ABSTRACT
This retrospective cohort study evaluated the comparative vaccine effectiveness (cVE) of licensed standard‐dose cell‐based versus egg‐based influenza vaccines in preventing influenza hospitalization among adults 18–64 years during the 2022–2023 season. The cohort included eligible Kaiser Permanente Southern California members who received ≥ 1 dose of influenza vaccine (n = 848,334). The adjusted cVE against influenza hospitalization was −10.1% (95% CI: −49.8%, 37.8%) in the 18‐ to 49‐year‐old cohort. In the 50‐ to 64‐year‐old cohort, the adjusted cVE was 14.9% (−33.8%, 52.1%). Cell‐based and egg‐based influenza vaccines conferred comparable protection against influenza hospitalization in adults 18–64 years of age in the 2022–2023 season.
Keywords: epidemiology, Influenza, influenza vaccine, vaccine effectiveness
1. Introduction
Seasonal influenza causes significant clinical burden with an estimated 5 million cases of severe illness and up to 650,000 deaths worldwide [1, 2]. Vaccines represent the best option for prevention and control of influenza. In individuals younger than 65 years of age, the available influenza vaccines in the United States include inactivated influenza vaccine (IIV), recombinant influenza vaccine, and live attenuated influenza vaccine, with no preferential recommendation [3]. IIVs, including egg‐based and cell‐based vaccines, are widely produced and administered in the United States. Egg‐based IIVs represent the majority of the influenza vaccines available on the market. However, there are several limitations to this method of manufacturing, including the length of production, production capacity, and possibility of egg‐adapted changes occurring during production [4, 5].
Some observational studies have reported greater protection against influenza or influenza‐related medical encounters among adults who received cell‐based compared to egg‐based influenza vaccines [6]. However, there was considerable heterogeneity in these observations across study design, study setting, age group, and influenza season. The purpose of this analysis was to evaluate the comparative vaccine effectiveness (cVE) of the licensed cell‐based versus egg‐based standard‐dose (SD) influenza vaccines in preventing influenza‐related hospitalization among adults younger than 65 years during the 2022–2023 influenza season.
2. Methods
2.1. Study Setting
Kaiser Permanente Southern California (KPSC) is a large, integrated health care system with over 4.8 million members with demographic and socioeconomic characteristics representative of the population of Southern California [7]. Comprehensive patient information, including vaccinations, laboratory tests, diagnoses, and procedures, are captured in electronic health records (EHR). Vaccinations received outside of KPSC are imported into the EHR from external sources, including the California Immunization Registry, Care Everywhere, claims, and vaccination self‐reports with valid documentation. Care received outside KPSC is added to the EHR through claims reimbursement. This study was approved by the KPSC Institutional Review Board, with a waiver of informed consent.
2.2. Study Population
The study population included adults 18–64 years of age who received ≥ 1 dose of influenza vaccine during the accrual period 08/01/2022 to 12/31/2022, with follow‐up until 5/20/2023. The 2022–2023 season was characterized by early influenza activity that peaked in December 2022 [8]. The index date was defined as the date when the first influenza vaccination was administered during the accrual period. Individuals were required to have continuous KPSC membership (allowing for a 31‐day gap) for at least one year prior to and 14 days following the index date (Figure 1). Individuals were followed from 14 days after the index date until the outcome of interest (influenza‐related hospitalization), death, disenrollment (allowing for a 31‐day gap), receipt of another dose of influenza vaccine, or end of follow‐up, whichever came first. We excluded individuals who: (1) received an influenza vaccine ≤ 180 days prior to the index date; or (2) had evidence of influenza infection, including a listed diagnosis code or polymerase chain reaction (PCR)‐positive test ≤ 180 days prior to or within 14 days following the index date.
FIGURE 1.
