ABSTRACT
Background
To investigate the impact of the number of positive lymph nodes (PLNs) on long‐term survival and pathological nodal stage in patients with oral tongue squamous cell carcinoma (OTSCC).
Materials and Methods
Newly diagnosed and nonmetastatic adult patients with OTSCC who underwent curative resection were identified between January 2010 and December 2020. External validation was performed via the SEER registry. Multivariate Cox proportional hazards model was employed to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of pathological nodal features. Propensity score matching (PSM) was used to assess effect of adjuvant chemoradiotherapy (ACRT).
Results
Among 518 curative‐intent OTSCC patients, the number of PLNs independently predicted overall survival (OS), surpassing other pathological nodal features, including extranodal extension, laterality, and lower neck involvement. Patients with 1 or 2 PLNs had comparable worse OS than those with no PLN (median OS of 1 PLN vs. 2 PLNs vs. 0 PLN: 35.1 vs. 30.5 vs. 40.2 months), but better than those with ≥ 3 PLNs (median OS of 1–2 PLNs vs. 3 PLNs: 32.1 vs. 19.0 months). A proposed nodal category with 0, 1–2 PLNs, and ≥ 3 PLNs exhibited increasingly worse OS (HR of 1–2 PLNs and ≥ 3 PLNs vs. 0 PLN: 2.98 [95% CI: 1.89–4.71], p < 0.001; 5.47 [95% CI: 3.33–9], p < 0.001; respectively) and showed improved prediction power versus current pN staging (C‐index: 0.717 vs. 0.713, p < 0.001). PSM analysis revealed that ACRT benefited patients with advanced nodal disease (≥ 3 PLNs) and improved OS. These findings were validated in SEER registry.
Conclusion
The number of PLNs is a better predictor of overall tumor burden for OTSCC and could be a more accurate metric for survival estimation, which should be considered in future simplified pathological nodal staging for better risk stratification and decision‐making in subsites of the oral cavity.
Keywords: AJCC, lymph node metastasis, oral tongue, squamous cell carcinoma, TNM staging
1. Introduction
Squamous cell carcinoma (SCC) of the head and neck ranks as the seventh most common cancer worldwide [1]. SCC of the oral tongue (OTSCC) is biologically distinct from cancers arising in other subsites of the head and neck and is characterized by non‐HPV‐associated cancer [2], increasing incidence [3], and a more aggressive clinical presentation [4, 5]. Although the therapeutic practice has evolved, with chemotherapy, irradiation, and immunotherapy now available in the curative protocol, glossectomy combined with neck resection remains the cornerstone of treatment. Unfortunately, tumor control remains unsatisfactory, with 5‐year overall survival (OS) rates ranging from approximately 30% to 66% among patients treated at high‐volume centers for curative‐intent resection of OTSCC [6, 7]. These findings highlight the need for greater focus on the OTSCC subtype.
Lymph node metastasis is a poor prognostic factor in head and neck SCC and other solid tumors [8, 9, 10]. Patients with even a single metastatic lymph node are classified as having advanced disease, with a 50% decrease in OS. The current American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) N‐staging system for oral cancers serves as a prognostic tool and includes size, laterality, extranodal extension (ENE), and the number of positive lymph nodes (PLNs) [11]. However, certain limitations persist in N staging [12, 13, 14], including the complex nodal staging schema, overlapping survival curves within the pN1‐N3b categories, and the narrow scopes of specific subgroups, consequently impacting its prognostic capability. For example, the pN2a status is only for patients with 1 PLN that is less than 3 cm across, whereas the pN3a status is reserved for patients with a node size greater than 6 cm in the absence of ENE. Moreover, applying a single nodal schema to oral entities with heterogeneous biological behaviors based on distinct anatomical backgrounds may not be adequate. Therefore, proposing a simplified and practical nodal category specific to OTSCC is important for precise survival estimation and treatment management.
Additionally, two large‐scale randomized trials, RTOG 95–012 [15] and EORTC 22931 [16], demonstrated the advantages of adjuvant chemoradiotherapy (ACRT) over adjuvant radiotherapy (RT) for patients with high‐risk head and neck cancers. These studies identified positive margins and ENE as appropriate criteria for adding chemotherapy. Despite numerous studies highlighting the benefits of concurrent ACRT over RT alone in head and neck cancers, data specifically addressing the survival benefit of ACRT in patients with OTSCC remain scarce. Therefore, determining the optimal lymph node metastatic burden threshold for OTSCC patients to benefit from ACRT in terms of survival is a further question that needs to be addressed. In this study, we evaluated the prognostic and therapeutic impact of the number of pathological PLNs among OTSCC patients at the institutional level with detailed clinicopathological data and applied the Surveillance, Epidemiology, and End Results (SEER) registry for external validation.
2. Material and Methods
2.1. Study Cohort and Ethics
We identified 948 consecutive adult patients with newly diagnosed, nonmetastatic OTSCC who underwent curative surgical treatment from January 2010 to December 2020. Among them, 430 were excluded due to prior/synchronous cancers (n = 107), incomplete histologic data/follow‐up (n = 126), no neck dissection (n = 74), > 15 days before surgery (n = 3), < 10 examined lymph nodes (n = 50), or neoadjuvant treatment (n = 70) (Figure 1). This study aimed to determine the significance of the pathological nodal characteristics of PLNs to optimize N staging. We specifically selected patients with at least ≥ 10 dissected lymph nodes to ensure precise pathological N staging as per the 8th edition of the AJCC/UICC guidelines. After applying the exclusion criteria, a total of 50 patients were excluded from further analysis. All patients were restaged according to the 8th AJCC TNM staging system [11]. Treatment adhered to University Cancer Center guidelines (Appendix S1). This retrospective study was conducted in accordance with the 1964 Helsinki Declaration. The study design was approved by the ethics committee (approval number B2019‐047‐Y02) on November 07, 2022. Our research adhered to the STROCSS criteria16 for reporting [17].
