Abstract
The Jun dimerization protein (Jdp2) gene is active in mouse cerebellar granule cells and its protein product plays a crucial role in the formation of the cerebellum lobes through programmed cell death. However, the role of Jdp2 in cellular differentiation and pluripotency in the cerebellum, and the effect of the antioxidation reaction on cell plasticity, remain unknown. N-acetyl-l-cysteine (NAC) induced the early commitment of the differentiation of granule cell precursors (GCPs) to neurons, especially Purkinje cells, via the γ-aminobutyric acid type A receptor α6 subunit (Gabra6) axis; moreover, Jdp2 depletion enhanced this differentiation program of GCPs. The antioxidative effect of NAC was the main driving force of this decision toward the neural differentiation of the GCP population in the presence of Gabra6 in vitro. This implies that antioxidative drugs are effective agents for rescuing oxidative-stress-induced GCP damages in the cerebellum and commit this Gabra6-positive cell population toward differentiation into Purkinje cells.
Subject terms: Gene expression, Cellular neuroscience
Introduction
Many neurodegenerative diseases included Alzheimer’s disease, Huntington’s disease (HD), Parkinson’s disease (PD), spinocerebellar atrophy, spinal muscular atrophy, and amyotrophic lateral sclerosis are characterized by the progressive loss of function and death of specific neurons that give rise to the clinical manifestation of the disease [1]. In general, the stem cells were sequentially differentiated into various kinds of neurons and glia with an effort made to mimic the process of development of the nervous system. Thus, the methods of the neural cell progenitors to differentiate or transdifferentiate into neuros should be developed and examined their preclinical application for these neurodegenerative diseases.
In some case, the alterations in specific proteins in neural generative diseases lead to dysfunction of different cellular pathways, including increased numbers of reactive oxygen species (ROS) derived from mitochondrial dysfunction, excitotoxicity, synaptic dysfunction, impairment of protein degradation, endoplasmic reticulum stress, DNA damage, inflammation, and cell cycle reentry [2]. The neural tissue is highly sensitive to oxidative stress, and this is a prominent factor in both chronic and acute neurodegeneration. Based on this knowledge, therapeutic strategies using antioxidant molecules towards redox equilibrium have been widely used for the treatment of several brain pathologies [3, 4].
Granule cell progenitors (GCPs) are the most abundant type of cells in the mammalian brain [5–7]. The generation of GCPs from embryonic stem cells (ESCs) has contributed remarkably to the control of the in vitro differentiation of a variety of neurons [8, 9]. The techniques used to differentiate GCPs into specific neuronal cells, such as Purkinje cells (PCs), enable the establishment of in vitro cell-based models as platforms for drug discovery and preclinical translational research.
Previously, we demonstrated that the animals derived from Jun dimerization protein 2 (Jdp2)-Cre/ZEG lines expressed green fluorescence protein signals in the brain, predominantly in the cerebellum. Moreover, β-Gal staining revealed that the signals were localized in the cerebellum of Jdp2-Cre/ZEG mice. In the absence of Jdp2, a complex of the cyclin-dependent kinase inhibitor 1 (p21Cip1) and Nrf2 bound to the antioxidant response elements of the Slc7a11 promoter and provided redox control to block ROS-mediated apoptosis [10, 11]. However, the role of Jdp2 in the neural differentiation of GCPs remains unknown.
It has been reported that mouse ESC-derived serum-free floating culture of embryoid-body-like aggregates can lead to their differentiation into PCs [12]. In a three-dimensional human ESC culture, polarized cerebellar self-organized neuroepithelial cells differentiated into PCs [13–15]. Granule cells (GCs) or GCPs can influence PC development from the moment PCs migrate in the primordial cerebellum to form the cerebellar networks [16]. γ-aminobutyric acid (Gaba) depolarized GCPs via Gaba type A (GabaA) receptors and led to calcium increases in GCPs [17]. In turn, loss of PCs in the cerebellum is a characteristic of dominantly inherited neurodegenerative diseases, such as spinocerebellar ataxia type 6 (SCA6). We investigated this protocol for the differentiation of GCPs to PCs and generated primary cultures of the sorted subpopulations of Gaba type A receptor α6 subunit (Gabra6)-positive (Gabra6+) GCPs from wild-type (WT) and Jdp2 knockout (KO) mice, then compared their proliferation and differentiation activities in the presence or absence of N-acetylcysteine (NAC), an antioxidation reagent, after triggering the cell-differentiation program of GCPs into the neurons. Here, we observed that most of the sorted Gabra6+ GCPs transdifferentiated into calbindin-positive PCs in the presence of NAC in our in vitro culture condition. Thus, we concluded that Jdp2 is a critical regulator that contributes to the blockage of the differentiation of Gabra6+-sorted GCPs into various types of neurons, especially PCs, which are of vital importance in the context of neurodegenerative diseases [18]. Taken together, our findings suggest that NAC induces the neural differentiation of GCPs, and that the deletion of Jdp2 enhances this differentiation program in Gabra6-sorted GCPs. This finding may contribute to the development of new therapeutics for the differentiation of predominant GCPs to PCs, to cure neurodegenerative disorders.
Results
Isolation of the purified Gabra6-positive subpopulation of GCPs
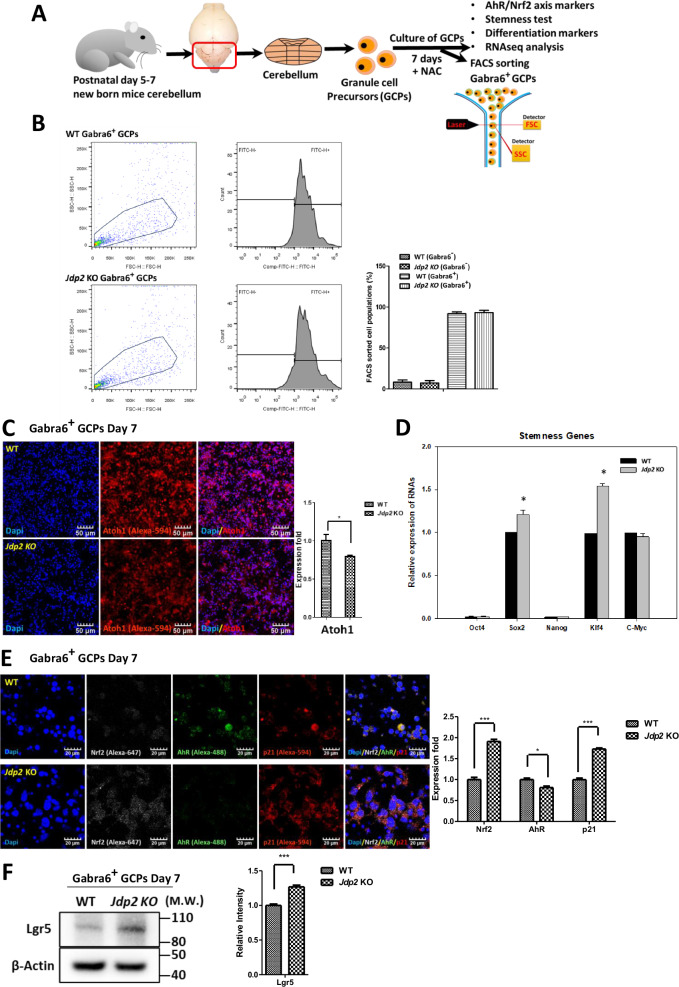
RNA sequencing of cell-type marker genes of the cerebellum after the cultivation of GCPs in the presence of NAC for 7 days was performed [10] (Fig. S1A). These values (P < 0.05) were summarized relative to the total number of cells. When the expression levels of these markers in each representative cell population of Jdp2-KO GCPs were compared with those detected in WT GCPs, the expression of granule cell marker genes as the dominant population was lower than that observed in WT GCPs; in contrast, the expression of the marker genes of PCs, astrocytes, and oligodendrocytes was 1.7–2.0-fold higher in Jdp2-KO GCPs compared with WT GCPs after cultivation with NAC. These data are similar to the results obtained from the recent single-cell RNA sequencing reported by Carter et al. [19]. In addition, the gamma-aminobutyric acid A receptor (Gabra) subunit alpha 6 (Gabra6) mRNA has been reported to be expressed in the cerebellum during development [20]. Thus, we examined the expression levels of proteins such as PC-related and calcium-channel-related molecules in the presence of NAC for 7 days using Western blotting (Fig. S1B). The expression of Cacn alpha1a (1.4-fold), calbindin (1.3-fold), Gabra1 (1.3-fold), Gabra6 (1.5-fold), Gabrb2 (1.3-fold), Grin2a (1.2-fold), Pcp4 (2.0-fold), and Vglut1 (1.8-fold) was significantly upregulated in Jdp2 KO vs. WT GCPs, indicating that Jdp2 might be the master regulator of Gaba-receptor-mediated neural differentiation into PCs [11]. Thus, we sorted the GCPs to isolate the Gabra6+ subpopulation after cultivation in the presence of NAC for 7 days using an anti-Gabra6 antibody (Fig. 1A, B); we found that more than 90% of the total cells were positive for Gabra6, whereas less than 5%–10% were negative for Gabra6 (Fig. 1B). These Gabra6+ GCPs were analyzed further for stemness, pluripotency, cell cycle, differentiation, and oxidation/antioxidation.