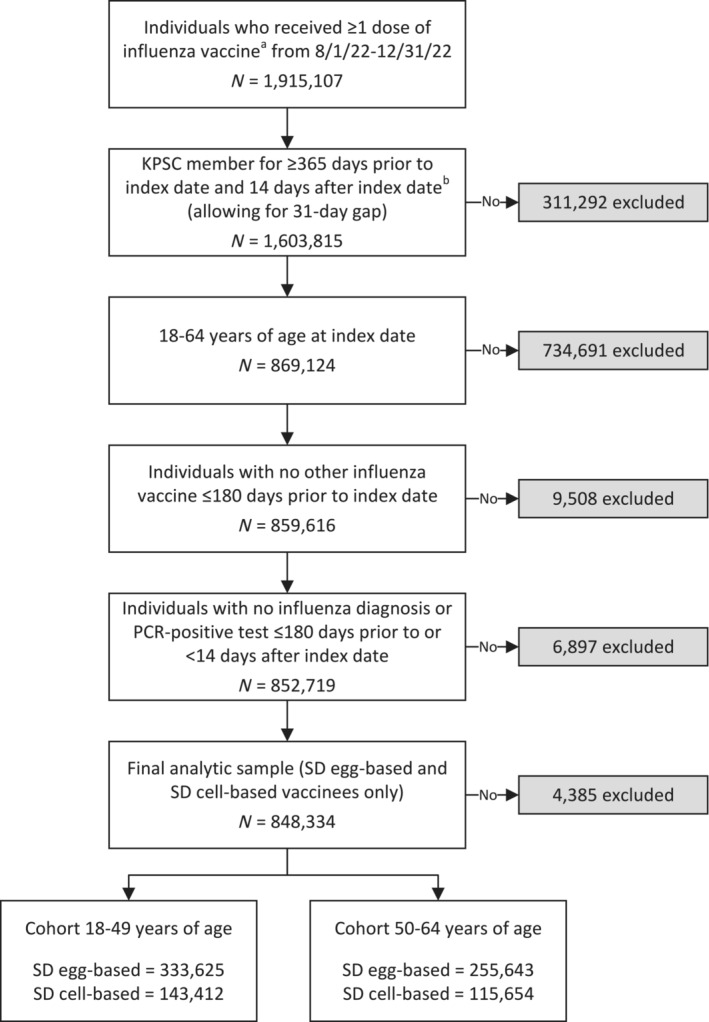
Flow diagram for the analytic cohort. KPSC = Kaiser Permanente Southern California; PCR = polymerase chain reaction; SD = standard dose. aInfluenza vaccines included SD egg‐based (CVX 150 and 158) and SD cell‐based (CVX 171 and 186) vaccines. bIndex date was defined as the date of the first dose of influenza vaccine received during the accrual period, based on the CVX codes above. Non‐index influenza vaccinations were not limited to the CVX codes above for identifying influenza vaccinations prior to index date (for exclusion criteria and covariates) and after index date (for censoring).
2.3. Exposure and Outcome
The exposure of interest was receipt of a pre‐specified licensed influenza vaccine during the accrual period identified using CVX (vaccine administered) codes (SD egg‐based = 150 and 158; SD cell‐based = 171 and 186). If an individual received > 1 influenza vaccine during the accrual period, follow‐up time related to the first vaccine was included, and the individual was censored at the time of receipt of the second vaccine. The primary outcome was PCR‐confirmed influenza‐related hospitalization, defined as a positive PCR test collected between 14 days prior to and 3 days following the inpatient admission date with an acute respiratory infection code [9].
2.4. Statistical Analysis
The study population was stratified into adults 18–49 and 50–64 years of age for statistical analysis. Baseline characteristics were described for each vaccine group; categorical variables were compared using the chi‐square test, and continuous variables were compared using the Kruskal–Wallis test. Stabilized inverse probability of treatment weighting (IPTW) was used to adjust for potential confounders [10]. Absolute standardized differences (ASD) were computed to assess the balance of covariates before and after weighting. An ASD < 0.10 was considered a negligible difference.
Weighted Cox proportional hazards regression models were used to estimate adjusted hazard ratios (aHR). Comparative vaccine effectiveness (cVE) (%) was calculated as (1 − aHR) × 100 when aHR ≤ 1, and ([1/aHR] − 1) × 100 when aHR > 1. All analyses were conducted using SAS (version 9.4; SAS Institute Inc, Cary, NC).
3. Results
Among the 1,915,107 individuals who received a dose of influenza vaccine during the accrual period, there were 477,037 individuals in the 18‐ to 49‐year‐old cohort and 371,297 in the 50‐ to 64‐year‐old cohort who met the eligibility criteria (Figure 1).
Patients were predominantly managed within the KPSC system at KPSC facilities where 795,169 (93.7%) of individuals included in this study received their influenza vaccines and where 105 (93.8%) of the 112 PCR‐confirmed influenza‐related hospitalizations were admitted. All patients with PCR‐confirmed influenza‐related hospitalizations had influenza A.
3.1. 18‐ to 49‐Year‐Old Cohort
In the 18–49‐year‐old cohort overall, 61.1% were female, 44.6% were Hispanic, and the median age was 37 years (interquartile range [IQR] 29–43; Table 1). In this age group, 333,625 (69.9%) received the egg‐based vaccine. The egg‐based and cell‐based vaccinees were similar in demographic and clinical characteristics. The cell‐based vaccinees received their vaccines earlier compared to egg‐based vaccinees, at 69.4% and 65.8%, respectively, during August–October 2022 (Table S1). Baseline demographic characteristics, clinical characteristics, and healthcare utilization were well balanced (ASD < 0.1) after IPTW.
