Abstract
We hypothesized that Escherichia coli cytotoxic necrotizing factor 1 (CNF1) might impair migration or proliferation of bladder cells and could potentially interfere with repair of the bladder epithelium. Using experimentally wounded human T24 bladder epithelial cell monolayers as an in vitro model, we found that both the number of T24 cells and the maximum distance they migrated into wounded regions was significantly decreased by bacterial extracts containing E. coli CNF1.
Escherichia coli cytotoxic necrotizing factor 1 (CNF1) is a 1,014-amino-acid cytotoxin epidemiologically associated with isolates from extraintestinal infections, primarily those from urinary tract infections (2, 3, 7, 19). CNF1 has pronounced effects on the cytoskeleton of cell lines in vitro (5, 6, 8), causing reorganization of actin into stress fibers, membrane ruffles, and promotion of cell spreading and multinucleation (10, 11). The epidemiological association of E. coli CNF1 with extraintestinal infection, its effects on cell function in vitro, and the identification of cnf1 on chromosomal pathogenicity island II, closely linked to genes for hemolysin and P fimbriae (4), all suggest that CNF1 is a potential virulence factor. However, a role for E. coli CNF1 in pathogenesis has not been established.
Biochemically, CNF1 deamidates glutamine 63 of the small Ras-related GTP-binding protein Rho (15, 23), resulting in a constitutively active GTPase (12, 13). The Rho family of GTPases, including Rho, Rac, and Cdc42, regulate actin organization and gene expression in response to extracellular signals and can affect a spectrum of cell functions (17). In particular, we noted that activated Rho (21) or bacterial toxins modifying Rho (1, 22) can alter cell migration in vitro. We hypothesized that CNF1 might impair migration or proliferation of bladder cells and could potentially interfere with repair of the bladder epithelium damaged by hemolysin or other mechanisms. To evaluate this hypothesis, we examined the ability of CNF1-containing extracts to inhibit repair of experimentally wounded bladder cell monolayers in vitro.
CNF1-containing and control extracts were prepared from E. coli DH5α transformed with either plasmid pISS392 or pGEM3, respectively. Plasmid pISS392 comprises the vector pGEM3 and a 3.5-kb AccI-StuI fragment containing the intact cnf1 gene from the urinary tract infection isolate E-B35 (9). Strains were inoculated into 100 ml of Trypticase soy broth (Becton Dickinson, BBL, Cockeysville, Md.) and grown overnight in a shaking incubator (200 rpm) at 37°C. Cultures were chilled on ice, harvested by centrifugation for 10 min at 10,000 rpm (Sorvall SS-34 rotor), and washed twice in cold Dulbecco’s phosphate-buffered saline (Bethesda Research Laboratories, Bethesda, Md.). The pellet was resuspended in 2 to 5 ml of Dulbecco’s phosphate-buffered saline and disrupted in a French pressure cell (American Scientific, Silver Spring, Md.) twice at 18,000 lb/in2. Cell debris was removed by centrifugation for 10 min at 10,000 rpm (Sorvall SS-34 rotor), and extracts were sterilized by filtration through a 0.2-μm-pore-size polyether-sulfone filter (Nalge, Rochester, N.Y.). Protein concentration was measured by the bicinchoninic acid method by using a protein assay kit (Sigma, St. Louis, Mo.) with bovine serum albumin as the standard. CNF1 activity in extracts was confirmed by using a bioassay for the formation of multinucleate cells, as previously described (20).
For wound assays, T24 bladder cells were seeded (106 cells) in 60-mm-diameter tissue culture dishes (Corning Glass Works, Corning, N.Y.); cultured 24 to 48 h (37°C, 5% CO2) to confluence in Dulbecco’s modified Eagle medium (DMEM) with glutamine, 10% fetal bovine serum, and 10 mM HEPES; and supplemented with antibiotic-antimycotic solution. A would 1 to 2 cm in width was made in the monolayer with a sterile single-edged razor blade. Fresh medium containing blinded 10-fold serial dilutions of control (DH5/pGEM3) or CNF1-containing (DH5/pISS392) bacterial extract was added, or no extract was added. All wounds were examined microscopically and then incubated for 24 h. At 24 h, dishes were fixed for 5 min with 95% ethanol and stained 1 h with Giemsa stain, and proceeding from one end of the wound, five segments of the wound margin were photographed. The mean number of nuclei entering the wounded region and the mean maximum distance the cells migrated were determined from the five photographic fields, and the percentages of these values obtained from wounds incubated in medium only were calculated. Data are expressed as percentages of the number of cell nuclei or the maximum distance the cells migrated in control wounds and are the means ± standard errors of the mean (SEM) of four (T24 cells) or six (Hs 738 cells) experiments. Data were compared in a two-way analysis of variance with t tests at each concentration of extract.
