Abstract
The present communication describes the construction of a new Escherichia coli-Treponema denticola shuttle vector based on the naturally occurring spirochete plasmid pTS1 and the expression of the heterologous T. pallidum flaA gene from the plasmid in T. denticola. This new shuttle vector system should prove useful in characterizing virulence factors from unculturable pathogenic spirochetes.
Spirochetes have unique morphology and motility. Their periplasmic flagella, located between the outer membrane and the cytoplasmic membrane, play an important role in cellular morphology and motility (5, 15). The Treponema genus contains several important pathogens, and many of these pathogenic spirochetes cannot be cultured in vitro. One of the most important spirochete pathogens is Treponema pallidum, the causative agent of syphilis, which can be grown experimentally only in rabbit testes, but no gene transfer system for the organism is available. For identifying the virulence factors of these pathogens, potential virulence genes must be expressed in heterologous systems. Although some T. pallidum genes can be expressed in Escherichia coli (6, 15), the distinct physiological differences between spirochetes and E. coli limits the use of the E. coli system for functional investigations.
One of the oral spirochetes, Treponema denticola, which has been shown to be associated with periodontitis (11, 12, 16), can be cultured in the laboratory. In addition, a gene transfer system for T. denticola was recently developed in our laboratory (8, 9). These advantages together with the similarity of T. denticola with other spirochetes suggest that T. denticola may serve as a suitable host for expressing heterologous spirochete genes.
Previously, the broad-host-range plasmid pKT210 was shown to serve as a shuttle vector in a variety of bacterial hosts, including T. denticola (8). However, this plasmid proved to be unstable in several host systems. Therefore, in the present study we constructed a novel E. coli-T. denticola shuttle vector based on the naturally occurring spirochete plasmid pTS1 (3) and demonstrated the expression of the heterologous T. pallidum flaA gene from the plasmid.
Construction of a novel shuttle vector and transformation of T. denticola.
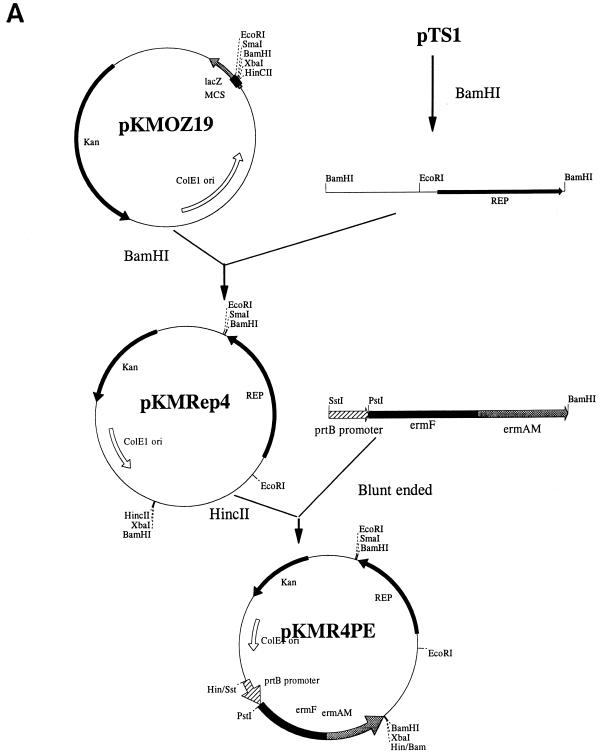
The cryptic plasmid pTS1 of T. denticola ATCC u9b (3) was used for shuttle vector construction. The sequence of pTS1 (3a) revealed an open reading frame homologous to a gene on plasmid pJDB23, a cryptic plasmid of Selenomonas ruminantium subsp. lactilytica (2). The fact that the gene on pJDB23 is responsible for the plasmid replication in E. coli (2) suggested that the open reading frame on pTS1 encodes a Rep protein. BamHI digestion of the pTS1 plasmid generated two fragments, and the larger, 2.8-kb fragment, which contains the potential Rep-encoding gene, was ligated into the BamHI site of an E. coli plasmid, pKMOZ19 (14), yielding the chimeric plasmid pKMRep4, which should replicate in both T. denticola and E. coli (Fig. 1A). The erythromycin resistance gene cassette (4), which has been shown to be expressed in T. denticola (9), was chosen as the selective marker for the shuttle vector. To ensure the transcription of the Emr cassette in T. denticola, the promoter of a T. denticola proteinase gene, prtB (1), was placed upstream of the Emr cassette. Both the Emr cassette and the prtB promoter were PCR amplified and cloned into the E. coli plasmid pBK-CMV (Stratagene, La Jolla, Calif.). The fragment which contained the promoter and the Emr cassette was removed from pBK-CMV, blunt ended, and ligated into the HincII site of plasmid pKMRep4 to generate the 7.7-kb pKMR4PE (Fig. 1A).
