Abstract
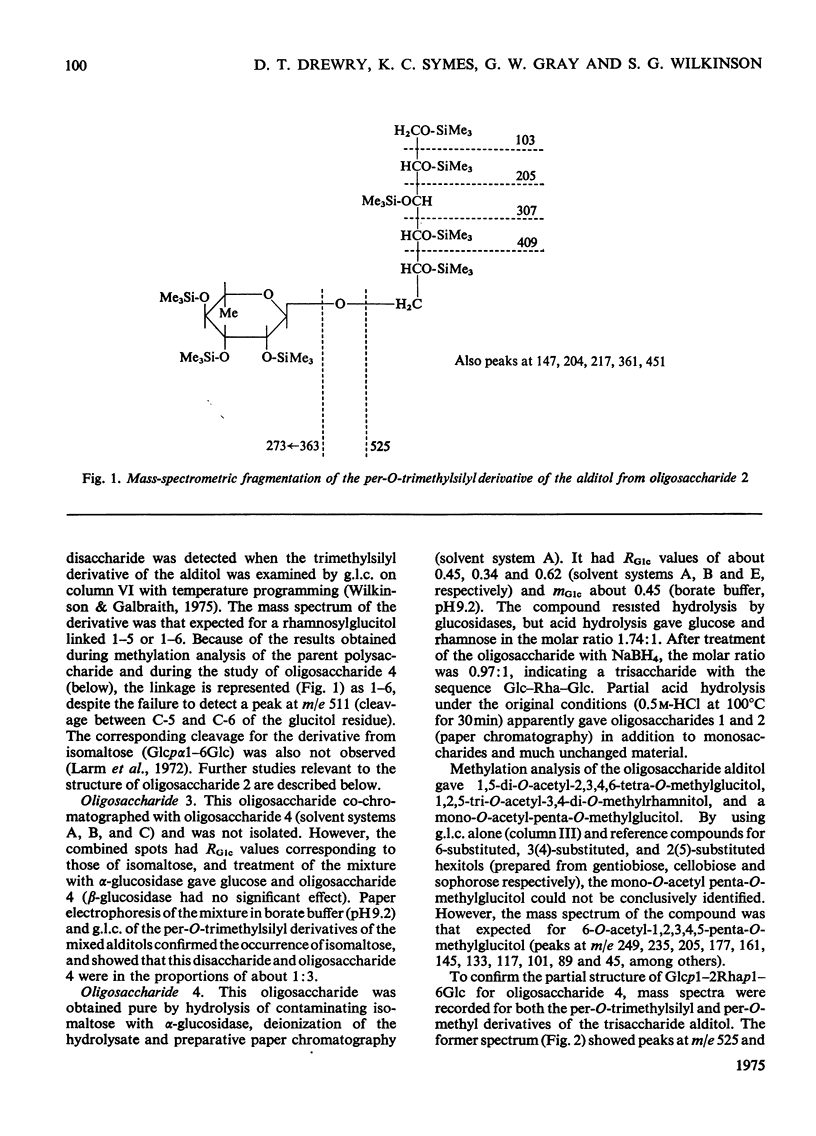
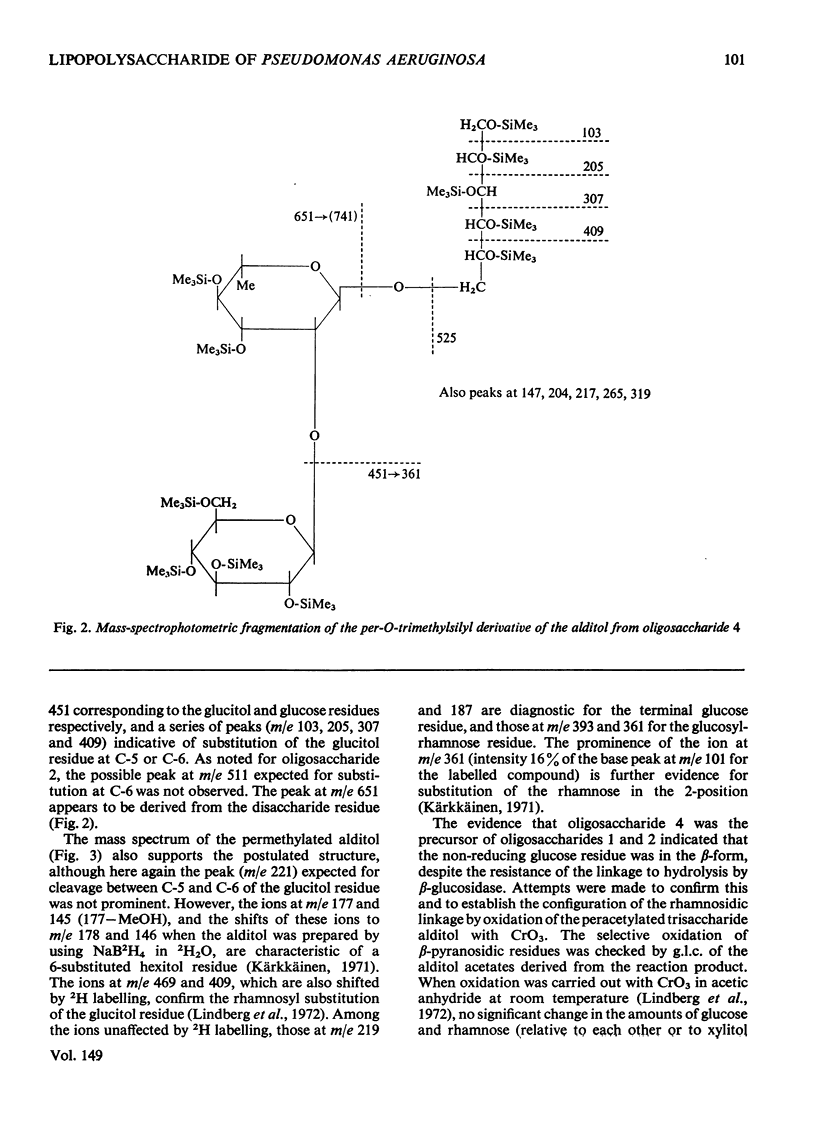
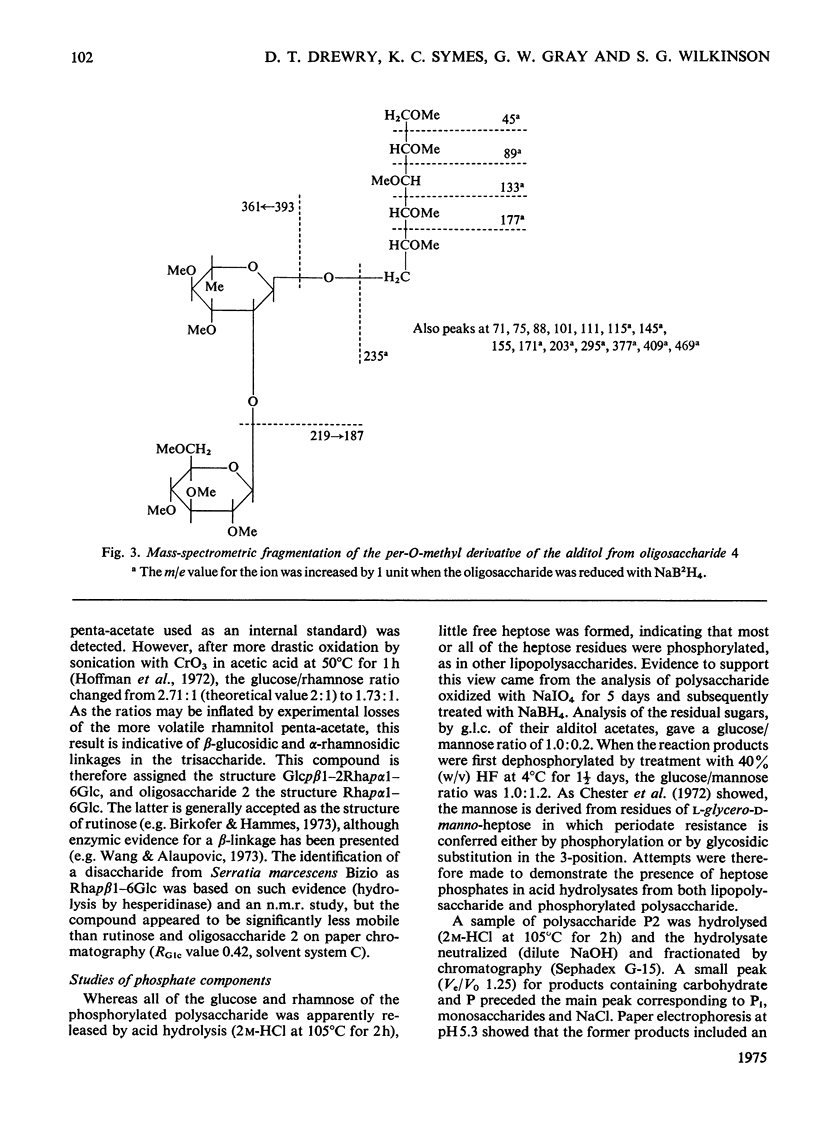
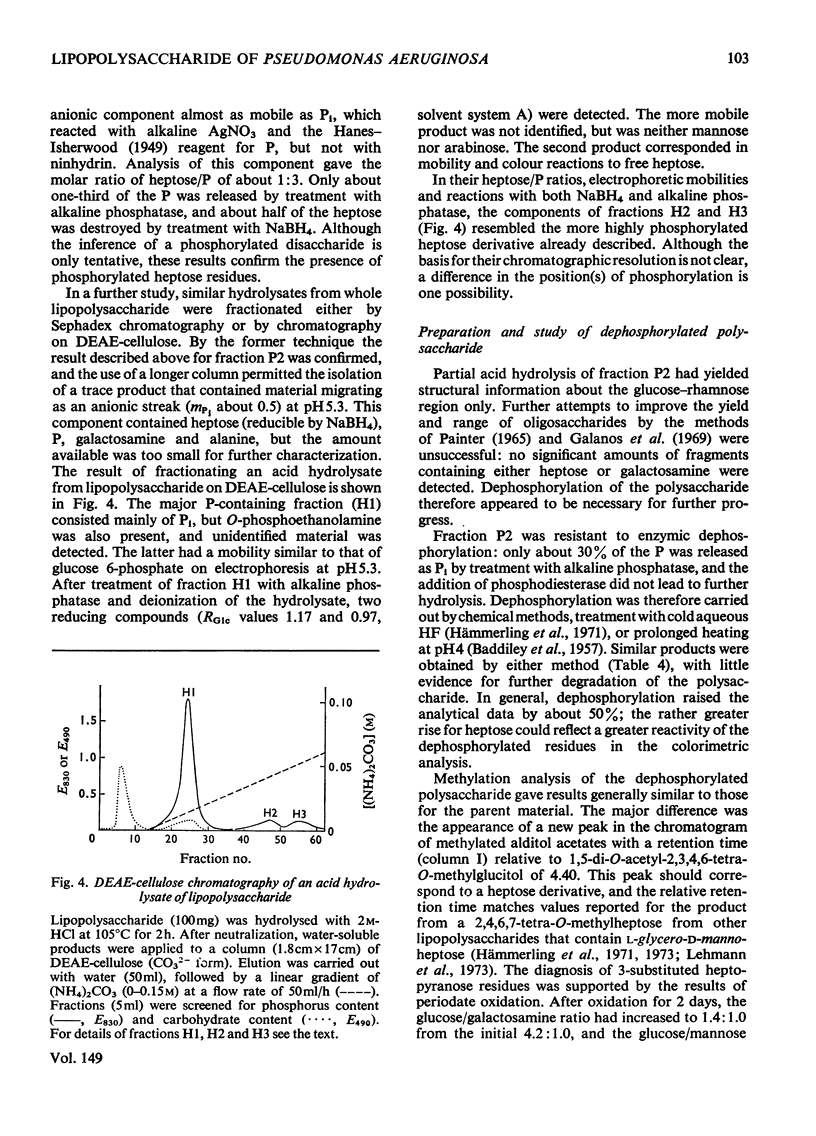
Two polymeric water-soluble fractions were isolated by gel filtration after mild acid hydrolysis of the lipopolysaccharide from Pseudomonas aeruginosa N.C.T.C. 1999. The fraction of higher molecular weight retained the O-antigenic specificity of the lipopolysaccharide and may be 'side-chain' material. This fraction was rich in N (about 10%) and gave several basic amino compounds on acid hydrolysis; fucosamine (at least 2.8% w/w) was the only specifc component identified. The fraction of lower molecular weight was a phosphorylated polysaccharide apparently corresponding to 'core' material. The major components of this fraction and their approximate molar proportions were: glucose (3-4); rhamnose (1); heptose (2); 3-deoxy-2-octulonic acid (1); galactosamine (1); alanine (1-1.5); phosphorus (6-7). In the intact lipopolysaccharide this fraction was probably linked to lipid A via a second residue of 3-deoxy-2-octulonic acid, and probably also contained additional phosphate residues and ethanolamine. The residues of 3-deoxy-2-octulonic acid were apparently substituted in the C-4 or C-5 position, and the phosphorylated heptose residues in the C-3 position. The rhamnose was mainly 2-substituted, though a little 3-substitution was detected. The glucose residues were either unsubstituted or 6-substituted. Four neutral oligosaccharides were produced by partial acid hydrolysis and were characterized by chemical, enzymic, chromatographic and mass-spectrometric methods of analysis. The structures assigned were: Glcpalpha1-6Glc; Glcpbeta1-2Rha; Rhapalpha1-6Glc; Glcpbeta1-2Rhapalpha1-6Glc. The galactosamine was substituted in the C-3 or C-4 position, the attachment of alanine was indicated, and evidence that the amino sugar linked the glucose-rhamnose region to the 'inner core' was obtained.