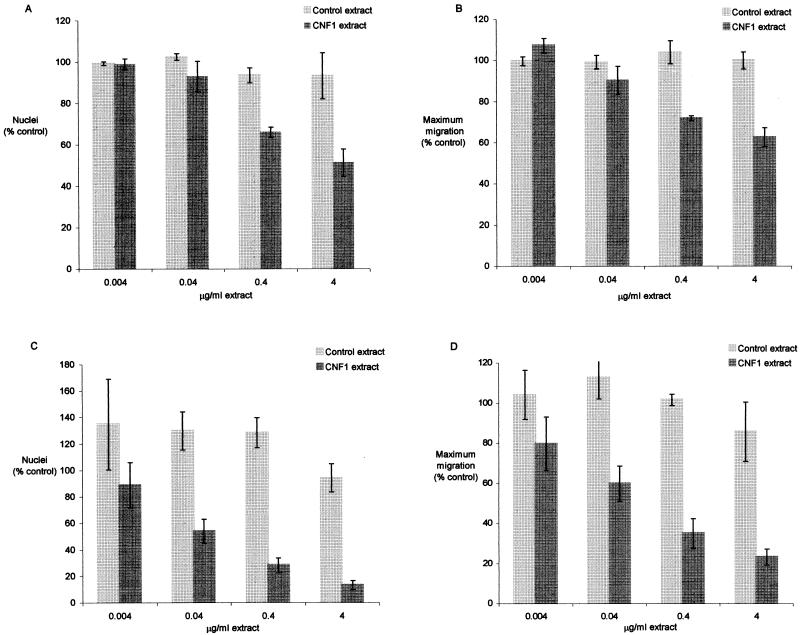
At 24 h after wounding, the number of T24 cells (nuclei were counted since cell borders were sometimes indistinct) advancing into the wounded region and the maximum distance they migrated were significantly lower (P ≤ 0.003) in monolayers exposed to CNF1-containing extracts (0.4 to 4 μg/ml) than they were in monolayers incubated with equivalent concentrations of control cell extract (Fig. 1 and 2). Movement of T24 bladder epithelial cells into the wounded region was not completely blocked; however, CNF1-treated cells (at 4 μg/ml of cell extract) reached only 55% of the control means for the number of nuclei and the maximum distance cells migrated from the original wound margin. This inhibition was dose dependent. The same experiment was also performed on fibroblastic Hs 738 normal human bladder cells (ATCC CRL7474), and repair of wounds in this cell type was also inhibited (P < 0.001) by CNF1-containing extracts (0.04 to 4 μg/ml) (Fig. 1). At higher extract concentrations (0.4 to 4 μg/ml), Hs 738 cells exposed to CNF1 essentially remained at the wound origin, with little appreciable migration into the wounded region.
FIG. 1.
Effect of CNF1-containing bacterial extracts on repair of wounded T24 (A and B) or Hs 738 (C and D) bladder cell monolayers. Confluent monolayers in 60-mm-diameter dishes were wounded and incubated with medium containing CNF1 (DH5/pISS392) or control (DH5/pGEM3) bacterial extracts for 24 h. The plates were fixed and stained with Giemsa stain. The mean number of nuclei entering the wounded region and the mean maximum distance the cells migrated were measured and expressed as percentages of the values obtained with the control wounds (incubated in medium only). Percentages of the number of cell nuclei (A and C) and maximum distance migrated by cells (B and D) in control wounds are the means ± SEM of four (T24 cells) or six (Hs 738 cells) experiments.
FIG. 2.
In vitro repair of wounded T24 bladder cell monolayers. Confluent T24 monolayers in 60-mm-diameter dishes were wounded and incubated with medium containing CNF1 (0.4 μg/ml) (right photo) or control (0.4 μg/ml) (left photo) bacterial extract for 24 h. Plates were fixed, stained with Giemsa stain, and photographed. The line indicates the original margin of the wound.