TABLE 1.
Characteristics of influenza vaccine recipients 18–49 and 50–64 years of age by vaccine type (after inverse probability of treatment weighting).
| n (%) | 18–49 years | 50–64 years | ||||||
|---|---|---|---|---|---|---|---|---|
| SD egg‐based | SD cell‐based | p value | ASD | SD egg‐based | SD cell‐based | p value | ASD | |
| n = 333,625 | n = 143,412 | n = 255,643 | n = 115,654 | |||||
| Demographic characteristics | ||||||||
| Age at index date, years | 0.926 | 0.000 | 0.810 | 0.001 | ||||
| Mean (std dev) | 35.6 (9.0) | 35.6 (9.0) | 57.4 (4.3) | 57.4 (4.3) | ||||
| Median (Q1, Q3) | 37 (29, 43) | 37 (29, 43) | 58 (54, 61) | 58 (54, 61) | ||||
| Min, max | 18, 49 | 18, 49 | 50, 64 | 50, 64 | ||||
| Age at index date, years | 0.423 | 0.004 | 0.770 | 0.001 | ||||
| 18–29 | 89,842 (26.9) | 38,808 (27.1) | N/A | N/A | ||||
| 30–39 | 112,293 (33.7) | 48,007 (33.5) | N/A | N/A | ||||
| 40–49 | 131,490 (39.4) | 56,597 (39.5) | N/A | N/A | ||||
| 50–59 | N/A | N/A | 160,538 (62.8) | 72,686 (62.8) | ||||
| 60–64 | N/A | N/A | 95,105 (37.2) | 42,968 (37.2) | ||||
| Sex | 0.907 | 0.000 | 0.969 | 0.000 | ||||
| Female | 203,787 (61.1) | 87,626 (61.1) | 141,495 (55.3) | 64,005 (55.3) | ||||
| Male | 129,838 (38.9) | 55,786 (38.9) | 114,148 (44.7) | 51,649 (44.7) | ||||
| Race/ethnicity | 0.723 | 0.005 | 0.815 | 0.004 | ||||
| Non‐Hispanic White | 81,286 (24.4) | 35,187 (24.5) | 82,048 (32.1) | 37,331 (32.3) | ||||
| Non‐Hispanic Black | 15,141 (4.5) | 6502 (4.5) | 18,343 (7.2) | 8293 (7.2) | ||||
| Hispanic | 148,706 (44.6) | 63,646 (44.4) | 102,332 (40.0) | 46,076 (39.8) | ||||
| Non‐Hispanic Asian | 59,937 (18.0) | 25,792 (18.0) | 38,777 (15.2) | 17,561 (15.2) | ||||
| Other/unknown | 28,555 (8.6) | 12,285 (8.6) | 14,142 (5.5) | 6393 (5.5) | ||||
| Medicaid | 38,146 (11.4) | 16,391 (11.4) | 0.967 | 0.000 | 19,547 (7.6) | 8849 (7.7) | 0.815 | 0.004 |
| Neighborhood median household income | 0.890 | 0.003 | 0.885 | 0.004 | ||||
| < $40,000 | 4997 (1.5) | 2148 (1.5) | 3732 (1.5) | 1686 (1.5) | ||||
| $40,000–$59,999 | 46,136 (13.8) | 19,688 (13.7) | 33,442 (13.1) | 14,989 (13.0) | ||||
| $60,000–$79,999 | 74,178 (22.2) | 31,827 (22.2) | 54,416 (21.3) | 24,608 (21.3) | ||||
| ≥ $80,000 | 207,784 (62.3) | 89,520 (62.4) | 163,358 (63.9) | 74,052 (64.0) | ||||
| Unknown | 531 (0.2) | 229 (0.2) | 696 (0.3) | 319 (0.3) | ||||
| Smoking a | 0.976 | 0.001 | 0.995 | 0.000 | ||||
| No | 263,105 (78.9) | 113,062 (78.8) | 193,424 (75.7) | 87,490 (75.6) | ||||
| Yes | 36,477 (10.9) | 15,709 (11.0) | 48,072 (18.8) | 21,764 (18.8) | ||||
| Unknown | 34,043 (10.2) | 14,641 (10.2) | 14,147 (5.5) | 6400 (5.5) | ||||
| Clinical characteristics | ||||||||
| Body mass index a , kg/m2 | 0.993 | 0.002 | 1.000 | 0.001 | ||||
| < 18.5 | 4557 (1.4) | 1971 (1.4) | 1557 (0.6) | 710 (0.6) | ||||
| 18.5 to < 25 | 79,831 (23.9) | 34,382 (24.0) | 48,830 (19.1) | 22,114 (19.1) | ||||
| 25 to < 30 | 85,028 (25.5) | 36,567 (25.5) | 81,159 (31.7) | 36,720 (31.7) | ||||