FIGURE 1.
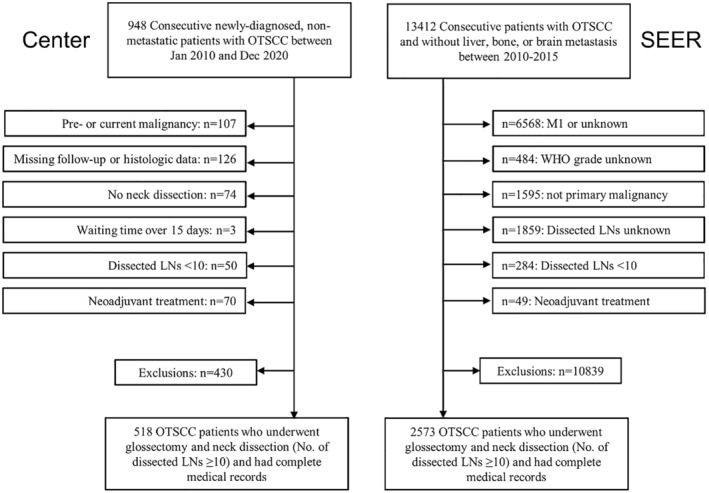
Flowchart of patient selection. OTSCC, oral tongue squamous cell carcinoma; SEER, Surveillance, Epidemiology, and End Results; LNs, lymph nodes.
2.2. Follow‐Up and Endpoints
The follow‐up frequency was at least once every 1–3 months during the first year and then once every 3–12 months thereafter. Follow‐up data up to December 2022 were collected via telephone or outpatient visits. The main clinical endpoint in this study was OS, defined as the period between the date of initial treatment and the date of death from any cause or censoring at the date of the last follow‐up. Disease‐free survival (DFS) was calculated from the date of surgery to the date of disease relapse, distant metastasis, or death from any cause, and locoregional‐free survival (LRFS) was defined as the date of surgery to the date of disease relapse.
2.3. Data Collection From the SEER Registry
Patients who underwent curative resection without brain, liver, or bone metastasis were identified in the SEER database between 2010 and 2015 via the 3rd edition of the International Classification of Disease for Oncology‐3 codes. Patient data were collected via the primary site code for the dorsal surface of the tongue (C02.0), border of the tongue (C02.1), ventral surface of the tongue (C02.2), anterior of the tongue (C02.3), overlapping lesions of the tongue (C02.8), and tongue (C02.9), as well as the histology code for squamous cells (8050–8089). Among the 13,412 OTSCC patients identified, those with 7th edition AJCC M1/unknown stage (n = 6568), unknown histological grade (n = 484), non‐primary tongue lesions (n = 1595), < 10/unknown dissected lymph nodes (n = 2143), and neoadjuvant treatment (n = 49) were excluded. The final validation cohort included 2573 patients (Figure 1).
2.4. Statistical Analysis
Categorical variables are described via frequency rates and percentages, whereas continuous data are presented as medians and interquartile ranges (IQRs). The reverse Kaplan–Meier method was used to calculate the median follow‐up [18]. Survival estimations were determined via the Kaplan–Meier method, and differences were compared via the log‐rank test. Univariate and multivariate analyses were conducted via Cox regression proportional hazards models, and hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) were estimated. Non‐nodal‐related clinicopathological variables with a p‐value < 0.05 in the univariate analysis were further included in the multivariate analysis for determining confounding factors. The optimal cutoff value was identified by analyzing the frequency distribution of metastatic lymph nodes [19], with subgroups exhibiting similar prognoses combined to optimize risk stratification [20]. Harrell's concordance index (C‐index) and its 95% CI were applied to evaluate the discrimination ability of the proposed nodal category and were used to compare the robustness between this system and the 8th AJCC pN‐staging system. Propensity score matching (PSM) was used to minimize selection bias and create a balance between baseline variables. Patients with and without ACRT after curative resection were matched via nearest‐neighbor matching (1:1) and a 0.2 caliper width. Because age was a categorical variable in the SEER cohort, age in our center cohort was categorized with a cutoff value of 45 for PSM analyses [21]. Statistical analyses were performed via the R package (Version 4.2.2; mice, rms, survival, SiZer, party libraries), with the two‐sided significance level set at 0.05.
3. Results
There were 518 cases of OTSCC in the institutional study population, comprising 349 men and 169 women. The median age was 52 years (range, 19–82 years). The median number of dissected lymph nodes was 20 (range, 10–89). At the time of surgical resection, 184 (35.5%) patients had one or more PLNs. During the postoperative period, 73 (39.7%) patients with at least 1 PLN and 23 (6.9%) patients without nodal disease received ACRT. After a median follow‐up of 44.8 months (95% CI, 42–47.5 months), 120 (23.2%) patients died, whereas 153 (29.5%) and 86 (16.6%) patients experienced disease progression and relapse, respectively. The overall median, 1‐, 3‐, and 5‐year OS rates were 36.8 months, 81.9%, 78.3%, and 73.3%, respectively.
3.1. The Impact of the Number of Positive Lymph Nodes on Long‐Term Survival
On multivariate analysis, age, diabetes, betel nut chewing, pathological differentiation, lymphovascular invasion, and pT stage independently predicted OS (all p < 0.05) and were selected as confounding factors for adjusting the impact of the nodal features (Table S1).