Fig. 1. Characterization of mouse Gabra6+ GCPs by fluorescence-activated cell sorting (FACS).
A Schematic model of the cultivation of mouse GCPs purified via discontinuous density gradient centrifugation from the cerebella (postnatal days 5–7) of mice [15, 16] in the presence of NAC (30 μM) for 7 days [10, 11], followed by characterization and FACS-based sorting using an anti-Gabra6 antibody. B FACS-based sorting to enrich the subfraction of Gabra6+ GCPs from WT and Jdp2KO mice. The left panel depicts a representative dot plot of FSC and SSC. The region was gated in dot plots and Gabra6+ cells were sorted by FACS analysis. The histogram was gated on a peak, to identify the percentage of GCPs that expressed Gabra6. C Staining of Gabra6+ GCPs from WT and Jdp2 KO mice with an anti-Atoh1 antibody. The nuclei of PGCs were stained with DAPI. Relative ratio of Atoh1-positive cells among Gabra6+ GCPs from WT and Jdp2 KO cells. Scale bars, 50 μm. D Relative values of the Oct4, Sox2, Nonog, Klf4, and c-Myc mRNAs between WT and Jdp2 KO Gabra6+ GCPs. The primer sequences used for qPCR are listed in Supplementary Table 2. The levels of the WT cells were taken as 1.0. E Immunostaining of Gabra6+ GCPs from WT and Jdp2 KO mice for Nrf2, p21Cip1, and AhR. The GC nuclei were stained with DAPI. The levels of the WT cells were taken as 1.0. Scale bars, 20 μm. F Comparative expression of the Lgr5 proteins, as assessed by Western blotting (left panel; the quantitation of each protein is shown on the right panel). The relative value was normalized to that of β-actin and presented as a ratio. Uncropped raw data was shown in Fig. S5. B–F: n = 5; *P < 0.05, **P < 0.01; ***P < 0.001.
Expression of genes related to stemness, differentiation, and the AhR–Nrf2 axis
For their characterization, the FACS-separated Gabra6-positive GCPs (Gabra6+ GCPs) were obtained after the cultivation of GCPs for 7 days in the presence of NAC and were then stained with an anti-Atoh1 antibody [10]. We found that Atoh1 expression was higher in WT compared with Jdp2 KO cells (Fig. 1C). The previous results of an RNA sequencing experiment in cerebellar cells derived from P6 mice showed that more than 95% of cells were positive for Atoh1 [10]. After cultivation with NAC for 7 days, the number of Atoh1-positive cells decreased to 70%. In fact, 70% of Gabra6+ GCPs remained positive for Atoh1 (data not shown). Furthermore, stemness markers such as Sox2 and Klf4 were upregulated in Jdp2 KO compared with WT cells. In turn, the levels of markers such as Oct4, Nanog, and c-Myc were lower, but not altered between WT and Jdp2 KO cells with NAC for 7 days (Fig. 1D). Western blotting also confirmed this finding (data not shown). Moreover, the WT and Jdp2 KO Gabra6+ GCPs were negative for alkaline phosphatase after cultivation in the presence of NAC for 7 days (data not shown). This indicates that cultivation with NAC resulted in the loss of the stemness character. The antioxidation-specific transcription factor Nrf2 and the cell-cycle regulator p21Cip1 were expressed at 1.9-fold and 1.7-fold higher levels in Jdp2 KO vs. WT cells, respectively. In contrast, the oxidative-stress-related factor AhR was expressed at 81% lower levels in Jdp2 KO compared with WT cells (Fig. 1E). The expression of Lgr5 in Jdp2 KO was higher by 1.2-fold than that detected in WT cells (Fig. 1F). Thus, the addition of NAC to Gabra6+ GCPs seems to initiate the differentiation program while maintaining the stemness character.
Cell proliferation and antioxidation
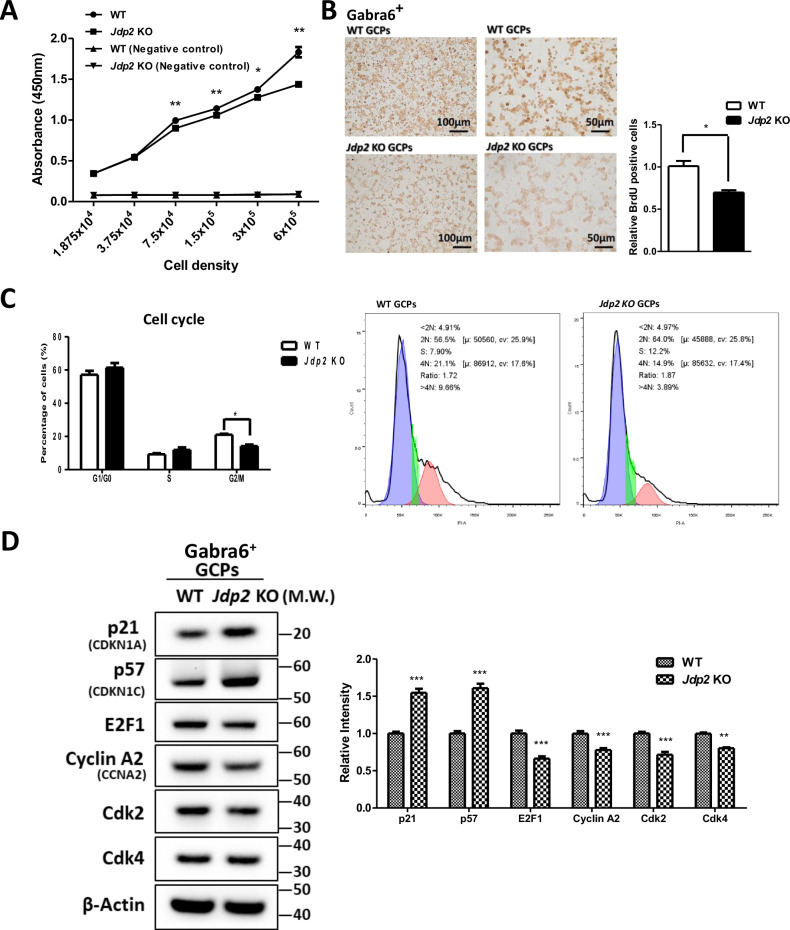
The BrdU incorporation activity of WT GCPs was higher than that of Jdp2 KO Gabra6+ GCPs (Fig. 2A). Moreover, immunocytochemistry demonstrated that the number of BrdU-positive Gabra6+ GCPs from WT mice was 1.4-fold higher than that from Jdp2 KO mice (Fig. 2B), suggesting that Jdp2 is an activator of the proliferation of Gabra6+ GCPs. In addition, a comparative cell-cycle analysis between WT and Jdp2 KO Gabra6+ GCPs showed that Jdp2 KO triggered a concomitant decrease in the number of cells in the G2/M phase (by 70%) (Fig. 2C). Furthermore, Western blotting revealed that cell-cycle-arrest–related proteins, such as p21Cip1 and p57Kip2, were expressed at 1.5-fold and 1.6-fold higher levels, respectively, in Jdp2 KO compared with WT cells. In contrast, cell-cycle-processing factors, such as E2F1, cyclin A2, Cdk4, and Cdk2, were decreased by about 60%–80% in Jdp2 KO vs. WT cells (Fig. 2D). These data suggest that Jdp2 plays a critical role in the control of the cell cycle in Gabra6+ GCPs. In addition, our previous studies demonstrated that the apoptotic activity of WT GCPs was higher than that of Jdp2 KO GCPs [11].