| ≥ 30 | 116,957 (35.1) | 50,207 (35.0) | 102,373 (40.0) | 46,280 (40.0) | ||||
| Unknown | 47,252 (14.2) | 20,284 (14.1) | 21,724 (8.5) | 9831 (8.5) | ||||
| Charlson comorbidity score b , c | 0.994 | 0.002 | 0.874 | 0.002 | ||||
| Mean (std dev) | 0.3 (0.8) | 0.3 (0.8) | 0.74 (1.43) | 0.7 (1.4) | ||||
| Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) | 0 (0, 1) | ||||
| Min, max | 0, 16 | 0, 13 | 0, 17 | 0, 16 | ||||
| Charlson comorbidity score b , c | 0.983 | 0.001 | 0.994 | 0.000 | ||||
| 0 | 279,325 (83.7) | 120,075 (83.7) | 164,922 (64.5) | 74,634 (64.5) | ||||
| 1 | 39,214 (11.8) | 16,839 (11.7) | 48,449 (19.0) | 21,907 (18.9) | ||||
| ≥ 2 | 15,086 (4.5) | 6499 (4.5) | 42,272 (16.5) | 19,113 (16.5) | ||||
| Frailty index b , d | 0.608 | 0.003 | 0.635 | 0.002 | ||||
| Mean (std dev) | 0.1 (0.0) | 0.1 (0.0) | 0.1 (0.0) | 0.1 (0.0) | ||||
| Median (Q1, Q3) | 0.1 (0.1, 0.1) | 0.1 (0.1, 0.1) | 0.1 (0.1, 0.1) | 0.1 (0.1, 0.1) | ||||
| Min, max | 0.0, 0.4 | 0.1, 0.4 | 0.0, 0.4 | 0.0, 0.4 | ||||
| Frailty index b , d | 0.983 | 0.001 | 1.000 | 0.000 | ||||
| Quartile 1 | 83,382 (25.0) | 35,805 (25.0) | 62,622 (24.5) | 28,312 (24.5) | ||||
| Quartile 2 | 45,896 (13.8) | 19,690 (13.7) | 65,184 (25.5) | 29,498 (25.5) | ||||
| Quartile 3 | 120,932 (36.2) | 51,996 (36.3) | 63,940 (25.0) | 28,932 (25.0) | ||||
| Quartile 4, most frail | 83,415 (25.0) | 35,921 (25.0) | 63,897 (25.0) | 28,911 (25.0) | ||||
| Chronic diseases b | ||||||||
| Kidney disease | 2468 (0.7) | 1061 (0.7) | 0.991 | 0.000 | 9224 (3.6) | 4162 (3.6) | 0.883 | 0.001 |
| Heart disease | 1558 (0.5) | 674 (0.5) | 0.882 | 0.001 | 6475 (2.5) | 2926 (2.5) | 0.961 | 0.000 |
| Liver disease | 7746 (2.3) | 3341 (2.3) | 0.870 | 0.001 | 12,162 (4.8) | 5502 (4.8) | 0.997 | 0.000 |
| Diabetes | 18,387 (5.5) | 7890 (5.5) | 0.891 | 0.000 | 51,824 (20.3) | 23,403 (20.2) | 0.797 | 0.001 |
| Immunocompromised e | 8878 (2.7) | 3818 (2.7) | 0.983 | 0.000 | 10,595 (4.1) | 4797 (4.1) | 0.963 | 0.000 |
| Respiratory conditions b | ||||||||
| Chronic obstructive pulmonary disease, chronic bronchitis, or emphysema | 3912 (1.2) | 1691 (1.2) | 0.854 | 0.001 | 6167 (2.4) | 2804 (2.4) | 0.819 | 0.001 |
| Asthma | 22,261 (6.7) | 9557 (6.7) | 0.913 | 0.000 | 16,762 (6.6) | 7591 (6.6) | 0.937 | 0.000 |
| Healthcare utilization | ||||||||
| Number of outpatient and virtual visits b | 0.995 | 0.001 | 0.999 | 0.001 | ||||
| 0 | 3912 (1.2) | 1691 (1.2) | 10,513 (4.1) | 4766 (4.1) | ||||
| 1–4 | 22,261 (6.7) | 9557 (6.7) | 69,446 (27.2) | 31,419 (27.2) | ||||
| 5–10 | 3912 (1.2) | 1691 (1.2) | 84,261 (33.0) | 38,130 (33.0) | ||||
| ≥ 11 | 22,261 (6.7) | 9557 (6.7) | 91,422 (35.8) | 41,338 (35.7) | ||||
| Number of emergency department visits b | 0.979 | 0.001 | 0.998 | 0.000 | ||||
| 0 | 284,111 (85.2) | 122,120 (85.2) | 215,586 (84.3) | 97,529 (84.3) | ||||
| 1 | 36,296 (10.9) | 15,623 (10.9) | 29,046 (11.4) | 13,147 (11.4) | ||||
