilize a specific sarcopenia definition as the field is currently evolving and transitioning into a unified global definition (27). Nevertheless, our cutpoints were derived from population-specific normative values for MRI-muscle, DXA-lean mass, and grip strength, which have been outlined in previous sarcopenia definitions (1,21).
Despite the overall poor diagnostic power of Cr:Cyc for our muscle-outcomes, it was noteworthy that pushing the cutpoint to the extreme improved the AUC, but it came with greater uncertainty due to a small number of cases, as reflected in the wider 95% confidence interval. A greater number of the “oldest-old” (eg, aged 70 or 80+) where low muscle volume (20), low ALM/BMI (28) or low grip strength (19) are more prevalent may have provided more insights in answering our research question. We will consider revisiting this research question in future years when the UK Biobank participants have aged and attended repeated MRI imaging visits that will allow analysis over a much longer follow-up. Until then (or further data that proves otherwise), we do not recommend Cr:Cyc as a suitable biomarker for identifying low muscle volume or low grip strength in community-dwelling older adults.
Our study is not without its limitations. We acknowledge that all observational studies are open to residual confounding, and we cannot determine cause-and-effect from such study designs as the one presented here. Nonetheless, we removed outliers and adjusted for demographics, lifestyle factors, and disease classifications (as well as adjustment for time differences between measures) in statistical models to reduce the influence of residual confounding. Despite the adjustment for time difference, our results may be affected by the fact that Cr:Cys (and covariates) were measured years prior to the imaging outcomes (mean difference: 9.2 ± 2.0 years; Figure 1). It is plausible that the diagnostic ability may be improved for outcomes in the short-term. As mentioned, including repeat measures of our biomarkers and outcomes would have strengthened the ability to answer the research question. Identifying the relationship between Cr:Cyc and whole-body D3Cr muscle mass (measured by stable isotopes) (29) may also prove fruitful, especially considering this measure has been consistently associated with poor health outcomes (30). We, and others, should consider these factors in future analysis on this research topic. Finally, although Cr:Cyc was not found to be a suitable biomarker in this study, the approach taken to account for heterogeneity in muscle volume and grip strength and target Cr:Cyc as a potential diagnostic marker for low values of these muscle-related outcomes represents an important application of the emerging field of precision gerontology (31,32).
To conclude, we found that Cr:Cyc was consistent associated with FFMV and other important muscle metrics such as ALM/BMI and grip strength. However, our findings suggest that Cr:Cyc may not be a suitable biomarker for identifying the risk of low muscle volume or low grip strength in older adults. This finding, drawn from a large sample size and the use of advanced medical imaging, marks an important contribution to the sarcopenia field.
Supplementary Material
Acknowledgments
Access to UK Biobank data was granted under application no. 92647 “Research to Inform the Field of Precision Gerontology” (PI: Richard H. Fortinsky). This research used data assets made available by National Safe Haven as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (research which commenced between 1 October 2020–31 March 2021 grant ref MC_PC_20029; 1 April 2021–30 September 2022 grant ref MC_PC_20058). This research also used data provided by patients and collected by the NHS as part of their care and support. Copyright © (year), NHS England. Re-used with the permission of the NHS England [and/or UK Biobank]. All rights reserved. The authors would like to thank the Australian Institute for Musculoskeletal Science (AIMSS) for supporting Ben Kirk.
Contributor Information
Ben Kirk, Department of Medicine, Western Health, Melbourne Medical School, University of Melbourne, Melbourne, Victoria, Australia; Australian Institute for Musculoskeletal Science (AIMSS), University of Melbourne and Western Health, Melbourne, Victoria, Australia.
Chia-Ling Kuo, The Cato T. Laurencin Institute for Translation in Regenerative Engineering, University of Connecticut Health, Farmington, Connecticut, USA; UConn Center on Aging, University of Connecticut, Farmington, Connecticut, USA.
Peiran Liu, The Cato T. Laurencin Institute for Translation in Regenerative Engineering, University of Connecticut Health, Farmington, Connecticut, USA.
Meiruo Xiang, The Cato T. Laurencin Institute for Translation in Regenerative Engineering, University of Connecticut Health, Farmington, Connecticut, USA.