Fig. 2. Comparison of the proliferation abilities and cell cycle regulation of Gabra6+ GCPs from WT and Jdp2 KO mice.
A Comparative incorporation assay of BrdU into the GCPs obtained from WT and Jdp2 KO mice. B Immunocytochemical analysis of Gabra6+ GCPs from WT and Jdp2 KO mice using anti-BrdU antibodies. Scale bars, 100 and 50 μm. Quantitation of the number of BrdU-stained GCPs from WT and Jdp2 KO mice. C The cell-cycle analysis of WT and Jdp2 KO Gabra6+ GCPs. The percentage of cells in the G2/M phase in Jdp2 KO Gabra6+ GCPs was lower by 70% than that observed in WT Gabra6+ GCPs. D Western blot analysis of the comparative expression of cell-cycle-related proteins in WT and Jdp2 KO Gabra6+ GCPs. The relative value was normalized to β-actin and presented as a ratio. The quantitation of relative expressions is summarized in the panels on the right. The levels of expression of all markers were significantly higher in Jdp2 KO vs. WT Gabra6+ GCPs. (All data were obtained, n = 5: *P < 0.05, **P < 0.01; ***P < 0.001). Uncropped raw data was shown in Fig. S5.
To clarify the mechanism underlying the upregulation of GSH in Gabra6+ GCPs in the presence of NAC, we examined the effect of NAC in WT and Jdp2 KO Gabra6+ GCPs, to compare their antioxidation activities. Under the NAC condition, the levels of ARE-luciferase activity in WT and Jdp2 KO cells were increased by 1.4–3.3-fold vs. those recorded in the absence of NAC (Fig. S2A). These results suggest that NAC plays a critical role in the antioxidation response; this effect was more sensitive in Jdp2 KO Gabra6+ GCPs compared with WT Gabra6+ GCPs.
The expression levels of Gsk3β [21] and NeuN [22] as neural markers were significantly lower in Jdp2 KO Gabra6+ GCPs in the presence of NAC. In contrast, the level of Gabra6 positivity was about 1.7-fold higher in Jdp2 KO vs. WT cells (Fig. S2B, C). Thus, Jdp2 might exert specific effects on Gabra6+ expression, but not on Gsk3β and NeuN-specific neural differentiation. Thus, we next focused on the triggering of the early commitment toward neural differentiation activity in Jdp2-depleted Gabra6+ GCPs, which indicated that Jdp2 might be a blocker of the NAC-mediated differentiation of Gabra6+ GCPs into the neural stages.
Neuronal differentiation protocol of Gabra6+ GCPs
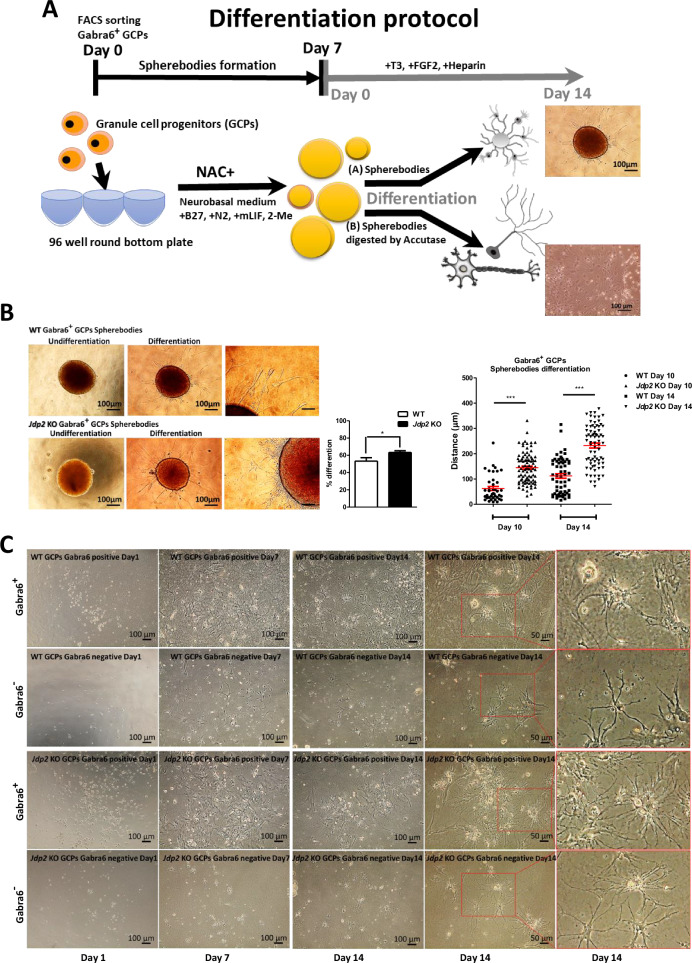
Here, we developed two methods to achieve the neural differentiation of Gabra6+ GCPs into PCs. One method (A) consisted in the generation of sphere bodies in low-attached 96-well plates of Gabra6+ GCPs in neurobasal medium (B27 supplement, N2 supplement, mouse leukemia inhibitor factor (mLIF), and 2-mercaptoethanol) plus NAC for about 1 week, followed by commitment toward differentiation by replacing with differentiation medium (Fig. 3A) [12, 13]. After the incubation of Gabra6+-GCP-derived neurosphere bodies in the differentiation medium for 7 days, we found that the differentiation efficiency of Jdp2 KO-derived neurosphere bodies was 1.2-fold faster than that of WT-derived neurosphere bodies (left panel, Fig. 3B). After further differentiation induction up to 10‒14 days, we found that the development of neurites in Jdp2 KO Gabra6+ GCP-derived neurosphere bodies was better compared with that of neurites from WT Gabra6+ GCP-derived neurosphere bodies (right panels, Fig. 3B). The second method (B) consisted in the use of flat cultivation after digesting the sphere bodies with accutase, followed by re-cultivation for an additional 7–14 days. Specific neuronal fibers were clearly apparent after cultivation for an additional 7 days (Fig. 3C). More than 80% of the subpopulation of Gabra6+ GCPs was stained with anti-calbindin antibodies, whereas less than 8% of each population was positive for anti-Atoh1 (GC-specific), anti-GFAP (astrocyte-specific), and anti-CD45 (glia-specific) antibodies (Fig. 4A, B). This suggests that about 80% of Gabra6+ GCPs can differentiate into PCs in vitro in differentiation medium containing NAC.