| ≥ 2 | 13,218 (4.0) | 5669 (4.0) | 11,011 (4.3) | 4977 (4.3) | ||||
| Number of hospitalizations b | 0.987 | 0.001 | 0.986 | 0.001 | ||||
| 0 | 317,205 (95.1) | 136,338 (95.1) | 245,846 (96.2) | 111,210 (96.2) | ||||
| 1 | 14,195 (4.3) | 6116 (4.3) | 7355 (2.9) | 3339 (2.9) | ||||
| ≥ 2 | 2226 (0.7) | 958 (0.7) | 2442 (1.0) | 1105 (1.0) | ||||
| Preventive care b , f | 122,802 (36.8) | 52,761 (36.8) | 0.902 | 0.000 | 156,647 (61.3) | 70,892 (61.3) | 0.902 | 0.000 |
| Receipt of influenza vaccine g | 244,696 (73.3) | 105,140 (73.3) | 0.822 | 0.001 | 213,891 (83.7) | 96,751 (83.7) | 0.926 | 0.000 |
| Receipt of COVID‐19 vaccine b | 249,791 (74.9) | 107,350 (74.9) | 0.896 | 0.000 | 212,000 (82.9) | 95,872 (82.9) | 0.806 | 0.001 |
| Concomitant vaccines h | 61,917 (18.6) | 26,582 (18.5) | 0.849 | 0.001 | 69,080 (27.0) | 31,303 (27.1) | 0.780 | 0.001 |
| Month of vaccination | 0.982 | 0.002 | 0.986 | 0.002 | ||||
| August 2022 | 11,838 (3.5) | 5088 (3.5) | 10,551 (4.1) | 4773 (4.1) | ||||
| September 2022 | 105,342 (31.6) | 45,326 (31.6) | 93,937 (36.7) | 42,532 (36.8) | ||||
| October 2022 | 105,870 (31.7) | 45,415 (31.7) | 84,077 (32.9) | 37,959 (32.8) | ||||
| November 2022 | 72,285 (21.7) | 31,050 (21.7) | 45,403 (17.8) | 20,527 (17.7) | ||||
| December 2022 | 38,290 (11.5) | 16,533 (11.5) | 21,675 (8.5) | 9863 (8.5) | ||||
Abbreviations: ASD = absolute standardized difference; Q1 = quartile 1; Q3 = quartile 3; SD = standard dose; std dev = standard deviation.
Defined in the two years prior to index date.
Defined in the one year prior to index date.
Possible range: 0–29 [11].
Possible range: 0–1 [12].
HIV/AIDS, leukemia/lymphoma, congenital/other immunodeficiencies, asplenia/hyposplenia, hematopoietic stem cell transplant/solid organ transplant, and receipt of immunosuppressive medications.
Includes screenings, preventive physical exams, and wellness visits.
During previous influenza season (August 2021 to April 2022).
Administered on index date.
The incidence rate (IR) of PCR‐confirmed influenza hospitalization per 1000 person‐years was 0.2 (95% confidence interval [CI]: 0.1–0.4) in those who received cell‐based vaccines and 0.2 (95% CI: 0.2–0.3) in those who received egg‐based vaccines (Table S2). The adjusted cVE was −10.1% (95% CI: −49.8%, 37.8%; Figure 2).
FIGURE 2.
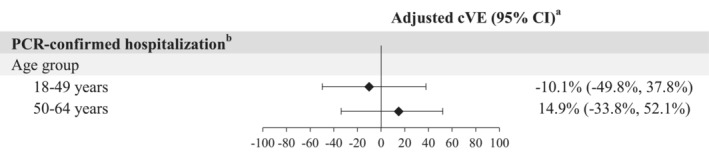
Adjusted cVE for the SD cell‐based influenza vaccine compared to the SD egg‐based vaccine. CI = confidence interval; cVE = comparative vaccine effectiveness (SD cell‐based vs. SD egg‐based); PCR = polymerase chain reaction; SD = standard dose. aWeighted using stabilized inverse probability of treatment weights. When the hazard ratio or its 95% CI was > 1, the cVE (%) or its 95% CI was transformed as ([1/hazard ratio] − 1) × 100. bPCR‐confirmed influenza‐related hospitalization (a positive PCR test collected between −14 and +3 days from the inpatient admission date) with an acute respiratory infection code [9].