According to the univariable analyses, the number of PLNs was strongly negatively associated with OS (p < 0.001; Table 1). In the adjusted multivariate Cox regression model without the number of PLNs, the presence of ENE (p = 0.013) and lower neck involvement (p < 0. 001) independently predicted 5‐year OS, whereas laterality approached significance (p = 0. 077) (Figure 2A). In contrast, when the model incorporated the number of PLNs, neither the presence of ENE, lower neck involvement, nor laterality independently predicted OS (Figure 2B). When the number of PLNs was considered along with ENE, lower neck involvement, and laterality, PLNs remained strongly associated with OS, and its significance still surpassed that of other adverse nodal pathological factors (Table S2).
TABLE 1.
Characteristics of patients with OTSCC.
| Variables | Total (N = 518) | 5‐years (%) | p |
|---|---|---|---|
| Age (years) | 52 (43–61) | — | 0.014 |
| Sex | |||
| Male | 349 (67.4%) | 70.72 | 0.075 |
| Female | 169 (32.6%) | 78.4 | |
| BMI (kg/m2) | 22.5 (20.5–24.8) | — | 0.313 |
| Smoking | |||
| No | 338 (65.3%) | 75.7 | 0.025 |
| Yes | 180 (34.7%) | 68.78 | |
| Alcohol use | |||
| No | 426 (82.2%) | 74.06 | 0.158 |
| Yes | 92 (17.8%) | 69.89 | |
| Betel net | |||
| No | 484 (93.4%) | 75.03 | 0.004 |
| Yes | 34 (6.6%) | 37.91 | |
| Hypertension | |||
| No | 431 (83.2%) | 73.2 | 0.399 |
| Yes | 87 (16.8%) | 74.2 | |
| Diabetes | |||
| No | 471 (90.9%) | 74.66 | 0.008 |
| Yes | 47 (9.1%) | 59.34 | |
| Family history | |||
| No | 478 (92.3%) | 73.98 | 0.276 |
| Yes | 40 (7.7%) | 65.02 | |
| Differentiation | |||
| Well | 307 (59.3%) | 68.51 | 0.018 |
| Moderate‐poor | 211 (40.7%) | 79.51 | |
| Lymphovascular involvement | |||
| No | 495 (95.6%) | 74.35 | 0.006 |
| Yes | 23 (4.4%) | 50.72 | |
| Perineural invasion | |||
| No | 333 (64.3%) | 79.59 | < 0.001 |
| Yes | 185 (35.7%) | 60.49 | |
| Margin status | |||
| Negative | 513 (99%) | 73.92 | 0.106 |
| Positive | 5 (1%) | 40 | |
| pT stage | |||
| T1 | 156 (30.1%) | 89.41 | < 0.001 |
| T2 | 188 (36.3%) | 73.88 | |
| T3 | 164 (31.7%) | 57.08 | |
| T4 | 10 (1.9%) | 28.57 | |
| pN stage | |||
| N0 | 334 (64.5%) | 86.32 | < 0.001 |
| N1 | 57 (11%) | 59.86 | |
| N2 | 106 (20.5%) | 45.93 | |
| N3 | 21 (4.1%) | 42.49 | |
| ACRT | |||
| No | 422 (81.5%) | 75.5 | 0.011 |
| Yes | 96 (18.5%) | 63.02 | |
| Neck dissection | |||
| Laterality | 491 (94.8%) | 73.87 | 0.078 |
| Bilaterality | 27 (5.2%) | 64.96 | |
| No. of positive lymph nodes | 0 (0–1) | — | < 0.001 |
| No. of dissected lymph nodes | 20 (15–26) | — | 0.122 |
| Lower neck involvement | |||
| No | 499 (96.3%) | 74.86 | < 0.001 |
| Yes | 19 (3.7%) | 30.62 | |
| ENE | |||
| Negative | 495 (95.6%) | 74.53 | < 0.001 |
| Positive | 23 (4.4%) | 42.29 | |
| Laterality (N2c) | |||
| Unilaterality | 514 (99.2%) | 73.79 | 0.001 |
| Bilaterality | 4 (0.8%) | NA | |
Note: Continuous data are medians, with IQRs in parentheses.
Abbreviations: ACRT, adjuvant chemoradiotherapy; BMI, body mass index; ENE, extranodal extension; IQR, interquartile ranges; NA, not available; OTSCC, oral tongue squamous cell carcinoma.
FIGURE 2.
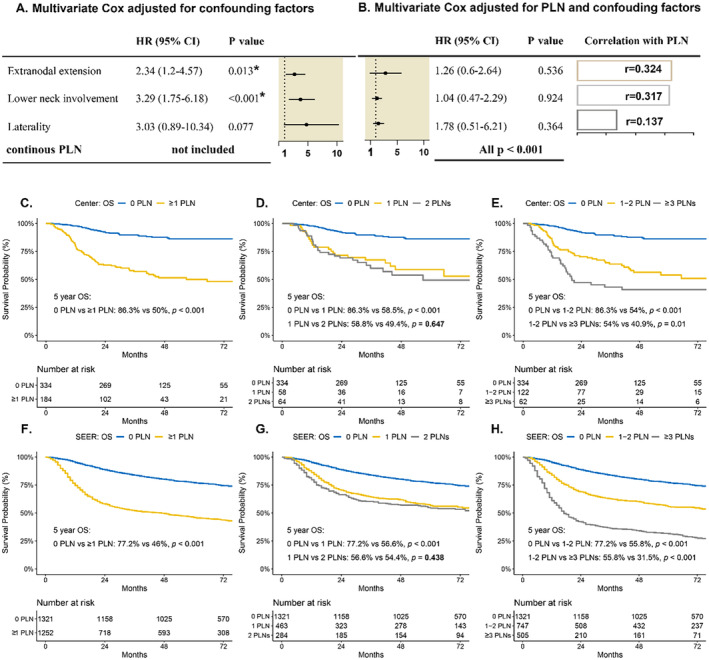
Forest plot and Kaplan–Meier analysis of overall survival by the PLN. (A) After adjusting for confounding factors (age, diabetes, betel nut chewing, differentiation, pT stage, and lymphovascular invasion), extranodal extension (ENE), and lower‐neck involvement were independent predictors of overall survival (OS), while laterality marginally approached significant. (B) After adjusting for the continuous number of PLNs and the same confounding factors, ENE, lower neck involvement, and laterality were not independent predictors of OS, but in each multivariate analysis, the number of PLNs was the independent predictor of OS. (C–H) A significant difference was observed between the patients with no nodal disease versus nodal disease, yet no significant difference was observed between the patients with one PLN versus those with two PLNs. Thus, we combined patients with one or two PLNs as one group. CI, confidence interval; HR, hazard ratio; OS, overall survival; PLN, positive lymph node; SEER, Surveillance, Epidemiology, and End Results Program.