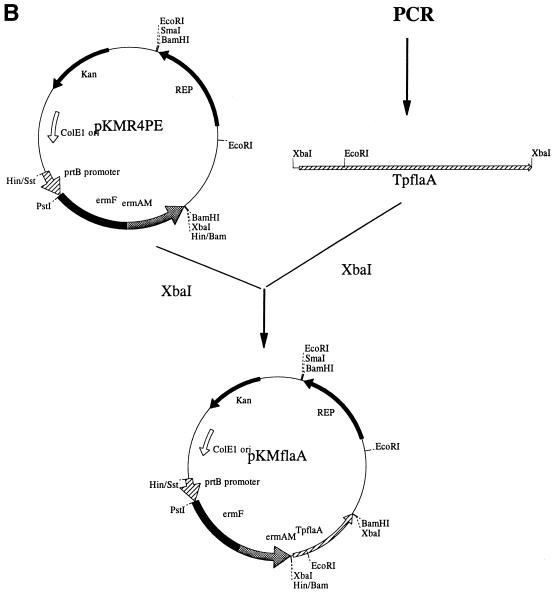
FIG. 1.
Construction of shuttle vectors pKMR4PE (A) and pKMflaA (B). The position and orientation of the putative Rep-encoding gene of pTS1 (Rep), the T. denticola prtB promoter (prtBp), and the Emr cassette (ermF and ermAM) are shown. Relevant restriction sites are indicated.
pKMR4PE was then transformed into T. denticola ATCC 33520 by electroporation as described previously (8). Ten micrograms of pKMR4PE plasmid (2 μg/μl) was used to transform 80 μl of fresh competent cells (about 4 × 109 cells). Transformants were selected on TYGVS plates supplemented with 0.8% SeaPlaque agarose (FMC BioProducts, Rockland, Maine) and erythromycin (40 μg/ml). All culturing was carried out at 37°C under anaerobic conditions. The erythromycin-resistant colonies began to appear after 7 to 10 days. The transformation efficiency was approximately 0.5 to 1 colony per μg of pKMR4PE. The individual colonies were then inoculated into 2 ml of TYGVS-erythromycin broth 2 to 3 days after their appearance and diluted to 10 ml at the mid-logarithmic growth phase. Plasmid DNA was isolated from T. denticola by using the Wizard Minipreps kit (Promega, Madison, Wis.) according to manufacturer’s protocol.
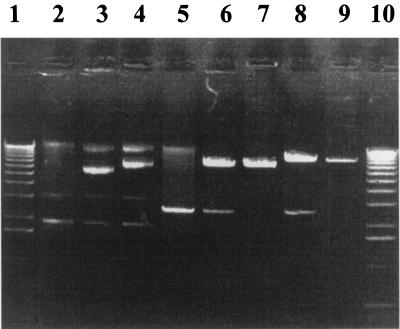
As demonstrated in Fig. 2, the wild-type strain ATCC 33520 carried the cryptic plasmid pTD1 of approximately 2.6 kb (7) (Fig. 2, lane 2). The pKMR4PE transformant also contained an additional plasmid (Fig. 2, lane 3). The linearized pKMR4PE in the transformant had the same size as the original pKMR4PE following cleavage with SmaI (Fig. 2, lanes 6 and 7). The T. denticola plasmids were next reintroduced into E. coli XL1-Blue cells (Stratagene). The rescued plasmids isolated from the erythromycin-resistant XL1-Blue colonies were characterized by restriction enzyme digestion. The analysis revealed that the rescued plasmids were indistinguishable from the original plasmids (data not shown). These results confirmed that the new shuttle vector pKMR4PE is capable of replicating independently and stably in T. denticola and that the open reading frame on the BamHI fragment of pTS1 encodes the Rep protein.
FIG. 2.
Electrophoretic analysis of plasmids isolated from T. denticola pKMR4PE and pKMflaA transformants. Lane 2, plasmid from wild-type T. denticola 33520; lane 3, plasmid from pKMR4PE transformants; lane 4, plasmid from pKMflaA transformants; lanes 5 to 9, SmaI digestions of plasmids from lane 2, lane 3, original pKMR4PE, lane 4, and original pKMflaA, respectively; lanes 1 and 10, 1-kb DNA ladder.