3.2. 50‐ to 64‐Year‐Old Cohort
In the 50‐ to 64‐year‐old cohort overall, 55.4% were female, 40.1% were Hispanic, and the median age was 58 years (IQR 54–61; Table 1). In this age group, 255,643 (68.9%) received egg‐based vaccines. The egg‐based and cell‐based vaccinees were similar in most demographic and clinical characteristics, although cell‐based vaccinees were vaccinated slightly earlier than egg‐based vaccinees, at 75.1% and 73.1%, respectively, during August to October 2022 (Table S1). Baseline demographic characteristics, clinical characteristics, and healthcare utilization were well balanced (ASD < 0.1) after IPTW. The IR of PCR‐confirmed influenza hospitalization per 1000 person‐years was 0.2 (95% CI: 0.1–0.4) with cell‐based vaccination and 0.3 (95% CI: 0.2–0.4) with egg‐based vaccination (Table S2). The adjusted cVE was 14.9% (95% CI: −33.8%, 52.1%; Figure 2).
4. Discussion
In this study, we evaluated the cVE of the SD cell‐based influenza vaccine, compared to the SD egg‐based vaccine. We observed that cell‐based and egg‐based influenza vaccines conferred comparable protection against PCR‐confirmed influenza‐related hospitalization in adults 18–64 years of age in the 2022–2023 season.
While this analysis reports the cVE of egg‐ and cell‐based influenza vaccines, influenza vaccinations provided moderate protection against influenza‐related hospitalizations in adults aged 18–64 in the 2022–2023 season (VE: 23%; 95% CI: 4%–39%) [13]. Previous studies have found that cell‐based vaccines may provide moderately higher levels of protection against influenza‐related outcomes in adults younger than 64 years. In a meta‐analysis of IIVs, the overall relative VE of cell‐based vaccines in preventing medical encounters, compared to egg‐based vaccines, in persons 4–64 years of age was estimated to be 16.2% (95% CI: 7.6%–24.8%), 6.1% (95% CI: 4.9%–7.3%), 10.2% (95% CI: 6.3%–14.0%) for the 2017–2018, 2018–2019, and 2019–2020 seasons, respectively [6]. However, there was considerable heterogeneity across studies (I 2 = 79%), which may be related to variations in study design, study setting, age group, and influenza season.
All PCR‐confirmed influenza‐related hospitalizations were caused by influenza A, which was the predominant circulating virus subtype in the 2022–2023 influenza season [8]. Based on recommendations from WHO, the SD egg‐based and SD cell‐based influenza vaccines for the season consisted of comparable influenza A (H1N1 and H3N2) and influenza B (Victoria and Yamagata) strains [8]. Any differences in vaccine performance should not have been based on potential strain mismatch.
Our study has several notable strengths, including the use of a large cohort with comprehensive capture of demographic and clinical information by EHR. Second, confounding by indication was minimized as all individuals in the study were vaccinated. The distribution of covariates, except index month, was well balanced even before weighting (Table S1), and vaccine type administered was based on availability as opposed to patient choice. Finally, PCR results were used to increase the specificity of our outcome definition.
Nevertheless, our study has some limitations. Despite incentivizing KPSC members to receive vaccines within the KPSC system and the importing of external vaccination data, it is possible that a small number of influenza vaccines received outside the system could be missed. Misclassification of influenza‐related hospitalization is possible due to the reliance on molecular test results; however, molecular tests for influenza are highly sensitive [14]. Outcome misclassification was likely non‐differential by vaccine type. Although we adjusted for covariates, residual confounding may still exist but is likely to be minimal based on good balance of most measured confounders even prior to weighting. Our results address cVE against hospitalized influenza, but we did not assess less severe influenza‐associated medical encounters. Finally, these findings may not be generalizable to individuals who receive care in different types of health systems in the United States, in uninsured populations, or in other countries, but KPSC members are racially and ethnically diverse and generally representative of the Southern California population [15].
In summary, our results indicate that cell‐based and egg‐based influenza vaccines conferred comparable protection against PCR‐confirmed influenza‐related hospitalization in adults 18–64 years of age during the 2022–2023 season. Ongoing evaluations of existing influenza vaccines are crucial in guiding recommendations, revision of current vaccine candidates, and development of new influenza vaccines that balance protection, risk, and ease and speed of production.