Jesse Zanker, Department of Medicine, Western Health, Melbourne Medical School, University of Melbourne, Melbourne, Victoria, Australia; Australian Institute for Musculoskeletal Science (AIMSS), University of Melbourne and Western Health, Melbourne, Victoria, Australia.
Konstantinos Prokopidis, Department of Musculoskeletal Biology, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, UK.
Marc Sim, Nutrition and Health Innovation Research Institute, School of Medical and Health Sciences, Edith Cowan University, Joondalup, Western Australia, Australia; Medical School, University of Western Australia, Crawley, Western Australia, Australia.
Richard H Fortinsky, UConn Center on Aging, University of Connecticut, Farmington, Connecticut, USA.
George A Kuchel, UConn Center on Aging, University of Connecticut, Farmington, Connecticut, USA.
Gustavo Duque, Bone, Muscle & Geroscience Group, Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada; Dr. Joseph Kaufmann Chair in Geriatric Medicine, Department of Medicine, McGill University, Montreal, Quebec, Canada.
Funding
Access to UK Biobank data was granted under application no. 92647 “Research to Inform the Field of Precision Gerontology” (PI: Richard H. Fortinsky), funded by the Claude D. Pepper Older American Independence Centers (OAIC) program: P30AG067988 (MPIs: George A. Kuchel and Richard H. Fortinsky). C.L.K. and G.A.K. are partially supported by P30AG067988. M.S. is supported by a Career Advancement Fellowship from the Royal Perth Hospital Research Foundation and an Emerging Leader Fellowship from the Future Health Research and Innovation Fund (Department of Health, Western Australia).
Conflict of Interest
None.
References
- 1. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. https://doi.org/ 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirk B, Feehan J, Lombardi G, Duque G.. Muscle, bone, and fat crosstalk: the biological role of myokines, osteokines, and adipokines. Curr Osteoporos Rep. 2020;18(4):388–400. https://doi.org/ 10.1007/s11914-020-00599-y [DOI] [PubMed] [Google Scholar]
- 3. Tieland M, Trouwborst I, Clark B.. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2017;9(1):3–19. https://doi.org/ 10.1002/jcsm.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beaudart C, Demonceau C, Reginster J, et al. Sarcopenia and health-related quality of life: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14:1228–1243. https://doi.org/ 10.1002/jcsm.13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10:jcsm.12411. https://doi.org/ 10.1002/jcsm.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ladang A, Beaudart C, Reginster JY, et al. Biochemical markers of musculoskeletal health and aging to be assessed in clinical trials of drugs aiming at the treatment of sarcopenia: consensus paper from an expert group meeting organized by the European Society for clinical and economic aspects of O. Calcif Tissue Int. 2023;112(2):197–217. https://doi.org/ 10.1007/s00223-022-01054-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. https://doi.org/ 10.1056/nejmoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okamura M, Konishi M, Butler J, Kalantar-Zadeh K, von Haehling S, Anker SD.. Kidney function in cachexia and sarcopenia: facts and numbers. J Cachexia Sarcopenia Muscle. 2023;14(4):1589–1595. https://doi.org/ 10.1002/jcsm.13260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kashani KB, Frazee EN, Kukrálová L, et al. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med. 2017;45(1):e23–e29. https://doi.org/ 10.1097/CCM.0000000000002013 [DOI] [PubMed] [Google Scholar]
- 10. He Q, Jiang J, Xie L, Zhang L, Yang M.. A sarcopenia index based on serum creatinine and cystatin C cannot accurately detect either low muscle mass or sarcopenia in urban community-dwelling older people. Sci Rep. 2018;8(1):11534. https://doi.org/ 10.1038/S41598-018-29808-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cawthon PM, Visser M, Arai H, et al. Defining terms commonly used in sarcopenia research: a glossary proposed by the Global Leadership in Sarcopenia (GLIS) Steering Committee. Eur Geriatr Med. 2022;13(6):1239–1244. https://doi.org/ 10.1007/s41999-022-00706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buckinx F, Landi F, Cesari M, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. 2018;9(2):269–278. https://doi.org/ 10.1002/jcsm.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kusunoki H, Tabara Y, Tsuji S, et al. Estimation of muscle mass using creatinine/cystatin C ratio in Japanese community-dwelling older people. J Am Med Dir Assoc. 2022;23(5):902.e21–902.e31. https://doi.org/ 10.1016/j.jamda.2021.07.029 [DOI] [PubMed] [Google Scholar]