3.2. Impact of the Number of Positive Lymph Nodes on Nodal Staging
The number of PLNs is a better predictor of overall tumor burden than other variables and could be a more accurate metric for nodal staging. Patients with nodal involvement had a worse OS than those without nodal involvement (median OS: 28.2 months for those with ≥ 1 PLN vs. 40.2 months for those with 0 PLN, p < 0.001) (Figure 2C). The distribution of PLNs is detailed in Figure S1, with a marked decline in patient numbers beyond three PLNs. Stratification by PLN count revealed prognostic differences: patients with one or two PLNs had a worse OS than patients with no nodal disease (median OS: 35.1 months for one PLN, 30.5 months for two PLNs, and 40.2 months for 0 PLN) (Figure 2D), and patients with three or more PLNs had the poorest prognosis (median OS: 19.0 months for ≥ 3 PLNs vs. 32.1 months for 1–2 PLNs, p = 0.01) (Figure 2E). Similar results were observed for DFS (median OS: 35.6 months for 0 PLN, 26.7 months for 1–2 PLNs, and 10.7 months for ≥ 3 PLNs; all p < 0.05) and LRFS (35.9 months vs. 30.5 months vs. 17.3 months; all p < 0.05) (Figure S2). For the SEER population, patients with 0, 1–2, and ≥ 3 PLNs had increasingly worse OS (median OS: 66.0 months for 0 PLN, 54.0 months for 1–2 PLNs, and 17.0 months for ≥ 3 PLNs; all p < 0.001) (Figure 2F–H).
A simplified nodal categorization consisting of the number of PLNs was developed (0 PLN, 1–2 PLNs, and ≥ 3 PLNs). In the adjusted multivariate analysis, the proposed nodal category independently predicted OS, with a 5‐year OS of 86.3% for the 0‐PLN group, 54% for the 1–2‐PLNs group (HR: 2.98 [1.89, 4.71]; p < 0.001), and 40.9% for the ≥ 3‐PLNs group (HR: 5.47 [3.33, 9]; p < 0.001) (Table 2). The C‐index for the proposed nodal category showed better predictive ability (0.717, 95% CI [0.673 to 0.761]) than did the 8th edition AJCC N‐staging (0.713, 95% CI [0.669 to 0.757]) (p = 0.005), which was validated in the SEER registry (0.674, 95% CI [0.659–0.69] vs. 0.666, 95% CI [0.65 to 0.681]; p < 0.001).
TABLE 2.
Multivariate Cox regression analyses for patients with OTSCC regarding overall survival.
| Variables | HR (95% CI) | p |
|---|---|---|
| Age | 1.02 (1, 1.03) | 0.015 |
| Betel nut | ||
| No | ||
| Yes | 2.72 (1.48, 5) | 0.001 |
| Diabetes | ||
| No | ||
| Yes | 1.94 (1.13, 3.32) | 0.016 |
| pT stage | ||
| T1 | ||
| T2 | 1.96 (1.08, 3.58) | 0.027 |
| T3 | 2.72 (1.52, 4.87) | 0.001 |
| T4 | 2.23 (0.72, 6.92) | 0.164 |
| Differentiation | ||
| Well | ||
| Moderate‐poor | 0.74 (0.5, 1.11) | 0.142 |
| Lymphovascular involvement | ||
| No | ||
| Yes | 1.57 (0.78, 3.19) | 0.208 |
| Simplified nodal category | ||
| 0 PLN | ||
| 1–2 PLNs | 2.98 (1.89, 4.71) | < 0.001 |
| ≥ 3 PLNs | 5.47 (3.33, 9) | < 0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; OTSCC, oral tongue squamous cell carcinoma; PLN, positive lymph node.
Given the similar prognoses of patients with one PLN and two PLNs, we further divided the 59 patients with current pN2 and 1–2 PLNs into pN1, with descending stage shifts of pN1 57 (11%) and pN2 106 (20.5%) to pN1 116 (22.4%) and pN2 47 (9.1%). After downstaging current pN2 with 1–2 PLNs to pN1, the survival curves of pN1 and pN2 were separated (before adjustment, p = 0.088; after adjustment, p = 0.015), with a significantly improved C‐index of the whole pN stage (C‐index before vs. after adjustment, 0.713 95% CI [0.669 to 0.757] vs. 0.716 95% CI [0.673 to 0.76], p = 0.004). In the SEER registry, a total of 253 patients with pN2 and 1–2 PLNs were downstaged to pN1, also showing a decreasing sample size of pN1 532 (20.7%) and pN2 704 (27.4%) to pN1 785 (30.5%) and pN2 451 (17.5%) and a significantly improved C‐index (0.673 95% CI [0.658 to 0.689] vs. 0.666 95% CI [0.65 to 0.681], p < 0.001). These findings underscore the importance of considering a stratified number of PLNs (1 to 2) in future optimizations of pN staging.