Author Contributions
Emily Rayens: writing – original draft, investigation. Jennifer H. Ku: investigation, conceptualization, methodology. Lina S. Sy: conceptualization, methodology, investigation, project administration. Lei Qian: conceptualization, methodology, formal analysis, investigation, data curation. Bradley K. Ackerson: conceptualization, methodology, investigation. Yi Luo: conceptualization, methodology, investigation, formal analysis, data curation. Julia E. Tubert: conceptualization, methodology, investigation, formal analysis, data curation. Gina S. Lee: investigation, project administration. Punam P. Modha: investigation, project administration. Yoonyoung Park: conceptualization, methodology, funding acquisition, project administration, investigation, supervision. Tianyu Sun: conceptualization, methodology, investigation. Evan J. Anderson: conceptualization, methodology, investigation, supervision. Hung Fu Tseng: conceptualization, methodology, investigation, funding acquisition, project administration, supervision.
Ethics Statement
The study was approved by the Institutional Review Board (IRB) of Kaiser Permanente Southern California (KPSC) (reference number 13638).
Conflicts of Interest
E.R., J.H.K., L.S.S., L.Q., B.K.A., Y.L., J.E.T., G.S.L., P.P.M., and H.F.T. are employees of Kaiser Permanente Southern California, which has been contracted by Moderna, Inc. to conduct this study. Y.P., T.S., and E.J.A. are employees of and shareholders in Moderna, Inc. E.R. received funding from GlaxoSmithKline unrelated to this manuscript. J.H.K. received funding from GlaxoSmithKline and Moderna unrelated to this manuscript. L.S.S. received funding from GlaxoSmithKline, Dynavax, and Moderna unrelated to this manuscript. L.Q. received funding from GlaxoSmithKline, Dynavax, and Moderna unrelated to this manuscript. B.K.A. received funding from GlaxoSmithKline, Dynavax, Genentech, and Moderna unrelated to this manuscript. Y.L. received funding from GlaxoSmithKline, Pfizer, and Moderna unrelated to this manuscript. J.E.T. received funding from GlaxoSmithKline and Moderna unrelated to this manuscript. G.S.L. received funding from GlaxoSmithKline and Moderna unrelated to this manuscript. P.P.M. received funding from GlaxoSmithKline unrelated to this manuscript. H.F.T. received funding from GlaxoSmithKline and Moderna unrelated to this manuscript; H.F.T. also served on advisory boards for Janssen Pharmaceuticals and Pfizer Inc.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/irv.70025.
Supporting information
Table S1. Characteristics of influenza vaccine recipients 18–49 and 50–64 years of age by vaccine type (before inverse probability of treatment weighting).
Table S2. Incidence rates and comparative vaccine effectiveness of SD cell‐based influenza vaccines in preventing PCR‐confirmed influenza‐related hospitalization.
Acknowledgements
The authors thank the patients of Kaiser Permanente for their partnership with us to improve their health. Their information, collected through our electronic health record systems, leads to findings that help us improve care for our members and can be shared with the larger community.
Funding: This work was supported by Moderna, Inc.
Data Availability Statement
Individual‐level data reported in this study involving human research participants are not publicly shared due to potentially identifying or sensitive patient information. Upon request to the corresponding author [ER], and subject to review and approval of an analysis proposal, Kaiser Permanente Southern California (KPSC) may provide the deidentified aggregate‐level data that support the findings of this study within 6 months. Anonymized data (deidentified data including participant data as applicable) that support the findings of this study may be made available from the investigative team in the following conditions: (1) agreement to collaborate with the study team on all publications, (2) provision of external funding for administrative and investigator time necessary for this collaboration, (3) demonstration that the external investigative team is qualified and has documented evidence of training for human subjects protections, and (4) agreement to abide by the terms outlined in data use agreements between institutions.