These findings may result from effects on one or more cell functions, including migration and/or proliferation of cells, or they may be the result of cell death. The number of viable T24 or Hs 738 cells at 24, 48, and 72 h after the addition of the control or CNF1-containing cell extract was determined spectrophotometrically by bioreduction of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS; Promega, Madison, Wis.), to formazan. T24 or Hs 738 cells were seeded to 96-well microtiter plates at 1.0 × 103 cells/well in DMEM with 10 mM HEPES, and 22.5 μl of serial 10-fold dilutions (maximum, 20 μg/ml) of CNF1-containing or control extract was added to the wells. Microtiter plates were incubated at 37°C in 5% CO2 and removed at 24, 48, and 72 h, and the medium was replaced with 100 μl of DMEM containing 20 μl of phenazinemethosulfate-MTS solution (CellTiter96 AQueous cell proliferation assay; Promega). Incubation was continued at 37°C in 5% CO2 in the dark for 4 h, and reduced formazan was measured at optical densities of 490 and 540 nm (OD490–540) in a microtiter plate reader (Titertek Multiscan MCC; Flow Laboratories, Irvine, United Kingdom). In each experiment, the mean OD490–540 from three wells for each concentration of extract was used to determine the percentage of growth relative to that of the wells with medium containing no extract. Data are expressed as percentages of growth (means ± SEM) of untreated wells from two (T24 cells) or five (Hs 738 cells) experiments.
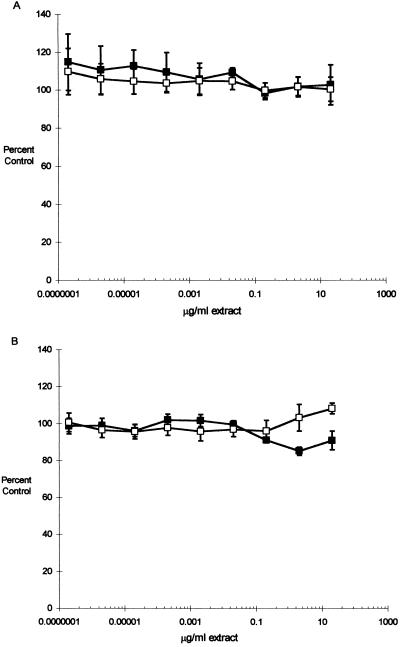
CNF1-containing (DH5/pISS392) cell extract had no effect on the growth of either T24 or Hs 738 cells at 24 or 48 h after seeding (data not shown), nor did we find any effect on the growth of T24 cells at 72 h (Fig. 3). However, a 16% difference (P = 0.004; 20 μg/ml) in the growth of Hs 738 cells after 72 h of incubation with CNF1-containing extracts suggests that proliferation in Hs 738 cells is to some extent inhibited.
FIG. 3.
Growth and viability of bladder cells exposed to CNF1-containing (■) and control (□) extracts. T24 (A) or Hs 738 (B) cells were seeded to 96-well microtiter plates (1.0 × 103 cells/well) and incubated with medium containing 10-fold serial dilutions of CNF1 (DH5/pISS392) or control (DH5/pGEM3) bacterial extract. At 24, 48, and 72 (shown) h, the medium in sample plates was replaced with 100 μl of fresh medium containing 20 μl of MTS solution (see the text). Incubation was continued in the dark for 4 h, and the reduction of MTS was quantitated spectrophotometrically. In each experiment, the growth in the control wells with medium containing no extract was determined and the data were expressed as percentages of control growth (means ± SEM) from two (T24 cells) or five (Hs 738 cells) experiments.
As another way of estimating proliferation, DNA synthesis was evaluated by measuring the incorporation of 5-bromo-2-deoxyuridine (BrdU) after 24 h of exposure to CNF1-containing or control extracts. T24 and Hs 738 cells in DMEM with 10 mM HEPES were seeded at 5.0 × 103 cells/well in 200 μl, and 22.5 μl of serial 10-fold dilutions of CNF1-containing or control extract was added to the wells. Plates were incubated at 37°C in 5% CO2, and at 24 h, 20 μl of medium was removed and replaced with 20 μl of medium containing BrdU (final concentration, 10 μM). BrdU incorporation was measured by enzyme-linked immunosorbent assay after 2 h of labeling according to the protocol of the manufacturer (BrdU labeling and detection kit III; Boehringer Mannheim, Indianapolis, Ind.). The mean OD450–690 from three wells for each concentration of extract was used to determine the percentage of incorporation in the control wells with medium containing no extract. Data are expressed as percentages of control incorporation (means ± SEM) from two experiments.