3.3. The Impact of the Proposed Nodal Category on Adjuvant Treatment
Among the 334, 122, and 62 patients with 0 PLN, 1–2 PLNs, and ≥ 3 PLNs, respectively, 23 (6.9%), 41 (33.6%), and 32 (51.6%) patients received ACRT, respectively. Patients with 0 PLN, 1–2 PLNs and ≥ 3 PLNs with and without ACRT were matched on a 1:1 basis, with age (≤ 44 vs. > 44 years), sex, body mass index, betel nut chewing, diabetes, pT stage, and the continuous number of dissected and metastatic lymph nodes as pairing factors. Owing to the balanced distribution of these matching factors among patients with ≥ 3 PLNs with and without ACRT, patients with ACRT in our center were matched with 23 and 36 patients without ACRT for 0 and 1–2 PLNs disease, respectively. ACRT further improved the OS of patients with ≥ 3 PLNs (5‐year OS: 26.2% vs. 55.3%; p = 0.01; Figure 3A and Table S3). This trend was observed in the SEER study population, where separate survival curves were generated for the ACRT and non‐ACRT groups after PSM (5‐year OS: 24.7% vs. 37.1%; p = 0.001; Figure 3B and Figure S3 and Table S4). In contrast, there was no significant difference in OS between patients who received ACRT and those who did not receive ACRT with 0 PLN (Figure S4 and Tables S5 and S6) or with 1–2 PLNs (Figure S5 and Tables S7 and S8), either at the institutional level or in the SEER registry (all p > 0.05).
FIGURE 3.
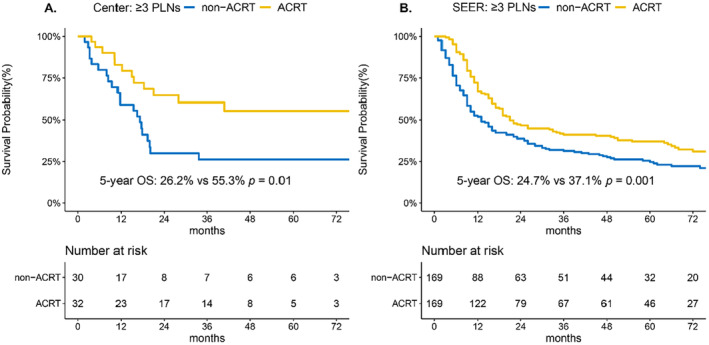
Kaplan–Meier curves of overall survival (OS) in patients with advanced nodal disease (≥ 3 PLNs) with and without ACRT. After PSM, ACRT significantly improved the OS of patients with ≥ 3 PLNs in (A) our center and (B) SEER registry cohort, respectively. ACRT, adjuvant chemoradiotherapy; PLN, positive lymph node; SEER, Surveillance, Epidemiology, and End Results Program.
4. Discussion
In this institutional‐level retrospective study, we found that the number of PLNs is a robust, independent predictor for OS, surpassing other nodal features. Additionally, the derived nodal category for OTSCC showed improved prediction performance over the 8th edition AJCC N‐staging. Moreover, we found that patients with ≥ 3 PLNs could benefit from ACRT. The external validation of the proposed nodal category in the SEER registry offers the potential for a simplified, comparably effective nodal framework in oral subgenus prognostication.
One important finding of this study is that the presence of ENE, lower neck involvement, and laterality were no longer independent predictors of survival when the number of PLNs for OTSCC was considered. A multicenter study involving 3704 patients with oral cancers emphasized the greater importance of the number of PLNs than of contralateral lymph node involvement [22]. These findings also align with a later larger‐scale study conducted by Ho et al. [8], which highlights the value of PLNs over nodal size and laterality. In their proposed staging system for N0, N1 (1 PLN/ENE‐), N2 (1 PLN/ENE+ or 2 PLNs), N3a (3–7 PLNs), and N3b (≥ 8 PLNs), the role of ENE in risk stratification, primarily among singularly affected nodes, is noteworthy. Another study [23] yielded analogous results, suggesting that patients with OTSCC with ≥ 2 PLNs fare worse than those with a single positive node, irrespective of ENE status. Given the correlation between the increased number of metastatic lymph nodes and increased ENE incidence [24, 25], segregating ENE status from mild or extensive nodal burden may have limited utility in prognostic stratification.
Spreading through the lymphatic chain vertically or invading surrounding tissues horizontally is a manifestation of tumor cell invasiveness in different dimensions. An increased number of metastatic lymph nodes signifies the inability of the body's immune system to effectively prevent cancer cells from disseminating or metastasizing via the lymphatic route [26]. For the presence of ENE, some authors associated its adverse prognosis with hematogenous dissemination, where cancer cells migrate into surrounding soft tissues, enter the blood circulation, and ultimately increase the risk of distant metastasis [27, 28]. Other studies have indicated that deposits of isolated tumor cells beyond the nodal capsule, which may increase the risk of regional recurrence [29, 30]. However, the underlying mechanism for the superior prognostic value of the number of metastatic lymph nodes compared with the presence of ENE remains elusive and requires further detailed exploration. In this study, we confirmed the dominant impact of the number of PLNs and grouped patients with one to two PLNs into a single‐risk subgroup due to their similar prognoses.
In recent studies, alternative methods for classifying lymph node metastasis, such as the lymph node ratio (LNR) [31, 32] and log odds of positive lymph nodes (LODDS) [33], have been proposed as prognostic indicators in patients with oral cancers. While one study demonstrated that the PLN, LNR, and LODDS offer comparable prognostic value [34], another found that only the PLN remained an independent prognostic factor for OS in multivariate analysis [35]. These findings suggest that the PLN may serve as a more straightforward and effective measure of nodal burden than complex mathematical models.