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYSON J. L., MITCHELL T. J. Improved spraying reagents for the detection of sugars on paper chromatograms. Nature. 1951 May 26;167(4256):864–864. doi: 10.1038/167864a0. [DOI] [PubMed] [Google Scholar]
- Björndal H., Lindberg B., Nimmich W. Structural studies on the lipopolysaccharide from Klebsiella K73-O10. I. Methylation analysis, identification and location of 3-O-methyl-L-rhamnose. Acta Chem Scand. 1970;24(9):3414–3415. doi: 10.3891/acta.chem.scand.24-3414. [DOI] [PubMed] [Google Scholar]
- Charon D., Szabó L. The synthesis of 3-deoxy-5-O-methyloctulosonic acid and its behaviour in the Warren reaction. Eur J Biochem. 1972 Aug 18;29(1):184–187. doi: 10.1111/j.1432-1033.1972.tb01973.x. [DOI] [PubMed] [Google Scholar]
- Chaudhari A. S., Bishop C. T., Fielder R. J. Structural studies on the specific type VII pneumococcal polysaccharide. Carbohydr Res. 1972 Nov;25(1):161–172. doi: 10.1016/s0008-6215(00)82756-1. [DOI] [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M., Pitt T. L. The relationship between the O-antigenic lipopolysaccharides and serological specificity in strains of Pseudomonas aeruginosa of different O-serotypes. J Gen Microbiol. 1973 Oct;78(2):305–318. doi: 10.1099/00221287-78-2-305. [DOI] [PubMed] [Google Scholar]
- Drewry D. T., Gray G. W., Wilkinson S. G. Low-molecular-weight solutes released during mild acid hydrolysis of the lipopolysaccharide of Pseudomonas aeruginosa. Biochem J. 1972 Nov;130(1):289–295. doi: 10.1042/bj1300289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry D. T., Gray G. W., Wilkinson S. G. Release of ethanolamine pyrophosphate during mild acid hydrolysis of the lipopolysaccharide of Pseudomonas aeruginosa. Eur J Biochem. 