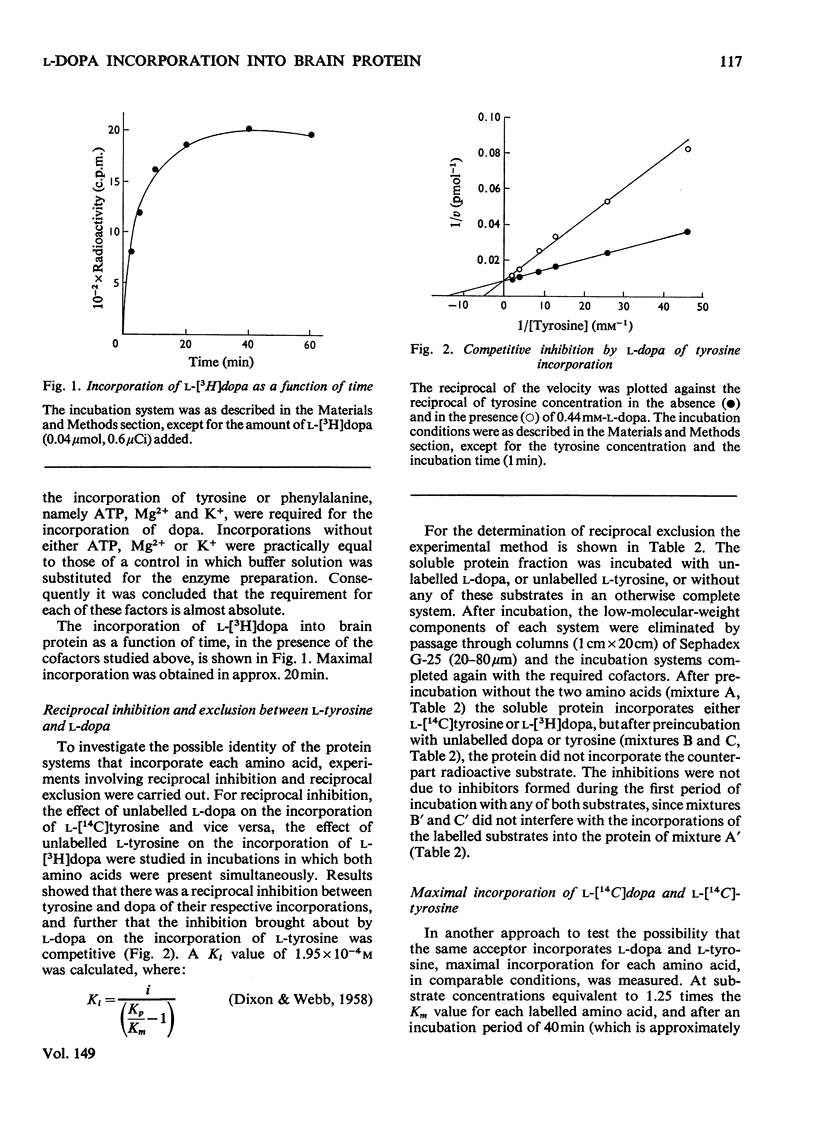
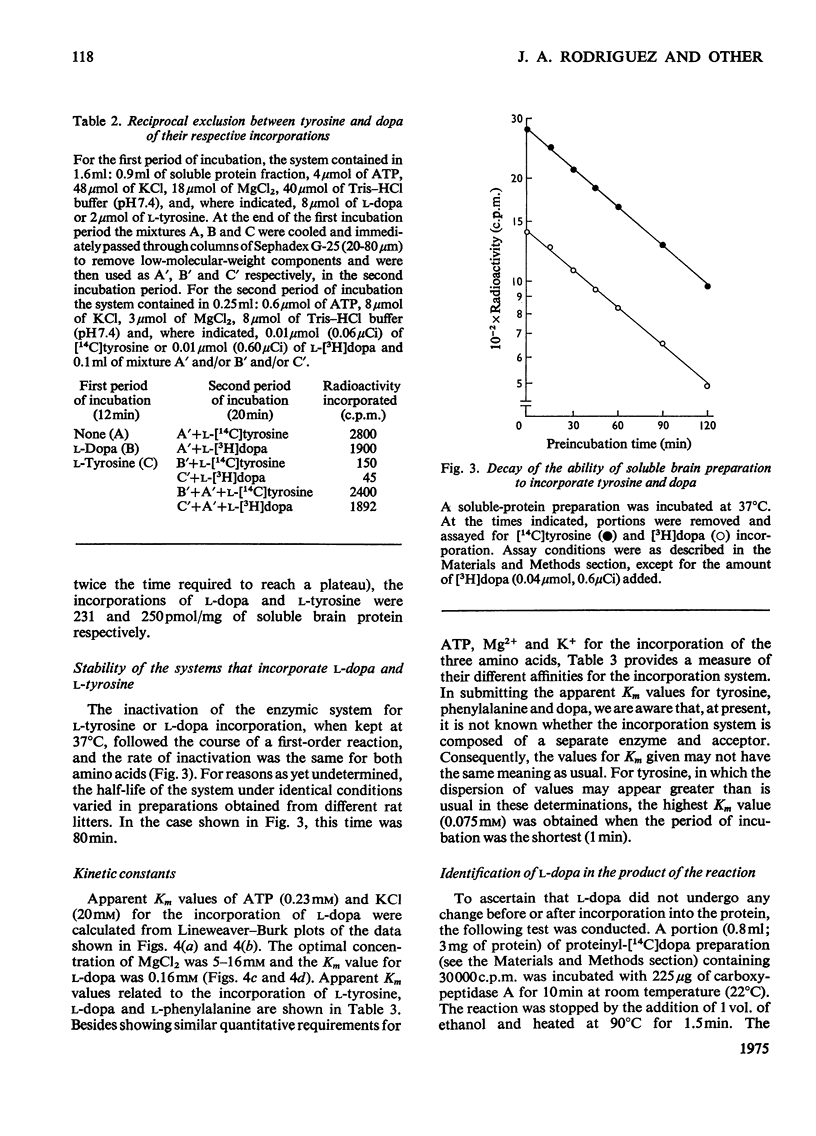
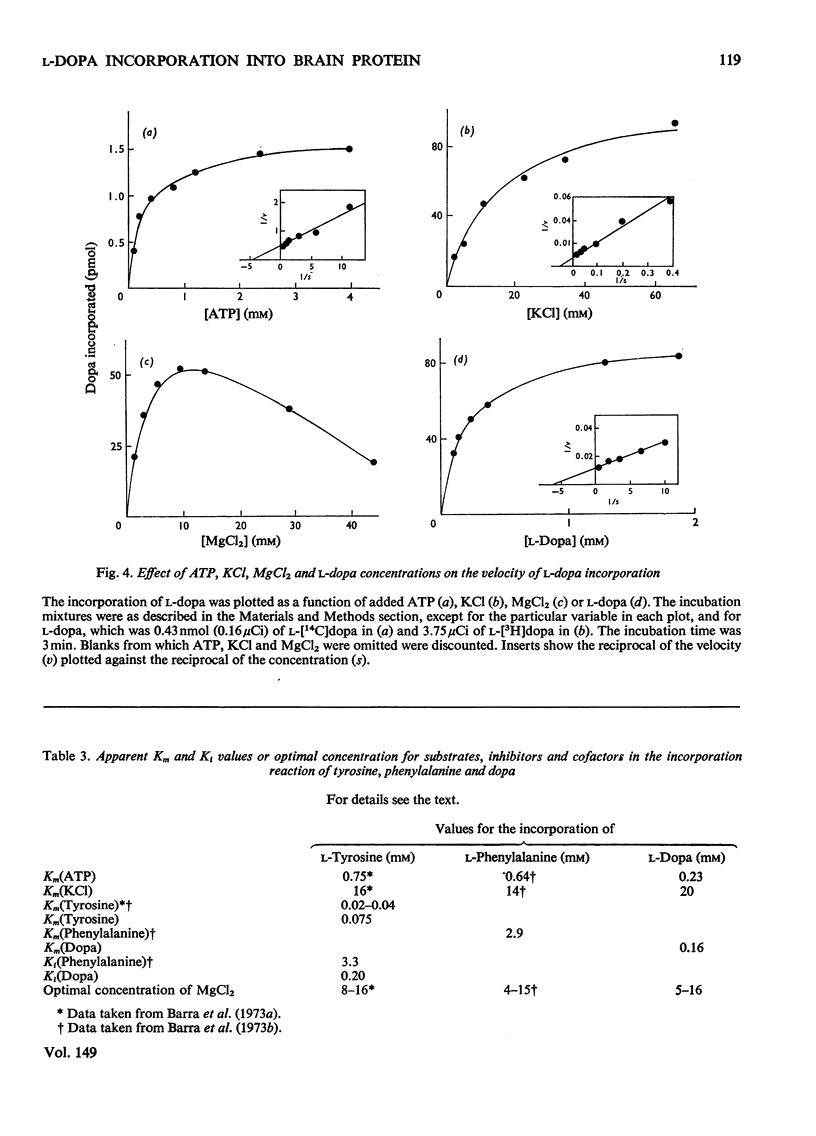
Abstract
Several compounds, structurally and metabolically related to phenylalanine and tyrosine, were tested for their effects on the incorporations of phenylalanine and tyrosine as single units into a protein of the soluble subcellular fraction of rat brain. Of the compounds tested, only L-dopa (L-3,4-dihydroxyphenylalanine) inhibited these incorporations. Further, L-dopa was incorporated into a protein of the same fraction in such a way that it excluded the incorporation of tyrosine as a single unit. Conversely, tyrosine inhibited and excluded the incorporation of L-dopa. The incorporation of L-dopa required ATP (apparent Km = 0.23mM), KCl (apparent Km = 20mM) and MgCl2 (optimal concentration range, 5-16mM). These requirements were similar to those previously determined for the incorporation of tyrosine and phenylalanine. The inactivation rate of the enzymic systems for L-tyrosine and L-dopa incorporations, when kept at 37 degrees C, was the same for both amino acids (half-life = 80 min). It is suggested that the acceptor for the incorporation of dopa is the same as that for the incorporation of tyrosine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal H. C., Davis J. M., Himwich W. A. Postnatal changes in free amino acid pool of rat brain. J Neurochem. 1966 Jul;13(7):607–615. doi: 10.1111/j.1471-4159.1966.tb11957.x. [DOI] [PubMed] [Google Scholar]
- Arce C. A., Barra H. S., Rodriguez J. A., Caputto R. Tentative identification of the amino acid that binds tyrosine as a single unit into a soluble brain protein. FEBS Lett. 1975 Jan 15;50(1):5–7. doi: 10.1016/0014-5793(75)81027-1. [DOI] [PubMed] [Google Scholar]