The transformation efficiency of T. denticola with the shuttle vector following electroporation is more than 100-fold higher when the plasmid isolated from T. denticola is used compared to the same plasmid isolated from E. coli. In addition, our experience (data not shown) and a previous report (7) have demonstrated that the EcoRI site of the plasmid is modified, probably methylated, in T. denticola but not in E. coli. Taken together, these results suggested that the restriction and modification systems are different in T. denticola and E. coli and that the DNA isolated from E. coli can be degraded by T. denticola restriction systems.
Expression of the T. pallidum flaA gene in T. denticola.
Our next step was to use the new shuttle vector to express heterologous spirochete genes. The gene of T. pallidum endoflagellum protein FlaA was chosen as a suitable gene because its sequence is known (5) and a monoclonal antibody, H9-2 (13), is available (gift from Sheila Lukehart, Harborview Medical Center, Seattle, Wash.). PCR primers were designed according to the T. pallidum flaA gene sequence (5), and the flaA gene was amplified from T. pallidum genomic DNA (gift from Kayla Hagman, University of Texas, Dallas). Our first attempt to clone the flaA gene together with its native promoter onto pKMR4PE in E. coli was not successful. This is consistent with previous reports that the strong expression of this flaA gene cannot be tolerated by E. coli (5). It was also known that the Emr cassette does not have transcriptional termination signals (10). The flaA gene was then placed downstream of the Emr cassette to be expressed from the prtB promoter. By using the XbaI restriction sites (underlined) which were incorporated into the forward and reverse PCR primers (5′-TTTTTTCTAGAGAGTGGTTATCTTATTGTGCG-3′ and 5′-TTTTTTCTAGATAGCCATCCTACCACGCATCC-3′, respectively) the amplified 1.25-kb flaA gene, which begins 24 bp upstream of its ribosome-binding site, was inserted into the unique XbaI site of pKMR4PE (Fig. 1B). The E. coli XL1-Blue colonies were screened by restriction endonuclease mapping for the flaA gene inserted in the same orientation as the Emr cassette. The resulting plasmid, pKMflaA, was transformed into T. denticola, and erythromycin-resistant T. denticola colonies were analyzed for plasmids and flaA gene expression. As shown in Fig. 2, pKMflaA-transformed T. denticola contained an additional band larger than that in pKMR4PE (Fig. 2, lane 4). Linearization of the plasmid with SmaI indicated that the plasmid had the same size as the original pKMflaA plasmid (Fig. 2, lanes 8 and 9). The pKMflaA plasmid from T. denticola was next retransformed into E. coli XL1-Blue cells. The rescued plasmids were further analyzed by restriction endonuclease mapping and proved to be identical to the original pKMflaA plasmid (data not shown).
The T. denticola pKMflaA transformants were then examined for expression of the T. pallidum FlaA protein by Western blot analysis. Monoclonal antibody H9-2, which is specific for T. pallidum FlaA protein (5), was used as the primary antibody. As shown in Fig. 3, H9-2 reacts with the 37-kDa FlaA band in the T. pallidum cell extract (gift from Kayla Hagman) (Fig. 3, lane 4) (5). A band with the same size was also detected by the H9-2 antibody in the T. denticola pKMflaA transformant cell extract (Fig. 3, lane 2). As a control, the T. denticola pKMR4PE cell extract doesn’t react with H9-2 (Fig. 3, lane 3). Compared to the wild type, ATCC 33520, the T. denticola pKMflaA transformants did not show any difference in growth rate or morphology under phase-contrast microscopy. The T. denticola pKMflaA transformants after three passages still expressed the T. pallidum FlaA protein (data not shown). While the size of the T. pallidum FlaA protein expressed in T. denticola corresponds to that of the protein expressed in the former organism, we cannot formally rule out the possibility of minor alternations in the protein expressed in the heterologous spirochete.
FIG. 3.
Western blot analysis of T. denticola pKMflaA transformants. Cell extracts were separated on a sodium dodecyl sulfate–12.5% polyacrylamide gel and transferred to a nitrocellulose membrane. Monoclonal antibody H9-2 of T. pallidum FlaA protein (1:10 dilution) was used as the primary antibody. Lanes 1 and 5, prestained sodium dodecyl sulfate-polyacrylamide gel electrophoresis standards (Bio-Rad, Hercules, Calif.); lane 2, cell extract of T. denticola pKMflaA transformants; lane 3, cell extract of T. denticola pKMR4PE transformants; lane 4, cell extract of T. pallidum.