In line with this, studies have identified key PLN thresholds, such as ≥ 5 PLNs in head and neck cancer [36], with Ho et al. [8] and Sinha et al. [9] further validating the prognostic significance of this threshold for risk stratification in oral cancer and p16+ oropharyngeal SCC, respectively. However, our study of OTSCC patients identified a lower cutoff at 3 PLNs, which aligns with the findings of Rajappa et al. [37], who proposed a cutoff of ≥ 3 PLNs for advanced N3 staging. Our proposed nodal classification, specific to the subsite of the oral cavity, offers a simplified nodal factor with fewer risk groups and clearer survival distinctions. In particular, current N‐staging considers only patients with ≥ 1 PLN for upstaging, and our nodal category broadens stratification with more nodes. In addition, given the similar prognoses of one PLN and two PLNs, we further downstage the 8th AJCC N2 with 1–2 PLNs to N1 and found an improved C‐index, which also provides solutions for optimizing current pN‐staging by refining the PLN threshold (1–2 nodes).
The current National Comprehensive Cancer Network (NCCN) guidelines recommend that adjuvant therapy be based on individual patient characteristics [38]. Zumsteg et al. [39] reported that in 7144 nonoropharyngeal head and neck SCCs from the National Cancer Database, increasing metastatic nodal burden was associated with increased benefit from postoperative adjuvant chemoradiation relative to radiotherapy alone, corresponding to nearly 8% and 23% absolute improvements in 3‐year OS for subgroup patients with 3–5 PLNs and ≥ 6 PLNs, respectively, over patients with 0–2 PLNs. In contrast, when the same data registry was used, Spiotto et al. [40] demonstrated that OTSCC with ≥ 2 PLNs was associated with a benefit from adjuvant systematic treatment compared with surgery plus postoperative radiation alone (3‐year OS: 67.5% vs. 57.1%; p = 0.01). In our study, which utilized a matching method for OTSCC, we observed a significant improvement in 5‐year OS in the proposed advanced nodal category (≥ 3 PLNs) at our institution and in the SEER registry. This finding supports the clinical practicality of this proposed simplified nodal scheme, which allows adjuvant chemoradiation to be administered individually.
Several limitations warrant acknowledgment. First, our institution lacks data on nodal size—a parameter whose prognostic value has diminished in previous studies and may thus have less impact on our analysis. Second, despite the advantages of the proposed nodal scheme, replacing the current pN stage with it would represent a significant adjustment. Nevertheless, we aimed to optimize AJCC N‐staging in the subsite of the head and neck by providing a more representative nodal feature rather than replacing the existing traditional staging system. Finally, patients with pN0 constituted more than half of the study population; these patients have heterogeneous prognoses, and the discovery of more biomarkers is needed to make generalizable predictions.
5. Conclusion
In summary, we found that the number of metastatic lymph nodes was the strongest factor for long‐term tumor control in patients with OTSCC. Patients with ≥ 3 PLNs derived greater relative and absolute improvements in OS from postoperative systematic treatment than patients with 0–2 PLNs did. Our study included an intricate analysis of clinicopathological variables, providing a new, simplified nodal schema with improved risk discrimination in OTSCC.
Author Contributions
Wenjie Huang: conceptualization (lead), data curation (lead), investigation (lead), resources (equal), software (lead), visualization (lead), writing – original draft (lead), writing – review and editing (lead). Yu Zhang: conceptualization (equal), data curation (equal), investigation (equal), resources (equal), supervision (equal), writing – review and editing (equal). Hao Li: investigation (equal), software (equal), writing – original draft (equal), writing – review and editing (equal). Zhiying Liang: data curation (supporting), investigation (supporting), writing – original draft (equal), writing – review and editing (equal). Shumin Zhou: investigation (supporting), visualization (supporting), writing – review and editing (supporting). Jie Pan: investigation (supporting), software (equal), visualization (equal), writing – review and editing (equal). Hui Xie: investigation (supporting), software (equal), visualization (equal), writing – review and editing (equal). Chao Luo: investigation (supporting), writing – original draft (equal), writing – review and editing (supporting). Shuqi Li: investigation (supporting), writing – original draft (equal), writing – review and editing (supporting). Guangying Ruan: data curation (supporting), investigation (supporting), writing – review and editing (supporting). Fei Ai: investigation (supporting), project administration (equal), supervision (equal), writing – review and editing (supporting). Yanfeng Chen: conceptualization (equal), investigation (supporting), project administration (equal), supervision (lead), writing – review and editing (equal).
Ethics Statement
This retrospective study received approval from the Clinical Research Ethics Committee of the Sun Yat‐Sen University Cancer Center (approval number B2019‐047‐Y02).
Consent
Informed consent was waived due to the retrospective nature of the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Data S1.
Acknowledgments
We want to thank all the seniors who have worked tirelessly and dedicatedly to this type of cancer. This work was supported by the National Natural Science Foundation of China [grant number 82171906] and the National Natural Science Foundation of China‐Regional Science Foundation Project (grant number 82260358). Special Fund for Major R&D of China National Health Commission (No. 2021KYSHX01508). The funding source did not have any role in the study design, collection, analysis, or interpretation of the data, writing of the report, or decision to submit the article for publication.
Funding: This work was supported by National Natural Science Foundation of China (82171906), National Natural Science Foundation of China‐Regional Science Foundation Project (82260358), and Major R&D of China National Health Commission (2021KYSHX01508).
Wenjie Huang, Yu Zhang, Hao Li and Zhiying Liang.
Contributor Information
Fei Ai, Email: aifei@sysucc.org.cn.
Yanfeng Chen, Email: chenyf@sysucc.org.cn.
Data Availability Statement
The data described in this study have been deposited at the Sun Yat‐Sen University Cancer Center for future reference (www.researchdata.org.cn) and are available upon reasonable request.