Fig. 3. Differentiation of Gabra6+ GCPs into neural cells.
A Two differentiation protocols of Gabra6+ GCPs were used: the sphere body method and the 2D-accutase culture method. A 96-well non-attached plate was used to generate sphere bodies, which were cultured in mouse GC medium supplemented with mLIF and 2-mercaptoethanol (2-ME) for 7 days. Then, the spherebodies were transferred to a differentiation medium containing brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), thyroid 3 (T3), and fibroblast growth factor 2 (FGF2), to induce Purkinje cells (PCs). The spherebodies were treated with accutase and plated in the 2D-plates and then committed to the differentiation as described in Materials and Methods. After 14 days, the differentiated cells were shown in respective panels. Scale bars: 100 and 50 μm. B Differentiation process of spherebodies derived from WT and Jdp2 KO Gabra6+ GCPs. Undifferentiated GCP sphere bodies (left) and differentiated GCP sphere bodies (middle and right) are shown. Scale bars, 100 and 50 μm. The quantitative ratio of the sphere bodies differentiated from WT (n = 43) and Jdp2 KO (n = 44) GCPs was measured. The neurite growth distance was measured on days 10 and 14. The Jdp2 KO GCPs exhibited longer neurites from differentiated sphere bodies compared with WT GCP-derived neurites (n = 5: *P < 0.05, ***P < 0.001). C The differentiation of WT and Jdp2 KO Gabra6+ GCPs was performed as described in the Materials and Methods. After digesting the respective sphere bodies with accutase, the cells were transferred into the differentiation medium and cultivated further for 1, 7, and 14 days. Two-dimensional (2D) cell cultivation from Jdp2 KO Gabra6+ GCPs yielded a greater number of dendrites compared with WT Gabra6+ GCPs. After 14 days of differentiation, the bright-field images showed the presence of Purkinje cells (PCs). The Purkinje cells were stained using neuronal markers, for quantification, as shown in Fig. 4B. Scale bars, 100 and 50 μm.
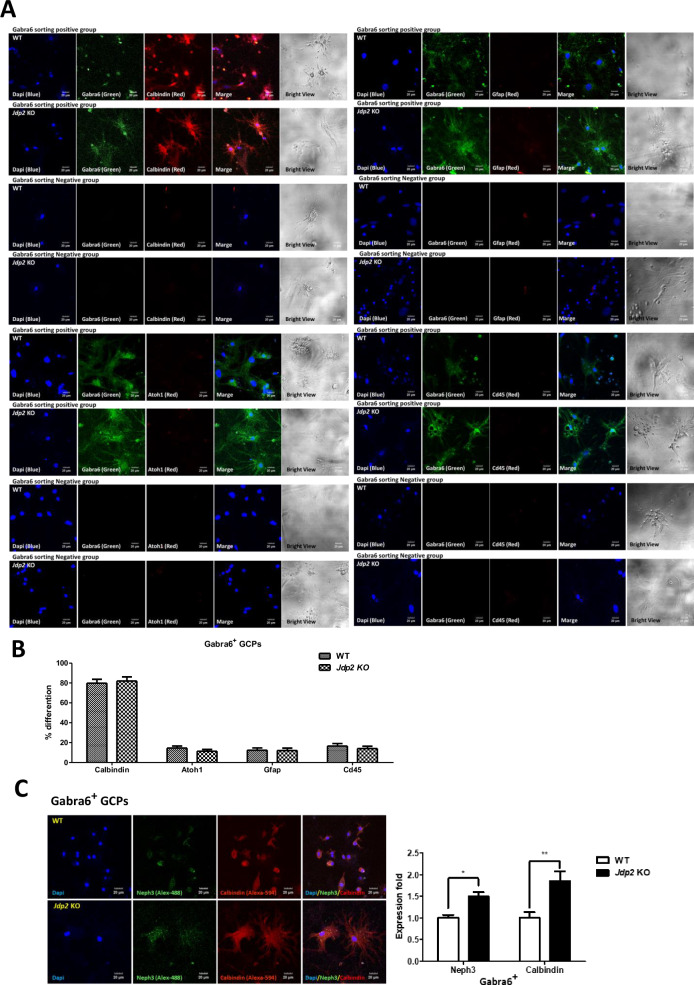
Fig. 4. Comparison of expression of neuronal markers in Gabra6+ GCPs from WT and Jdp2 KO mice after differentiation method of 2D cultivation-accutase treatment of the spherebodies for 14 days.
A Gabra6+ GCPs from WT and Jdp2 KO mice were generated the spherebodies for 7 days culture and digested with accutase and then cultured on 2D cells and then cultured for 14 days and examined the expression of differentiation markers on immunostaining. The different neurons were stained for the following marker proteins: Calbindin for Purkinje cells, Atoh1 for granule cells, GFAP for astrocytes, and CD45 for microglia. The blue color corresponds to DAPI staining, the green color corresponds to Gabra6 (as a control), and the neuronal markers were colored in red. We also present bright-field images. Scale bars, 20 μm. B In Gabra6+ GCPs sorted cell populations, the quantitation of different cell types was performed by counting double-positive signals for Gabra6 and neuronal markers. About 80% of the cell populations were Purkinje cells. The other cell types were less than 10% after differentiation for 14 days. C Immunostaining for Neph3 and calbindin in accutase-treated 2D-cultured Jdp2 KO Gabra6+ GCPs and WT Gabra6+ GCPs after differentiation for 7 days. Scale bars, 20 μm. The expression of Neph3 and calbindin was compared between WT and Jdp2 KO Gabra6+ GCPs. (n = 5; *P < 0.05; **P < 0.01).
To define the role of exposure to NAC in this differentiation process, we compared the expression of Purkinje progenitor cell markers, such as Neph3 [23, 24] and calbindin, between the condition of NAC exposure and that of the absence of NAC in Gabra6+ PGCs for 7 days. After NAC exposure of Gabra6+ PGCs prepared using an accutase-2D cell method, we found that PC makers, such as calbindin 3 and Neph3, were predominant (about 1.8-fold- and 1.5-fold-higher expression, respectively) in Jdp2 KO vs. WT cells after 2 weeks of the neural differentiation protocol (Fig. 4C). These results suggest that Jdp2 controls the frequency and the speed of differentiation of GCPs into PCs. Therefore, the Jdp2 protein itself can prevent the neural differentiation of Gabra6+ GCPs into Purkinje neurons.