1971 Aug 16;21(3):400–403. doi: 10.1111/j.1432-1033.1971.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Drewry D. T., Lomax J. A., Gray G. W., Wilkinson S. G. Studies of lipid A fractions from the lipopolysaccharides of Pseudomonas aeruginosa and Pseudomonas alcaligenes. Biochem J. 1973 Jul;133(3):563–572. doi: 10.1042/bj1330563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W., Lehmann V., Lüderitz O., Westphal O. Structural investigations on the 2-keto-3-deoxyoctonate region of lipopolysaccharides. Eur J Biochem. 1970 May 1;14(1):175–184. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Fensom A. H., Gray G. W. The chemical composition of the lipopolyacarideof Pseudomonas aeruginosa. Biochem J. 1969 Sep;114(2):185–196. doi: 10.1042/bj1140185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensom A. H., Meadow P. M. Evidence for two regions in the polysaccharide moiety of the lipopolysaccharide of Pseudomonas aeruginosa 8602. FEBS Lett. 1970 Jul 29;9(2):81–84. doi: 10.1016/0014-5793(70)80318-0. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Himmelspach K. The partial acid hydrolysis of polysaccharides: a new method for obtaining oligosaccharides in high yield. Eur J Biochem. 1969 Apr;8(3):332–336. doi: 10.1111/j.1432-1033.1969.tb00532.x. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Hough L., Jones J. V., Wusteman P. On the automated analysis of neutral monosaccharides in glycoproteins and polysaccharides. Carbohydr Res. 1972 Jan;21(1):9–17. doi: 10.1016/s0008-6215(00)81725-5. [DOI] [PubMed] [Google Scholar]
- Hämmerling G., Lehmann V., Lüderitz O. Structural studies on the heptose region of Salmonella lipopolysaccharides. Eur J Biochem. 1973 Oct 18;38(3):453–458. doi: 10.1111/j.1432-1033.1973.tb03079.x. [DOI] [PubMed] [Google Scholar]