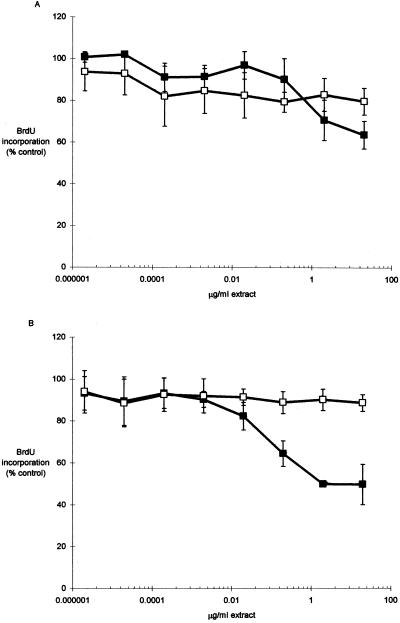
The incorporation of BrdU in cultures treated with CNF1-containing extracts was 22% lower (P = 0.194; 20 μg/ml) in T24 cells and 44% lower (P = 0.002; 20 μg/ml) in Hs 738 cells (Fig. 4) than that in cultures treated with control extract. Thus, there was a significant difference in proliferation in Hs 738 cells, and this inhibition may in part account for decreases in in vitro wound repair. The proliferation of T24 cells was not significantly decreased, but there was an apparent trend toward lower incorporation at higher concentrations (Fig. 4).
FIG. 4.
BrdU incorporation in bladder cells exposed to CNF1-containing (■) and control (□) extracts. T24 (A) or Hs 738 (B) cells were seeded to 96-well microtiter plates (5.0 × 103 cells/well) and incubated with medium containing 10-fold serial dilutions of CNF1 (DH5/pISS392) or control (DH5/pGEM3) bacterial extract for 24 h. Cells were labeled for 2 h (final concentration, 10 μM BrdU) (see the text), and the incorporation of BrdU was determined by enzyme-linked immunosorbent assay. In each experiment, the mean percentages of incorporation in the control wells with medium containing no extract were determined; data are expressed as percentages of control incorporation (means ± SEM) from two experiments for each cell type.
We hypothesized that CNF1 might impair the migration or proliferation of bladder cells and could potentially interfere with repair of the bladder epithelium. Using bladder cell monolayers as an in vitro model, we found that repair of experimental wounds was inhibited by CNF1-containing bacterial extracts. In the case of T24 bladder epithelial cells, repair was not completely blocked, but both the number of cells and the maximum distance they migrated into wounded regions were decreased. More pronounced inhibition was observed in fibroblastic Hs 738 bladder cells, which moved only marginally from the wound origin at higher concentrations of CNF1-containing extract. Since the growth and viability of bladder cells exposed to extracts for 24 to 48 h were not significantly lower based on bioreduction of MTS, it is unlikely that our observations are the result of high levels of cell death in CNF1-treated monolayers. Nevertheless, cell proliferation (based on incorporation of BrdU) was significantly decreased in Hs 738 cells and showed a similar but insignificant trend in T24 cells. The basis for differences in sensitivity between cell types is not known.
In broad terms, our experiments indicate that one or more cell functions potentially involved in the repair of bladder monolayers are altered by the effects of CNF1 in vitro. These include but are not limited to migration and proliferation of bladder cells (e.g., cell-cell interactions were not explored). Determining whether these in vitro results reflect the impact of CNF1 on bladder epithelial cells in vivo will require additional studies. If CNF1 is an E. coli virulence factor, this and other recent works (10, 14, 16, 18, 20) suggest that the mechanism(s) through which it acts is potentially complex.
Acknowledgments
This work was supported by Public Health Service research grants R29 DK52591-01 and PO1 DK49720-01 from the National Institutes of Health.
We thank Richard Hebel for advice on statistical analysis and V. Falbo for providing pISS392.