- Barra H. S., Arcce C. A., Rodriguez J. A., Caputto R. Uncorporation of phenylalanine as a single unit into rat brain protein: reciprocal inhibition by phenylalanine and tyrosine of their respective incorporations. J Neurochem. 1973 Nov;21(5):1241–1251. doi: 10.1111/j.1471-4159.1973.tb07578.x. [DOI] [PubMed] [Google Scholar]
- Barra H. S., Arce C. A., Rodríguez J. A., Caputto R. Some common properties of the protein that incorporates tyrosine as a single unit and the microtubule proteins. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1384–1390. doi: 10.1016/0006-291x(74)90351-9. [DOI] [PubMed] [Google Scholar]
- Barra H. S., Rodriguez J. A., Arce C. A., Caputto R. A soluble preparation from rat brain that incorporates into its own proteins ( 14 C)arginine by a ribonuclease-sensitive system and ( 14 C)tyrosine by a ribonuclease-insensitive system. J Neurochem. 1973 Jan;20(1):97–108. doi: 10.1111/j.1471-4159.1973.tb12108.x. [DOI] [PubMed] [Google Scholar]
- Barra H. S., Uñates L. E., Sayavedra M. S., Caputto R. Capacities for binding amino acids by tRNAs from rat brain and their changes during development. J Neurochem. 1972 Oct;19(10):2289–2297. doi: 10.1111/j.1471-4159.1972.tb01282.x. [DOI] [PubMed] [Google Scholar]
- Bayer S. M., McMurray W. C. The metabolism of amino acids in developing rat brain. J Neurochem. 1967 Jul;14(7):695–706. doi: 10.1111/j.1471-4159.1967.tb10303.x. [DOI] [PubMed] [Google Scholar]
- Daniels M. P. Colchicine inhibition of nerve fiber formation in vitro. J Cell Biol. 1972 Apr;53(1):164–176. doi: 10.1083/jcb.53.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A. Properties of rat brain tubulin. J Biol Chem. 1974 Mar 10;249(5):1407–1416. [PubMed] [Google Scholar]