- Hämmerling G., Lüderitz O., Westphal O., Mäkelä P. H. Structural investigations on the core polysaccharide of Escherichia coli 0100. Eur J Biochem. 1971 Oct 14;22(3):331–344. doi: 10.1111/j.1432-1033.1971.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Key B. A., Gray G. W., Wilkinson S. G. The purification and chemical composition of the lipopolysaccharide of Pseudomonas alcaligenes. Biochem J. 1970 Dec;120(3):559–566. doi: 10.1042/bj1200559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larm O., Lindberg B., Svensson S., Kabat E. A. Structural studies on pneumococcus type II capsular polysaccharide. Carbohydr Res. 1972 May;22(2):391–397. doi: 10.1016/s0008-6215(00)81290-2. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Hämmerling G., Nurminen M., Minner I., Ruschmann E., Lüderitz O., Kuo T. T., Stocker B. A. A new class of heptose-defective mutant of Salmonella typhimurium. Eur J Biochem. 1973 Jan 15;32(2):268–275. doi: 10.1111/j.1432-1033.1973.tb02607.x. [DOI] [PubMed] [Google Scholar]
- Lindberg B., Lönngren J., Thompson J. L. Structural studies of the Klebsiella type 9 capsular polysaccharide. Carbohydr Res. 1972 Nov;25(1):49–57. doi: 10.1016/s0008-6215(00)82745-7. [DOI] [PubMed] [Google Scholar]
- Lomax J. A., Gray G. W., Wilkinson S. G. Studies of the polysaccharide fraction from the lipopolysaccharide of Pseudomonas alcaligenes. Biochem J. 1974 Jun;139(3):633–643. doi: 10.1042/bj1390633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi M., Yamakawa T. Gas-liquid chromatography of oligosaccharides released from red cell glycosphingolipids by ozonolysis. J Lipid Res. 1973 Nov;14(6):698–700. [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Different core regions in the lipopolysaccharides of O group 8. Eur J Biochem. 