- 14. Sim M, Dalla Via J, Scott D, et al. Creatinine to cystatin C ratio, a biomarker of sarcopenia measures and falls risk in community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2022;77(7):1389–1397. https://doi.org/ 10.1093/gerona/glab369 [DOI] [PubMed] [Google Scholar]
- 15. Linge J, Heymsfield SB, Leinhard O.. On the definition of sarcopenia in the presence of aging and obesity-initial results from UK Biobank. J Gerontol A Biol Sci Med Sci. 2020;75(7):1309–1316. https://doi.org/ 10.1093/GERONA/GLZ229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tukey JW. Exploratory Data Analysis. Vol 2. Reading, MA: Addison-wesley; 1977. [Google Scholar]
- 17. West J, Leinhard OD, Romu T, et al. Feasibility of MR-based body composition analysis in large scale population studies. PLoS One. 2016;11(9):e0163332. https://doi.org/ 10.1371/JOURNAL.PONE.0163332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Linge J, Petersson M, Forsgren MF, Sanyal AJ, Dahlqvist Leinhard O.. Adverse muscle composition predicts all-cause mortality in the UK Biobank imaging study. J Cachexia Sarcopenia Muscle. 2021;12(6):1513–1526. https://doi.org/ 10.1002/jcsm.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. Vina J, ed. PLoS One. 2014;9(12):e113637. https://doi.org/ 10.1371/journal.pone.0113637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dai L, Huang X, Lu Y, et al. Defining reference values for body composition indices by magnetic resonance imaging in UK Biobank. J Cachexia Sarcopenia Muscle. 2023;14(2):992–1002. https://doi.org/ 10.1002/jcsm.13181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–558. https://doi.org/ 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. https://doi.org/ 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirk B, Zanker J, Duque G.. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11(3):609–618. https://doi.org/ 10.1002/jcsm.12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cassidy S, Chau JY, Catt M, Bauman A, Trenell MI.. Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233 110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes. BMJ Open. 2016;6(3):e010038. https://doi.org/ 10.1136/bmjopen-2015-010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Potok OA, Ix JH, Shlipak MG, et al. Cystatin C- and creatinine-based glomerular filtration rate estimation differences and muscle quantity and functional status in older adults: the health, aging, and body composition study. Kidney Med. 2022;4(3):100416. https://doi.org/ 10.1016/j.xkme.2022.100416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–1316. https://doi.org/ 10.1097/JTO.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
- 27. Kirk B, Cawthon PM, Arai H, et al. The conceptual definition of sarcopenia: Delphi consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing. 2024;53(3):afae052. https://doi.org/ 10.1093/ageing/afae052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirk B, Bani Hassan E, Brennan‐Olsen S, et al. Body composition reference ranges in community‐dwelling adults using dual‐energy X‐ray absorptiometry: the Australian Body Composition (ABC) Study. J Cachexia Sarcopenia Muscle. 2021;12(4):880–890. https://doi.org/ 10.1002/jcsm.12712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kirk B, Harrison SL, Zanker J, et al. Interactions between HR-pQCT bone density and D3 Cr muscle mass (or HR-pQCT bone structure and HR-pQCT muscle density) in predicting fractures: the osteoporotic fractures in men study. J Bone Miner Res. 2023;38:1245–1257. https://doi.org/ 10.1002/jbmr.4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cawthon PM, Blackwell TMS CS.. Muscle mass assessed by D3-Creatine dilution method and incident disability and mortality in community dwelling older men. 2019. [DOI] [PMC free article] [PubMed]
- 31. Ferrucci L, Kuchel GA.. Heterogeneity of aging: individual risk factors, mechanisms, patient priorities, and outcomes. J Am Geriatr Soc. 2021;69(3):610–612. https://doi.org/ 10.1111/jgs.17011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuchel GA. Function begets function and resilience in old age: is precision gerontology possible? J Am Geriatr Soc. 2017;65(6):1141–1144. https://doi.org/ 10.1111/jgs.14901 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.