REFERENCES
- 1.Aepfelbacher M, Essler M, Huber E, Sugai M, Weber S C. Bacterial toxins block endothelial wound repair. Evidence that Rho GTPases control cytoskeletal rearrangements in migrating endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:1623–1629. doi: 10.1161/01.atv.17.9.1623. [DOI] [PubMed] [Google Scholar]
- 2.Alonso P, Blanco J, Blanco M, Gonzalez E A. Frequent production of toxins by Escherichia coli strains from urinary tract infections: relation with hemagglutination. FEMS Microbiol Lett. 1987;48:391–396. [Google Scholar]
- 3.Blanco J, Blanco M, Alonso M P, Blanco J E, Gonzalez E A, Garabal J I. Characteristics of haemolytic Escherichia coli with particular reference to production of cytotoxic necrotizing factor type 1 (CNF1) Res Microbiol. 1992;143:869–878. doi: 10.1016/0923-2508(92)90074-x. [DOI] [PubMed] [Google Scholar]
- 4.Blum G, Falbo V, Caprioli A, Hacker J. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and alpha-hemolysin form the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol Lett. 1995;126:189–195. doi: 10.1111/j.1574-6968.1995.tb07415.x. [DOI] [PubMed] [Google Scholar]
- 5.Caprioli A, Donelli G, Falbo V, Possenti R, Roda L, Roscetti G, Ruggeri F. A cell division-active protein from E. coli. Biochem Biophys Res Commun. 1984;118:587–593. doi: 10.1016/0006-291x(84)91343-3. [DOI] [PubMed] [Google Scholar]
- 6.Caprioli A, Falbo V, Roda L G, Ruggeri F M, Zona C. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect Immun. 1983;39:1300–1306. doi: 10.1128/iai.39.3.1300-1306.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caprioli A, Falbo V, Ruggeri F M, Baldassarri L, Bisicchia R, Ippolito G, Romoli E, Donelli G. Cytotoxic necrotizing factor production by hemolytic strains of Escherichia coli causing extraintestinal infections. J Clin Microbiol. 1987;25:146–149. doi: 10.1128/jcm.25.1.146-149.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Rycke J, González E A, Blanco J, Oswald E, Blanco M, Boivin R. Evidence for two types of cytotoxic necrotizing factor in human and animal clinical isolates of Escherichia coli. J Clin Microbiol. 1990;28:694–699. doi: 10.1128/jcm.28.4.694-699.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falbo V, Pace T, Picci L, Pizzi E, Caprioli A. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect Immun. 1993;61:4909–4914. doi: 10.1128/iai.61.11.4909-4914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falzano L, Fiorentini C, Donelli G, Michel E, Kocks C, Cossart P, Cabanie L, Oswald E, Boquet P. Induction of phagocytic behavior in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol Microbiol. 1993;9:1247–1254. doi: 10.1111/j.1365-2958.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 11.Fiorentini C, Arancia G, Caprioli A, Falbo V, Ruggeri F, Donelli G. Cytoskeletal changes induced in Hep-2 cells by the cytotoxic necrotizing factor of Escherichia coli. Toxicon. 1988;26:1047–1056. doi: 10.1016/0041-0101(88)90203-6. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentini C, Donelli G, Matarrese P, Fabbri A, Paradisi S, Boquet P. Escherichia coli cytotoxic necrotizing factor 1: evidence for induction of actin assembly by constitutive activation of the p21 Rho GTPase. Infect Immun. 1995;63:3936–3944. doi: 10.1128/iai.63.10.3936-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorentini C, Fabbri A, Flatau G, Donelli G, Matarrese P, Lemichez E, Falzano L, Boquet P. Escherichia coli cytotoxic necrotizing factor 1 (CNF1), a toxin that activates the Rho GTPase. J Biol Chem. 1997;272:19532–19537. doi: 10.1074/jbc.272.31.19532. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentini C, Fabbri A, Matarrese P, Falzano L, Boquet P, Malorni W. Hinderance of apoptosis and phagocytic behavior induced by Escherichia coli cytotoxic necrotizing factor 1: two related activities in epithelial cells. Biochem Biophys Res Commun. 1997;241:341–346. doi: 10.1006/bbrc.1997.7723. [DOI] [PubMed] [Google Scholar]
- 15.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 16.Gerhard R, Schmidt G, Hofmann F, Aktories K. Activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor 1 increases intestinal permeability in Caco-2 cells. Infect Immun. 1998;66:5125–5131. doi: 10.1128/iai.66.11.5125-5131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 18.Hofman P, Flatau G, Selva E, Gauthier M, Le Negrate G, Fiorentini C, Rossi B, Boquet P. Escherichia coli cytotoxic necrotizing factor 1 effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect Immun. 1998;66:2494–2500. doi: 10.1128/iai.66.6.2494-2500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hostacka A. Production of enterotoxin, verotoxin, hemolysin, and cytotoxic necrotizing factor by Escherichia coli of intestinal and extraintestinal origin. Folia Microbiol. 1994;39:79–82. doi: 10.1007/BF02814536. [DOI] [PubMed] [Google Scholar]
- 20.Island M D, Cui X, Foxman B, Marrs C F, Stamm W E, Stapleton A E, Warren J W. Cytotoxicity of hemolytic, cytotoxic necrotizing factor 1-positive and -negative Escherichia coli to human T24 bladder cells. Infect Immun. 1998;66:3384–3389. doi: 10.1128/iai.66.7.3384-3389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridley A J, Comoglio P M, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos M F, McCormack S A, Guo Z, Okolicany J, Zheng Y, Johnson L R, Tigyi G. Rho proteins play a critical role in cell migration during the early phase of mucosal restitution. J Clin Investig. 1997;100:216–225. doi: 10.1172/JCI119515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]