1969 Oct;10(3):501–510. doi: 10.1111/j.1432-1033.1969.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Simmons D. A. The immunochemistry of Shigella flexneri O-antigens. The structure and biosynthesis of the O-specific side chains of some representative serotypes. Eur J Biochem. 1969 Dec;11(3):554–575. doi: 10.1111/j.1432-1033.1969.tb00808.x. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Volk W. A., Galanos C., Lüderitz O. The occurrence of 4-amino-4-deoxy-L-arabinose as a constituent in Salmonella lipopolysaccharide preparations. Eur J Biochem. 1970 Dec;17(2):223–229. doi: 10.1111/j.1432-1033.1970.tb01157.x. [DOI] [PubMed] [Google Scholar]
- WARREN L. Thiobarbituric acid spray reaction for deoxy sugars and sialic acids. Nature. 1960 Apr 16;186:237–237. doi: 10.1038/186237a0. [DOI] [PubMed] [Google Scholar]
- Wang C. S., Alaupovic P. Composition and structure of the O-specific side chain of endotoxin from Serratia marcescens Bizio. Biochemistry. 1973 Jan 16;12(2):309–315. doi: 10.1021/bi00726a021. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbraith L., Lightfoot G. A. Cell walls, lipids, and lipopolysaccharides of Pseudomonas species. Eur J Biochem. 1973 Feb 15;33(1):158–174. doi: 10.1111/j.1432-1033.1973.tb02666.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbrath L. Studies of lipopolysaccharides from Pseudomonas aeruginosa. Eur J Biochem. 1975 Mar 17;52(2):331–343. doi: 10.1111/j.1432-1033.1975.tb04001.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G. Studies on the cell walls of pseudomonas species resistant to ethylenediaminetetra-acetic acid. J Gen Microbiol. 1968 Dec;54(2):195–213. doi: 10.1099/00221287-54-2-195. [DOI] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]