References
- 1. Chow L. Q. M., “Head and Neck Cancer,” New England Journal of Medicine 382, no. 1 (2020): 60–72, 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 2. Vered M., Dayan D., and Salo T., “The Role of the Tumour Microenvironment in the Biology of Head and Neck Cancer: Lessons From Mobile Tongue Cancer,” Nature Reviews. Cancer 11, no. 5 (2011): 382; author reply 382, 10.1038/nrc2982-c1. [DOI] [PubMed] [Google Scholar]
- 3. Stepan K. O., Mazul A. L., Larson J., et al., “Changing Epidemiology of Oral Cavity Cancer in the United States,” Otolaryngology and Head and Neck Surgery 168, no. 4 (2022): 761–768, 10.1177/01945998221098011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rusthoven K., Ballonoff A., Raben D., and Chen C., “Poor Prognosis in Patients With Stage I and II Oral Tongue Squamous Cell Carcinoma,” Cancer 112, no. 2 (2008): 345–351, 10.1002/cncr.23183. [DOI] [PubMed] [Google Scholar]
- 5. Far hood Z., Simpson M., Ward G. M., Walker R. J., and Osazuwa‐Peters N., “Does Anatomic Subsite Influence Oral Cavity Cancer Mortality? A SEER Database Analysis,” Laryngoscope 129, no. 6 (2019): 1400–1406, 10.1002/lary.27490. [DOI] [PubMed] [Google Scholar]
- 6. Myers J. N., Greenberg J. S., Mo V., and Roberts D., “Extracapsular Spread. A Significant Predictor of Treatment Failure in Patients With Squamous Cell Carcinoma of the Tongue,” Cancer 92, no. 12 (2001): 3030–3036, . [DOI] [PubMed] [Google Scholar]
- 7. Faisal M., Dhanani R., Ullah S., et al., “Prognostic Outcomes of Treatment naïve Oral Tongue Squamous Cell Carcinoma (OTSCC): A Comprehensive Analysis of 14 Years,” European Archives of Oto‐Rhino‐Laryngology 278, no. 8 (2021): 3045–3053, 10.1007/s00405-020-06482-x. [DOI] [PubMed] [Google Scholar]
- 8. Ho A. S., Kim S., Tighiouart M., et al., “Metastatic Lymph Node Burden and Survival in Oral Cavity Cancer,” Journal of Clinical Oncology 35, no. 31 (2017): 3601–3609, 10.1200/jco.2016.71.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinha P., Kallogjeri D., Gay H., et al., “High Metastatic Node Number, Not Extracapsular Spread or N‐Classification Is a Node‐Related Prognosticator in Transorally‐Resected, Neck‐Dissected p16‐Positive Oropharynx Cancer,” Oral Oncology 51, no. 5 (2015): 514–520, 10.1016/j.oraloncology.2015.02.098. [DOI] [PubMed] [Google Scholar]
- 10. Deng J., Yamashita H., Seto Y., and Liang H., “Increasing the Number of Examined Lymph Nodes Is a Prerequisite for Improvement in the Accurate Evaluation of Overall Survival of Node‐Negative Gastric Cancer Patients,” Annals of Surgical Oncology 24, no. 3 (2017): 745–753, 10.1245/s10434-016-5513-8. [DOI] [PubMed] [Google Scholar]
- 11. Amin M., Edge S., Greene F., et al., AJCC Cancer Staging Manual (New York, NY: Springer, 2016). [Google Scholar]
- 12. Almangush A., Pirinen M., Youssef O., Mäkitie A. A., and Leivo I., “Risk Stratification in Oral Squamous Cell Carcinoma Using Staging of the Eighth American Joint Committee on Cancer: Systematic Review and Meta‐Analysis,” Head & Neck 42, no. 10 (2020): 3002–3017, 10.1002/hed.26344. [DOI] [PubMed] [Google Scholar]
- 13. Moeckelmann N., Ebrahimi A., Tou Y. K., et al., “Prognostic Implications of the 8th Edition American Joint Committee on Cancer (AJCC) Staging System in Oral Cavity Squamous Cell Carcinoma,” Oral Oncology 85 (2018): 82–86, 10.1016/j.oraloncology.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 14. Cramer J. D., Reddy A., Ferris R. L., Duvvuri U., and Samant S., “Comparison of the Seventh and Eighth Edition American Joint Committee on Cancer Oral Cavity Staging Systems,” Laryngoscope 128, no. 10 (2018): 2351–2360, 10.1002/lary.27205. [DOI] [PubMed] [Google Scholar]
- 15. Cooper J. S., Pajak T. F., Forastiere A. A., et al., “Postoperative Concurrent Radiotherapy and Chemotherapy for High‐Risk Squamous‐Cell Carcinoma of the Head and Neck,” New England Journal of Medicine 350, no. 19 (2004): 1937–1944, 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 16. Bernier J., Domenge C., Ozsahin M., et al., “Postoperative Irradiation With or Without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer,” New England Journal of Medicine 350, no. 19 (2004): 1945–1952, 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 17. Mathew G., Agha R., Albrecht J., et al., “STROCSS 2021: Strengthening the Reporting of Cohort, Cross‐Sectional and Case‐Control Studies in Surgery,” International Journal of Surgery 96 (2021): 106165, 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 18. Schemper M. and Smith T. L., “A Note on Quantifying Follow‐Up in Studies of Failure Time,” Controlled Clinical Trials 17, no. 4 (1996): 343–346, 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 19. Li J., Zhao Q., Zhang Y., et al., “Prognostic Value of Quantitative Cervical Nodal Necrosis Burden on MRI in Nasopharyngeal Carcinoma and Its Role as a Stratification Marker for Induction Chemotherapy,” European Radiology 32, no. 11 (2022): 7710–7721, 10.1007/s00330-022-08785-5. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X. F., Xue F., Dong D. H., et al., “Number and Station of Lymph Node Metastasis After Curative‐Intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis,” Annals of Surgery 274, no. 6 (2021): e1187–e1195, 10.1097/sla.0000000000003788. [DOI] [PubMed] [Google Scholar]