To our knowledge, this is the first report of heterologous gene expression from a shuttle vector in a spirochete. Therefore, T. denticola can serve as a potential system for characterizing virulence genes from unculturable spirochetes. PCR fragments of potential virulence genes from other spirochetes could be inserted into the shuttle vector, and the function of the expressed proteins could be examined. At present, the virulence factors of pathogenic spirochetes remain largely undefined. This new shuttle vector system should prove useful in identifying virulence factors from these organisms.
Acknowledgments
This investigation was supported by National Institutes of Health grant DE09821.
REFERENCES
- 1.Arakawa S, Kuramitsu H K. Cloning and sequence analysis of a chymotrypsinlike protease from Treponema denticola. Infect Immun. 1994;62:3424–3433. doi: 10.1128/iai.62.8.3424-3433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attwood G T, Brooker J D. Complete nucleotide sequence of a Selenomonas ruminantium plasmid and definition of a region necessary for its replication in Escherichia coli. Plasmid. 1992;28:123–129. doi: 10.1016/0147-619x(92)90043-a. [DOI] [PubMed] [Google Scholar]
- 3.Chan E C, Klitorinos A, Gharbia S, Caudry S D, Rahal M D, Siboo R. Characterization of a 4.2-kb plasmid isolated from periodontopathic spirochetes. Oral Microbiol Immunol. 1996;11:365–368. doi: 10.1111/j.1399-302x.1996.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 3a.Chauhan, S., and H. K. Kuramitsu. Unpublished data.
- 4.Fletcher H M, Schenkein H A, Morgan R M, Bailey K A, Berry C R, Macrina F L. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect Immun. 1995;63:1521–1528. doi: 10.1128/iai.63.4.1521-1528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isaacs R D, Hanke J H, Guzman-Verduzco L M, Newport G, Agabian N, Norgard M V, Lukehart S A, Radolf J D. Molecular cloning and DNA sequence analysis of the 37-kilodalton endoflagellar sheath protein gene of Treponema pallidum. Infect Immun. 1989;57:3403–3411. doi: 10.1128/iai.57.11.3403-3411.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaacs R D, Radolf J D. Expression in Escherichia coli of the 37-kilodalton endoflagellar sheath protein of Treponema pallidum by use of the polymerase chain reaction and a T7 expression system. Infect Immun. 1990;58:2025–2034. doi: 10.1128/iai.58.7.2025-2034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivic A, MacDougall J, Russell R R, Penn C W. Isolation and characterization of a plasmid from Treponema denticola. FEMS Microbiol Lett. 1991;62:189–193. doi: 10.1016/0378-1097(91)90156-5. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Kuramitsu H K. Development of a gene transfer system in Treponema denticola by electroporation. Oral Microbiol Immunol. 1996;11:161–165. doi: 10.1111/j.1399-302x.1996.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Ruby J, Charon N, Kuramitsu H. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J Bacteriol. 1996;178:3664–3667. doi: 10.1128/jb.178.12.3664-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limberger, R. J. Personal communication.
- 11.Listgarten M A, Hellden L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978;5:115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- 12.Loesche W J, Laughon B E. Role of spirochetes in periodontal diseases. In: Mergenhagen G A, editor. Host-parasite interactions in periodontal diseases. Washington, D.C: American Society for Microbiology; 1982. pp. 67–75. [Google Scholar]
- 13.Lukehart S A, Tam M R, Hom J, Baker-Zander S A, Holmes K K, Nowinski R C. Characterization of monoclonal antibodies to Treponema pallidum. J Immunol. 1985;134:585–592. [PubMed] [Google Scholar]
- 14.Sato Y, Yamamoto Y, Suzuki R, Kizaki H, Kuramitsu H K. Construction of scrA::lacZ gene fusions to investigate regulation of the sucrose PTS of Streptococcus mutants. FEMS Microbiol Lett. 1991;63:339–345. doi: 10.1111/j.1574-6968.1991.tb04552.x. [DOI] [PubMed] [Google Scholar]
- 15.Schouls L M, van der Heide H G, van Embden J D. Characterization of the 35-kilodalton Treponema pallidum subsp. pallidum recombinant lipoprotein TmpC and antibody response to lipidated and nonlipidated T. pallidum antigens. Infect Immun. 1991;59:3536–3546. doi: 10.1128/iai.59.10.3536-3546.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simonson L G, Goodman C H, Bial J J, Morton H E. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988;56:726–728. doi: 10.1128/iai.56.4.726-728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]