Enhancement of Ca2+ signals in Jdp2 KO Gabra6+ GCPs
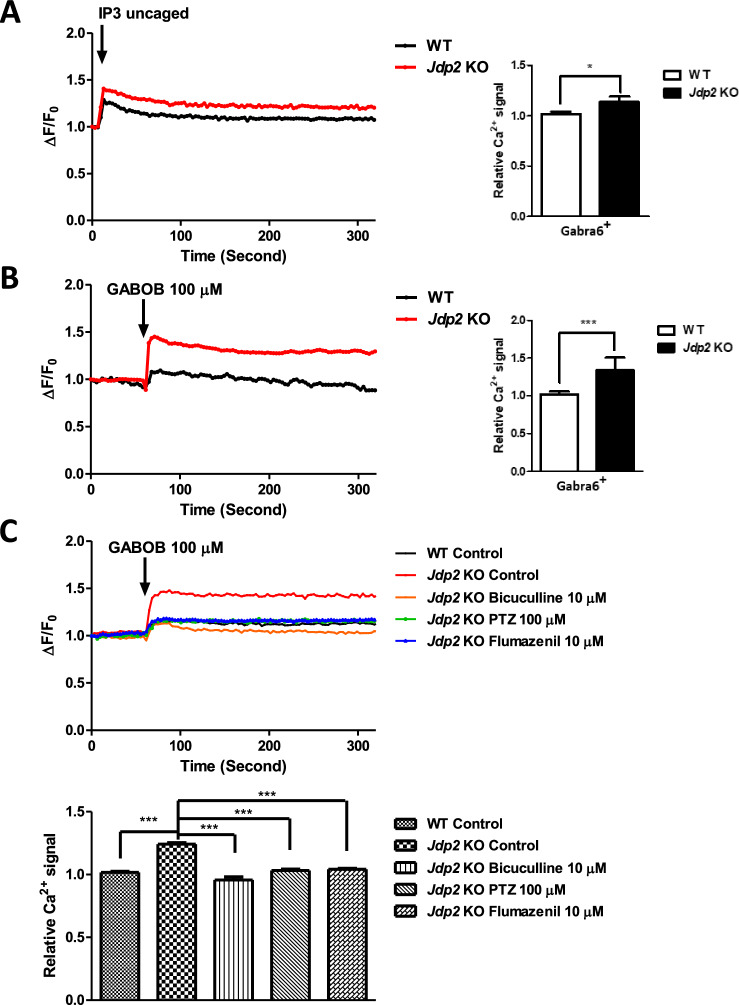
A previous study demonstrated that the Gaba receptor is involved in the regulation of the differentiation of rodent neural progenitor cells [23]. The Gaba receptor is a G-protein-coupled receptor that is associated with inositol 1,4,5-triphosphate (IP3)-induced Ca2+ signals [25–27]. We hypothesized that the Gaba-receptor-mediated Ca2+ signals are involved in Jdp2-regulated neural differentiation. To investigate the effect of Jdp2 on Gaba-receptor-mediated Ca2+ signals, we examined the caged inositol IP3-mediated Ca2+ uptake, which leads to local calcium release, in accutase-2D-differentiated Gabra6+ GCPs (Fig. 5). In the case of calcium release in Jdp2 KO cells, the level of uncaged IP3 was 1.1-fold higher than that detected in WT cells (Fig. 5A). This uncaged-IP3-mediated Ca2+ uptake was modulated by the Gaba receptor. Thus, to confirm the Gaba-receptor-regulated IP3-mediated calcium release, the Gaba receptor agonist GABOB was added to the culture medium. The calcium uptake in Jdp2 KO accutase-2D-differentiated Gabra6+ GCPs was 1.3-fold higher than that observed in WT cells after GABOB stimulation (Fig. 5B). Taken together, these results suggest that the ability to trigger calcium signaling is evoked by a higher calcium uptake in Jdp2-depleted accutase-2D-differentiated Gabra6+ GCPs via intracellular uncaging. To examine the role of the Gaba receptor in inositol IP3-mediated Ca2+ uptake in Jdp2 KO accutase-2D-differentiated Gabra6+ GCPs, we used various Gaba receptor inhibitors, such as bicuculline, PTZ, and flumazenil (Fig. 5C). The calcium uptake in Jdp2 KO accutase-2D-differentiated Gabra6+ GCPs after GABOB stimulation was reversed to the original levels detected in control GCPs.
Fig. 5. Calcium uptake studies in accutase-treated 2D-cultured cells on day 7 of differentiation.
A Calcium-uptake activities of WT and Jdp2 KO Gabra6+ GCPs. Inositol triphosphate (IP3)-mediated Ca2+ liberation was assessed in neuronal cells using a two-beam-signal cell simulation system. The IP3 (0.5 μM) stimulation ΔF/F ratio and the calcium-uptake activities were evoked to a higher degree in Jdp2 KO GCPs after IP3 uncaging (n = 5; *P < 0.05). B A Gaba receptor agonist (GABOB) (100 μM) stimulated a greater release of Ca2+ in Jdp2 KO Gabra6+ GCPs (1.34-fold higher compared with WT GCPs) (n = 5; ***P < 0.001). C The effects of Gaba receptor inhibitors, i.e., bicuculline (10 μM), PTZ (100 μM), and flumazenil (10 μM), on GABOB stimulated the release of Ca2+ in WT and Jdp2 KO Gabra6+ GCPs. The protocol used for the liberation of Ca2+ was as described in the Materials and Methods (n = 5; *P < 0.05; **P < 0.01; ***P < 0.001).
Expression of PC-related and Gaba-receptor-related genes
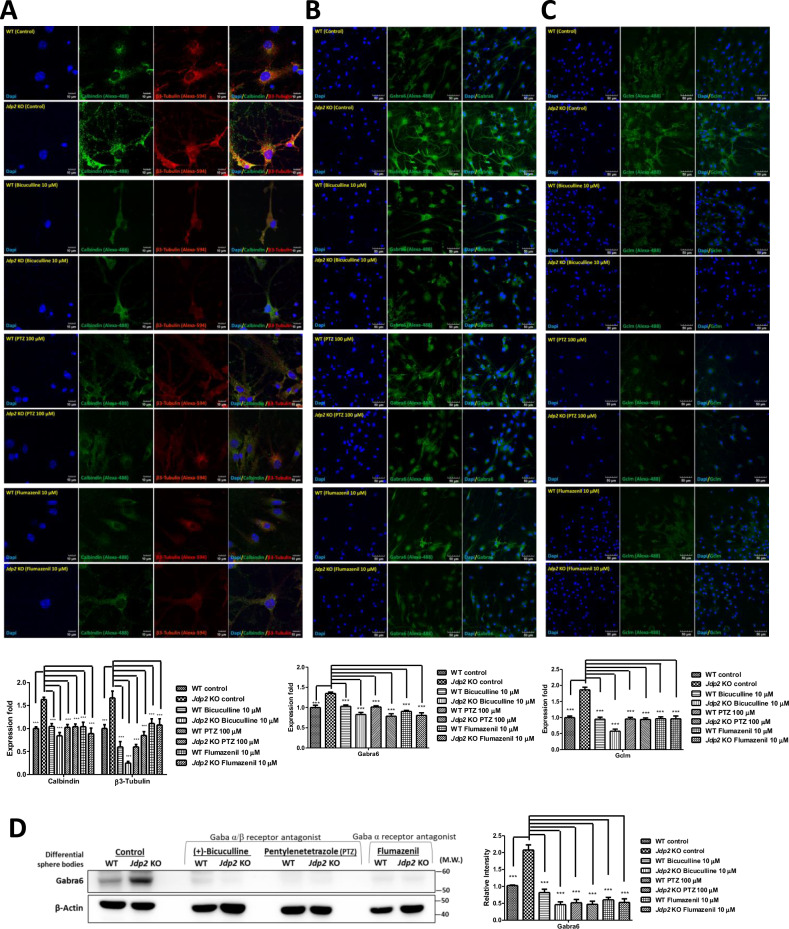
To examine the role of the Gabra6, we used various GABA receptor inhibitors [28], such as bicuculline and PTZ (specific for α/β), and flumazenil (a competitive antagonist at the benzodiazepine-binding site on α) and assessed whether they inhibited neural differentiation (Fig. 6A–D). These inhibitors blocked the expression of calbindin and βIII tubulin (Fig. 6A), Gabra6 (Fig. 6B), and the glutamate–cysteine ligase modifier subunit (Gclm, known as gamma-glutamyl cysteine; glycine ligase or glutathione synthetase) [29] (Fig. 6C). These results indicate that the Gabra6 plays a critical role in NAC-mediated antioxidation, for the accumulation of GSH, by increasing the glutamine–cysteine pump including Gclm, and determines the neural network in Jdp2-depleted Gabra6+ GCPs, including PC neurons.