References
- 1. Krammer F., Smith G. J. D., Fouchier R. A. M., et al., “Influenza,” Nature Reviews. Disease Primers 4, no. 1 (2018): 3, 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iuliano A. D., Roguski K. M., Chang H. H., et al., “Estimates of Global Seasonal Influenza‐Associated Respiratory Mortality: A Modelling Study,” Lancet 391, no. 10127 (2018): 1285–1300, 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grohskopf L. A., Blanton L. H., Ferdinands J. M., et al., “Prevention and Control of Seasonal Influenza With Vaccines: Recommendations of the Advisory Committee on Immunization Practices ‐ United States, 2022‐23 Influenza Season,” MMWR ‐ Recommendations and Reports 71, no. 1 (2022): 1–28, 10.15585/mmwr.rr7101a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore K. A., Ostrowsky J. T., Kraigsley A. M., et al., “A Research and Development (R&D) Roadmap for Influenza Vaccines: Looking toward the future,” Vaccine 39, no. 45 (2021): 6573–6584, 10.1016/j.vaccine.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 5. Ortiz de Lejarazu‐Leonardo R., Montomoli E., Wojcik R., et al., “Estimation of Reduction in Influenza Vaccine Effectiveness Due to Egg‐Adaptation Changes‐Systematic Literature Review and Expert Consensus,” Vaccines (Basel) 9, no. 11 (2021): 1255, 10.3390/vaccines9111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coleman B. L., Gutmanis I., McGovern I., and Haag M., “Effectiveness of Cell‐Based Quadrivalent Seasonal Influenza Vaccine: A Systematic Review and Meta‐Analysis,” Vaccines (Basel) 11, no. 10 (2023): 1607, 10.3390/vaccines11101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koebnick C., Langer‐Gould A. M., Gould M. K., et al., “Sociodemographic Characteristics of Members of a Large, Integrated Health Care System: Comparison With US Census Bureau Data,” Permanente Journal 16, no. 3 (2012): 37–41, 10.7812/TPP/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control , “Influenza Activity in the United States During the 2022–23 Season and Composition of the 2023–24 Influenza Vaccine”. Centers for Disease Control and Prevention. Updated 2023 September 28, accessed March 21, 2024, https://www.cdc.gov/flu/whats‐new/22‐23‐summary‐technical‐report.html.
- 9. Tenforde M. W., Weber Z. A., DeSilva M. B., et al., “Vaccine Effectiveness Against Influenza‐Associated Urgent Care, Emergency Department, and Hospital Encounters During the 2021‐2022 Season, VISION Network,” The Journal of Infectious Diseases 228, no. 2 (2023): 185–195, 10.1093/infdis/jiad015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tartof S. Y., Qian L., Rieg G. K., et al., “Safety of Seasonal Influenza Vaccination in Hospitalized Surgical Patients: A Cohort Study,” Annals of Internal Medicine 164, no. 9 (2016): 593–599, 10.7326/M15-1667. [DOI] [PubMed] [Google Scholar]
- 11. Quan H., Li B., Couris C. M., et al., “Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries,” American Journal of Epidemiology 173, no. 6 (2011): 676–682, 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 12. Kim D. H., Schneeweiss S., Glynn R. J., Lipsitz L. A., Rockwood K., and Avorn J., “Measuring Frailty in Medicare Data: Development and Validation of a Claims‐Based Frailty Index,” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 73, no. 7 (2018): 980–987, 10.1093/gerona/glx229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tenforde M. W., Weber Z. A., Yang D. H., et al., “Influenza Vaccine Effectiveness Against Influenza‐A‐Associated Emergency Department, Urgent Care, and Hospitalization Encounters Among U.S. Adults, 2022‐2023,” Journal of Infectious Diseases 230 (2023): 141–151, 10.1093/infdis/jiad542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention , “Overview of Influenza Testing Methods: Rapid Molecular Assays,” Updated 2020. August 31. 2023 July 18, https://www.cdc.gov/flu/professionals/diagnosis/overview‐testing‐methods.htm.
- 15. Davis A. C., Voelkel J. L., Remmers C. L., Adams J. L., and McGlynn E. A., “Comparing Kaiser Permanente Members to the General Population: Implications for Generalizability of Research,” Permanente Journal 27 (2023): 87–98, 10.7812/tpp/22.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of influenza vaccine recipients 18–49 and 50–64 years of age by vaccine type (before inverse probability of treatment weighting).
Table S2. Incidence rates and comparative vaccine effectiveness of SD cell‐based influenza vaccines in preventing PCR‐confirmed influenza‐related hospitalization.
Data Availability Statement
Individual‐level data reported in this study involving human research participants are not publicly shared due to potentially identifying or sensitive patient information. Upon request to the corresponding author [ER], and subject to review and approval of an analysis proposal, Kaiser Permanente Southern California (KPSC) may provide the deidentified aggregate‐level data that support the findings of this study within 6 months. Anonymized data (deidentified data including participant data as applicable) that support the findings of this study may be made available from the investigative team in the following conditions: (1) agreement to collaborate with the study team on all publications, (2) provision of external funding for administrative and investigator time necessary for this collaboration, (3) demonstration that the external investigative team is qualified and has documented evidence of training for human subjects protections, and (4) agreement to abide by the terms outlined in data use agreements between institutions.