- Fernandez H. L., Burton P. R., Samson F. E. Axoplasmic transport in the crayfish nerve cord. The role of fibrillar constituents of neurons. J Cell Biol. 1971 Oct;51(1):176–192. doi: 10.1083/jcb.51.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hier D. B., Arnason B. G., Young M. Studies on the mechanism of action of nerve growth factor. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2268–2272. doi: 10.1073/pnas.69.8.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. C., Gold G. J., Clouet D. H. An improved method for the assay of dopa. Anal Biochem. 1973 Jul;54(1):129–136. doi: 10.1016/0003-2697(73)90255-8. [DOI] [PubMed] [Google Scholar]
- Kreutzberg G. W. Neuronal dynamics and axonal flow. IV. Blockage of intra-axonal enzyme transport by colchicine. Proc Natl Acad Sci U S A. 1969 Mar;62(3):722–728. doi: 10.1073/pnas.62.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rodriguez J. A., Arce C. A., Barra H. S., Caputto R. Release of tyrosine incorporated as a single unit into rat brain protein. Biochem Biophys Res Commun. 1973 Sep 5;54(1):335–340. doi: 10.1016/0006-291x(73)90927-3. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims K. L., Davis G. A., Bloom F. E. Activities of 3,4-dihydroxy-L-phenylalanine and 5-hydroxy-L-tryptophan decarboxylases in rat brain: assay characteristics and distribution. J Neurochem. 1973 Feb;20(2):449–464. doi: 10.1111/j.1471-4159.1973.tb12144.x. [DOI] [PubMed] [Google Scholar]
- Sjöstrand J., Frizell M., Hasselgren P. O. Effects of colchicine on axonal transport in peripheral nerves. J Neurochem. 1970 Nov;17(11):1563–1570. doi: 10.1111/j.1471-4159.1970.tb03726.x. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]