Fig. 6. Gaba receptor antagonists block the neuronal differentiation of Gabra6+ GCPs from Jdp2 KO mice to PCs.
Immunostaining of accutase-treated 2D cultures of WT and Jdp2 KO Gabra6+ GCPs for 7 days in the presence of NAC and neurites after induction toward neural differentiation via Gaba receptor inhibitors, i.e., bicuculline (10 μM), PTZ (100 μM), and flumazenil (10 μM), using antibodies against calbindin and tubulin βIII (A), Gabra6 (B), and Gclm (C). The staining was performed as described in the Materials and Methods. The nucleus was stained with DAPI. Scale bars, 10 μm. The expression of each protein in WT Gcbra6+ GCPs was set as 1.0 (n = 3; ***P < 0.001). D Western blot analysis of the expression of Gabra6 in accutase-treated 2D-cultivated Jdp2 KO vs. WT Gabra6+ GCPs for 7 days in the presence of NAC and in the presence of inhibitors, i.e., bicuculline (10 μM), PTZ (100 μM), and flumazenil (10 μM). The expression of each protein in WT Gabra6+ GCPs was set as 1.0 (n = 3, ***P < 0.001). The relative value was normalized to β-actin and is presented as a ratio. Uncropped raw data was shown in Fig. S5.
In an attempt to search for mutations in the GABRA6, GABRA1, GABRB2 genes, as well as the antioxidative gene NRF2, cysteine transporters (such as SLC7A11 and CD44v), and their regulators (such as JDP2 and p21Cip1 (CDKN1A) in brain tumors, the cBioPortal (http://www.cbioportal.org/faq#how-do-i-cite-the-cbioportal) data were accessed. In total, 8139 patients and 8597 samples from 26 studies were grouped for each item, such as signaling, apoptosis, and cell-cycle-progression pathways [30–32]. The frequency of mutation in the GABRA6, GABRA1, and GABRB2 genes was significantly higher than that detected for other cell-cycle- and cell-proliferation-related genes; furthermore, their co-occurrence in patients with cancer was examined. Thus, alterations in the GABRA gene family seem to be closely related to cancer occurrence, not only in mice, but also in human patients with brain cancer, through the regulation of the antioxidative response genes to produce GSH via SLA7A11 and CD44v, as an xCT receptor. A synthetic analogue of GABA was reported to play roles in anti-inflammation and as an antioxidant in mice [33] and pigs [28]. As shown in Figs S3A–C and S4A, B, the mutation rates of GABA receptors were significantly increased [30–32]. Accordingly, molecules that control the antioxidation response, such as GSH production-related molecules (e.g., SLA7A11, CD44v, p21Cip1 (CDKN1A), and JDP2), were also mutated significantly by gene amplification and deep deletions, similar to the gene mutation events observed in the GABA receptor family. GABRA1 might be related to NRF2 and CD44v, GABRA2 is related to CD44v, and GABRA6 is related to SLC7a11 (Figs. S3 and S4). These databases suggest that JDP2 is a possible regulator of the GABA receptor family signaling which might be concerned with ROS-antioxidation balance and neural differentiation from PGCs to PCs.
Discussion
We developed a critical in vitro culture condition for mouse Jdp2-depleted Gabra6+ GCPs in the presence of NAC that allowed the trans-differentiation capacity to PCs. Because NAC is frequently used as an antioxidant [34–37], we tested the level of total glutathione (GSH) and ROS in this context. In general, NAC upregulates GSH and downregulates ROS in GCPs. We focused on the markers of brain stemness and differentiation at an early stage [38]. The Lgr5 stemness markers and Sox2/Klf4 were highly expressed in Jdp2 KO compared with WT cells (Fig. 1D, F). In addition, the expression of antioxidation-related factors, such as Nrf2 and p21Cip1, was increased, whereas that of AhR was decreased in Jdp2 KO vs. WT cells (Fig. 1E). This might be important to show the difference between these stemness and antioxidation markers in brain Gabra6+ GCPs with NAC in the case of trans-differentiation. The BrdU-incorporation experiment and cell-cycle analysis indicated that the G2/M ratio in Gabra6+-Jdp2 KO GCPs was lower than that observed in WT cells (Fig. 2A–C). In fact, during the differentiation-commitment stages, DNA synthesis was decreased, and apoptosis was increased in Jdp2 KO Gabra6+ GCPs. Moreover, the cells seemed to enter differentiation. Treatment with NAC enhanced this stage in Gabra6+ GCPs collected from the cerebella of Jdp2 KO mice, which might affect the antioxidation control.
We successfully generated a primary culture system of PGCs in the presence of NAC for more than 2 weeks in vitro. We also established a protocol for the generation of sphere bodies after 1 week of culture, and maintained the stemness of GCPs by adding mLIF, to avoid cell differentiation. Subsequently, the medium was changed to a differentiation medium that included T3, FGF2, and heparin. The proliferation of Gabra6+ GCPs from WT mice was greater than that of cells from Jdp2 KO mice. However, in the differentiation experiment, the number of neurosphere bodies obtained from Jdp2 KO mice was higher than that obtained from WT-derived neurosphere bodies; moreover, the onset of differentiation in Jdp2 KO cells was also higher than that of WT Gabra6+ GCPs after measuring the neurite growth from neurosphere bodies (Fig. 3B). In the case of pre-differentiation, the Jdp2 KO spheres committed to differentiation toward the neural cascade compared with WT spheres. Furthermore, we found that staining signals for PC-specific biomarkers were clearly detected during the differentiation stage. Moreover, a significantly higher level of expression of calbindin was detected in Jdp2 KO compared with WT Gabra6+ GCPs (Fig. 4B, C). The comparison of the differentiation frequency between the standard cultivation method with NAC and the in vitro differentiation culture condition revealed that the expression of calbindin in the differentiation medium in Jdp2 KO cells was higher compared with that observed for WT Gabra6+ GCPs; however, we detected a significant trans-differentiation of Gabra6+ GCPs to PCs, even in the NAC culture condition (Fig. 4C).
Previously, it was reported that calcium ions are among the most critical signaling molecules because they control almost all cellular functions and processes [25, 39]. Changes in intracellular free calcium concentrations are closely correlated with the signal transduction of G-protein-coupled receptors and various cellular pathophysiological conditions, such as spinocerebellar ataxia, PD, Gillespie syndrome, and HD [25–27, 40]. Therefore, the measurement of free intracellular calcium is critical for understanding calcium-dependent neuronal activity. As depicted in Fig. 5, the depletion of Jdp2 caused a higher Gaba receptor-IP3-mediated calcium release compared with that observed in WT GCPs. The expression levels of the Gaba receptor and Purkinje markers, as well as IP3-mediated calcium, were closely related to the function of Jdp2 depletion in the presence of NAC. In turn, the Gaba receptor antagonists bicuculline, PTZ, and flumazenil inhibited the differentiation of GCPs to neurons, the levels of calbindin (Fig. 6A). Gabra/Gabrb antagonists and a Gabra antagonist inhibited the expression of Gabra6 and Gclm (Fig. 6B, C). These data indicate that a strong correlation between Gabra and PCs is critical for the developmental control of Purkinje cell dendrites and tumor phenotypes [39, 41]. In addition, the inhibitory synapses that occur during neural development are regulated by GABAergic synaptic lateral diffusion dynamics, which are tuned by Ca2+ and glutamate [27]. In fact, the marker genes of the GABRA receptor families and cystine transporter xCT (SLA7A11 and CD44v), as well as antioxidation-controlled factors, such as JDP2, NRF2, and CDKN1A (p21Cip1), were significantly mutated in brain tumors, e.g., glioblastoma, glioma, and neuroblastoma (Fig. S4A, B). Taken together, our results suggest that Jdp2 is critical for inhibiting the normal differentiation of GCPs into functional PCs through an IP3-mediated calcium-uptake function via the Gaba receptor alpha 6 (Fig. 7). Further molecular studies are required to understand the Jdp2-mediated IP3 generation axis, Gaba receptor function, and calcium uptake, which were required here for the neural differentiation of GCPs into PCs. In addition, Jdp2 might be a key regulator of the suppression of the cell cycle progression and, eventually, of the differentiation program of Gabra6+ GCPs into PCs. Thus, the understanding of how Jdp2 controls the cell cycle and the differentiation of Gabra6+ GCPs into functional PCs through calcium uptake is critical for the development of the use of Jdp2 to treat PC-mediated genetic diseases and related behavioral disorders.
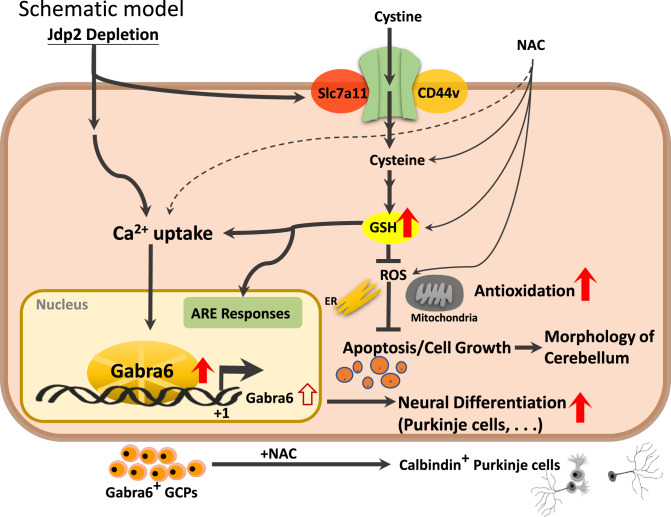
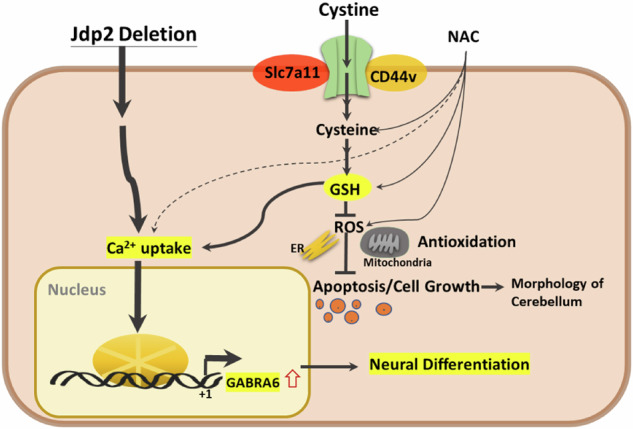
Fig. 7. Hypothetical model of the effect of Jdp2 depletion and Gaba receptor-family and antioxidation response in the initiation of neural differentiation.
Schematic models of the pathways of Jdp2 depletion in the presence of NAC for the neural differentiation of GCPs. NAC induces xCT channels and calcium uptake, to generate antioxidation, including GSH production, thus inducing GAabra1 and 6 gene activation, which might result in the neural induction of PGCs. The original of the figure presented in Fig. S4 was by C–C. Ku et al. [10, 11], with permission to reuse and modify.
In summary, the present study demonstrated first time that the trans-differentiation of Gabra6+ GCPs toward neuron lineages, especially PCs, was triggered by their exposure to the NAC, to induce antioxidation signaling, including calcium uptake and the activation of the Gabra6. The deletion of Jdp2 also activated the early differentiation program of Gabra6+ GCPs in vitro. Thus, mutation and deletion of the Jdp2 molecule in the urgent stress might trigger this determination of Gabra6+ GCPs toward neurons, including Purkinje neurons, after exposure to NAC. Therefore, we speculate that antioxidant drugs might be effective agents for rescuing the oxidative-stress-induced Gabra6+ GCP damages that occur in the absence of a normal functional Jdp2 in the cerebellum.
Supplementary information
Supplementary Figure 5, uncropped Western blots
Acknowledgements
We thank Y.L. Lee of Welgene Biotech for the RNA sequencing work; YH Yang, S. Kishikawa and K. Nakade for the animal, cell, and RNA experiments; and the NLAC for technical support in histology-related experiments, contract breeding, and testing services. We also thank the Center for Research Resources and Development of Kaohsiung Medical University for providing the Olympus FV1000 and BD LSR II systems. This work was supported by grants from the Ministry of Science and Technology (MOST111-2314-B-037-009), the National Health Research Institutes (NHRI-EX109-10720SI), Kaohsiung Medical University Hospital (KMUH111-1R77; KMUH-DK(A)112002) and National Yang Ming Chiao Tung University-Kaohsiung Medical University Joint Research Project (NYCUKMU-113-I006) and Kaohsiung Medical University (KMU-TC113A02).
Author contributions
C-CK designed experiments and performed research, analyzed data, provided new reagents/analytical tools, and wrote the manuscript. J-BP designed experiments and performed research, analyzed data, provided new reagents/analytical tools. KW analyzed data. W-LH analyzed data. KK provided new reagents/analytical tools. MN provided new reagents/analytical tools. YN provided new reagents/analytical tools. SS provided new reagents/analytical tools. C-YT analyzed data. Y-CL provided new reagents/analytical tools. D-CW provided new reagents/analytical tools, acquired the funds, and corresponding author. C-SL wrote the manuscript. KKY designed experiments and performed research, wrote the manuscript, acquired funds, and corresponding author.
Data availability
All studies are included in the article and/or datasets S1–S4 and S5 (uncropped Western blots).
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The animal welfare guidelines published by the Animal Care Committee of the RIKEN BioResource Research Center (BRC) in Japan (Kiteisv.intra.riken.jp/JoureiV5HTMLContents/act/print/print110000514.htm), the National Laboratory Animal Center (NLAC)(106022), and the Kaohsiung Medical University in Taiwan (106189; 107128; 108244) were used for the care of laboratory animals [10, 11]. Various organs were isolated from the Jdp2-KO mice (RIKEN Modified SHIRPA; https://ja.brc.riken.jp/lab/jmc/shirpa/en/) and evaluated for LacZ expression. The strategy used to produce the Jdp2-KO mouse has been described elsewhere [42, 43]. Primary GCPs were prepared as described previously [10, 11].
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Deng-Chyang Wu, Email: dechwu@kmu.edu.tw.
Kazunari K. Yokoyama, Email: kazu@kmu.edu.tw
Supplementary information
The online version contains supplementary material available at 10.1038/s41420-024-02262-2.
References
- 1.Chi H, Chang HY, Sang TK. Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci. 2018;19:3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houldsworth A. Role of oxidative stress in neurodegenerative disorders: a review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024;6:fcad356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moren C, deSouza RM, Giraldo DM, Uff C. Antioxidant therapeutic strategies in neurodegenerative diseases. Int J Mol Sci. 2022;23:9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olufunmilayo EO, Gerke-Duncan MB, Damian Holsinger KM. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants. 2023;12:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. [DOI] [PubMed] [Google Scholar]
- 6.Okano-Uchida T, Himi T, Komiya Y, Ishizaki Y. Cerebellar granule cell precursors can differentiate into astroglial cells. Proc Natl Acad Sci USA. 2004;101:1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, et al. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–72. [DOI] [PubMed] [Google Scholar]
- 8.Su HL, Muguruma K, Matsuo-Takasaki M, Kengaku M, Watanabe K, Sasai Y. Generation of cerebellar neuron precursors from embryonic stem cells. Dev Biol. 2006;290:287–96. [DOI] [PubMed] [Google Scholar]
- 9.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–97. [DOI] [PubMed] [Google Scholar]
- 10.Ku CC, Wuputra K, Kato K, Pan JB, Li CP, Tsai MH, et al. Deletion of Jdp2 enhances Slc7a11 expression in Atoh-1 positive cerebellum granule cell progenitors in vivo. Stem Cell Res Ther. 2021;12:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ku CC, Wuputra K, Kato K, Lin WH, Pan JB, Tsai SC, et al. Jdp2-deficient granule cell progenitors in the cerebellum are resistant to ROS-mediated apoptosis through xCT/Slc7a11 activation. Sci Rep. 2020;10:4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao O, Shimazaki T, Okada Y, Naka H, Kohda K, Yuzaki M, et al. Efficient generation of mature cerebellar Purkinje cells from mouse embryonic stem cells. J Neurosci Res. 2010;88:234–47. [DOI] [PubMed] [Google Scholar]
- 13.Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015;10:537–50. [DOI] [PubMed] [Google Scholar]
- 14.Muguruma K. Self-organized cerebellar tissue from human pluripotent stem cells and disease modeling with patient-derived iPSCs. Cerebellum. 2018;17:37–41. [DOI] [PubMed] [Google Scholar]
- 15.Ishida Y, Kawakami H, Kitajima H, Nishiyama A, Sasai Y, Inoue H, et al. Vulnerability of purkinje cells generated from spinocerebellar ataxia type 6 patient-derived iPSCs. Cell Rep. 2016;17:1482–90. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Jun S, Park H, Tanaka-Yamamoto K, Yamamoto Y. Regulation of cerebellar network development by granule cells and their molecules. Front Mol Neurosci. 2023;16:1236015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dave KA, Bordey A. GABA increases Ca2+ in cerebellar granule cell precursors via depolarization: implications for proliferation. IUBMB Life. 2009;61:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cajal SR. La rétine des vertébrés. Typ. de Joseph van In & Cie., 1892.
- 19.Carter RA, Bihannic L, Rosencrance C, Hadley JL, Tong Y, Phoenix TN, et al. A single-cell transcriptional atlas of the developing murine cerebellum. Curr Biol. 2018;28:2910–.e2912. [DOI] [PubMed] [Google Scholar]
- 20.Wisden W. Structure and distribution of multiple GABAA receptor subunits with special reference to the cerebellum. Ann N Y Acad Sci. 1995;757:506–15. [DOI] [PubMed] [Google Scholar]
- 21.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11:539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gusel’nikova VV, Korzhevskiy DE. NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta Nat. 2015;7:42–47. [PMC free article] [PubMed] [Google Scholar]
- 23.Wegner F, Kraft R, Busse K, Härtig W, Schaarschmidt G, Schwarz SC, et al. Functional and molecular analysis of GABA receptors in human midbrain-derived neural progenitor cells. J Neurochem. 2008;107:1056–69. [DOI] [PubMed] [Google Scholar]
- 24.Mizuhara E, Minaki Y, Nakatani T, Kumai M, Inoue T, Muguruma K, et al. Purkinje cells originate from cerebellar ventricular zone progenitors positive for Neph3 and E-cadherin. Dev Biol. 2010;338:202–14. [DOI] [PubMed] [Google Scholar]
- 25.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. [DOI] [PubMed] [Google Scholar]
- 26.Hisatsune C, Mikoshiba K. IP3receptor mutations and brain diseases in human and rodents. J Neurochem. 2017;141:790–807. [DOI] [PubMed] [Google Scholar]
- 27.Bannai H, Niwa F, Sakuragi S, Mikoshiba K. Inhibitory synaptic transmission tuned by Ca(2+) and glutamate through the control of GABA(A) R lateral diffusion dynamics. Dev Growth Differ. 2020;62:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Zhao J, Hu J, He H, Wei Y, Ji L, et al. Gama-aminobutyric acid (GABA) alleviates hepatic inflammation via GABA receptors/TLR4/NF-κB pathways in growing-finishing pigs generated by super-multiparous sows. Anim Nutr. 2022;9:280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gipp JJ, Bailey HH, Mulcahy RT. Cloning and sequencing of the cDNA for the light subunit of human liver gamma-glutamylcysteine synthetase and relative mRNA levels for heavy and light subunits in human normal tissues. Biochem Biophys Res Commun. 1995;206:584–9. [DOI] [PubMed] [Google Scholar]
- 30.Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45:1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dias JM, de Brito TV, de Aguiar Magalhães D, da Silva Santos PW, Batista JA, do Nascimento Dias EG, et al. Gabapentin, a synthetic analogue of gamma aminobutyric acid, reverses systemic acute inflammation and oxidative stress in mice. Inflammation. 2014;37:1826–36. [DOI] [PubMed] [Google Scholar]
- 34.Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharm. 1997;38:205–27. [PubMed] [Google Scholar]
- 35.Dekhuijzen PN. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004;23:629–36. [DOI] [PubMed] [Google Scholar]
- 36.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–42. [DOI] [PubMed] [Google Scholar]
- 37.Feng H, Moriyama T, Ohuchida K, Sheng N, Iwamoto C, Shindo K, et al. N-acetyl cysteine induces quiescent-like pancreatic stellate cells from an active state and attenuates cancer-stroma interactions. J Exp Clin Cancer Res. 2021;40:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flora A, Klisch TJ, Schuster G, Zoghbi HY. Deletion of Atoh1 disrupts sonic hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science. 2009;326:1424–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawaguchi K, Habara T, Terashima T, Kikkawa S. GABA modulates development of cerebellar Purkinje cell dendrites under control of endocannabinoid signaling. J Neurochem. 2010;114:627–38. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Rivera ML, Barragan-Galvez JC, Gasca Martínez D, Hidalgo-Figueroa S, Isiordia-Espinoza M, Alonso-Castro AJ. In vivo neuropharmacological effects of neophytadiene. Molecules. 2023;28:3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nietz A, Krook-Magnuson C, Gutierrez H, Klein J, Sauve C, Hoff I, et al. Selective loss of the GABA(Aα1) subunit from Purkinje cells is sufficient to induce a tremor phenotype. J Neurophysiol. 2020;124:1183–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan J, Nakade K, Huang YC, Zhu ZW, Masuzaki S, Hasegawa H, et al. Suppression of cell-cycle progression by Jun dimerization protein-2 (JDP2) involves downregulation of cyclin-A2. Oncogene. 2010;29:6245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakade K, Pan J, Yoshiki A, Ugai H, Kimura M, Liu B, et al. JDP2 suppresses adipocyte differentiation by regulating histone acetylation. Cell Death Differ. 2007;14:1398–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 5, uncropped Western blots
Data Availability Statement
All studies are included in the article and/or datasets S1–S4 and S5 (uncropped Western blots).