- 21. Siegelmann‐Danieli N., Hanlon A., Ridge J. A., Padmore R., Fein D. A., and Langer C. J., “Oral Tongue Cancer in Patients Less Than 45 Years Old: Institutional Experience and Comparison With Older Patients,” Journal of Clinical Oncology 16, no. 2 (1998): 745–753, 10.1200/jco.1998.16.2.745. [DOI] [PubMed] [Google Scholar]
- 22. Ebrahimi A., Gil Z., Amit M., et al., “The Prognosis of N2b and N2c Lymph Node Disease in Oral Squamous Cell Carcinoma Is Determined by the Number of Metastatic Lymph Nodes Rather Than Laterality: Evidence to Support a Revision of the American Joint Committee on Cancer Staging System,” Cancer 120, no. 13 (2014): 1968–1974, 10.1002/cncr.28686. [DOI] [PubMed] [Google Scholar]
- 23. Greenberg J. S., Fowler R., Gomez J., et al., “Extent of Extracapsular Spread: A Critical Prognosticator in Oral Tongue Cancer,” Cancer 97, no. 6 (2003): 1464–1470, 10.1002/cncr.11202. [DOI] [PubMed] [Google Scholar]
- 24. Mermod M., Tolstonog G., Simon C., and Monnier Y., “Extracapsular Spread in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta‐Analysis,” Oral Oncology 62 (2016): 60–71, 10.1016/j.oraloncology.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 25. Maxwell J. H., Ferris R. L., Gooding W., et al., “Extracapsular Spread in Head and Neck Carcinoma: Impact of Site and Human Papillomavirus Status,” Cancer 119, no. 18 (2013): 3302–3308, 10.1002/cncr.28169. [DOI] [PubMed] [Google Scholar]
- 26. Chan H. L. and Zhang X. H., “Node Foretold: Cancer Cells in Lymph Node Rewire The Immune System to Enable Further Metastases,” Cancer Cell 40, no. 8 (2022): 812–814, 10.1016/j.ccell.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 27. Grégoire V., Eisbruch A., Hamoir M., and Levendag P., “Proposal for the Delineation of the Nodal CTV in the Node‐Positive and the Post‐Operative Neck,” Radiotherapy and Oncology 79, no. 1 (2006): 15–20, 10.1016/j.radonc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 28. Mao Y., Wang S., Lydiatt W., et al., “Unambiguous Advanced Radiologic Extranodal Extension Determined by MRI Predicts Worse Outcomes in Nasopharyngeal Carcinoma: Potential Improvement for Future Editions of N Category Systems,” Radiotherapy and Oncology 157 (2021): 114–121, 10.1016/j.radonc.2021.01.015. [DOI] [PubMed] [Google Scholar]
- 29. Chang C. W., Wang C., Lu C. J., et al., “Incidence and Prognostic Significance of Extranodal Extension in Isolated Nodal Recurrence of Oral Squamous Cell Carcinoma,” Radiotherapy and Oncology 167 (2022): 81–88, 10.1016/j.radonc.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 30. Malik A., Nair S., Singh A., et al., “Soft Tissue Deposit in Neck Dissection Specimen Carries a Poor Prognosis in Oral Cancer: A Matched Pair Analysis,” Head & Neck 42, no. 8 (2020): 1783–1790, 10.1002/hed.26103. [DOI] [PubMed] [Google Scholar]
- 31. Talmi Y. P., Takes R. P., Alon E. E., et al., “Prognostic Value of Lymph Node Ratio in Head and Neck Squamous Cell Carcinoma,” Head & Neck 40, no. 5 (2018): 1082–1090, 10.1002/hed.25080. [DOI] [PubMed] [Google Scholar]
- 32. Tomo S., Araújo W. A. F., de Castro T. F., et al., “Potential of Lymph‐Node Ratio as a Prognostic Factor for Patients With Oral Squamous Cell Carcinoma,” Journal of Stomatology, Oral and Maxillofacial Surgery 123, no. 6 (2022): e814–e821, 10.1016/j.jormas.2022.07.013. [DOI] [PubMed] [Google Scholar]
- 33. Lee C. C., Ho H. C., Su Y. C., Lee M. S., Hung S. K., and Chen Y. L., “The Prognostic Ability of Log Odds of Positive Lymph Nodes in Oral Cavity Squamous Cell Carcinoma,” Medicine (Baltimore) 94, no. 27 (2015): e1069, 10.1097/md.0000000000001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Subramaniam N., Balasubramanian D., Kumar N., et al., “Lymph Node Staging Systems in Oral Squamous Cell Carcinoma: A Comparative Analysis,” Oral Oncology 97 (2019): 92–98, 10.1016/j.oraloncology.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 35. Tomo S., de Castro T. F., Araújo W. A. F., et al., “Influence of Different Methods for Classification of Lymph Node Metastases on the Survival of Patients With Oral Squamous Cell Carcinoma,” Journal of Stomatology, Oral and Maxillofacial Surgery 124, no. 2 (2023): 101311, 10.1016/j.jormas.2022.10.010. [DOI] [PubMed] [Google Scholar]
- 36. Roberts T. J., Colevas A. D., Hara W., Holsinger F. C., Oakley‐Girvan I., and Divi V., “Number of Positive Nodes Is Superior to the Lymph Node Ratio and American Joint Committee on Cancer N Staging for the Prognosis of Surgically Treated Head and Neck Squamous Cell Carcinomas,” Cancer 122, no. 9 (2016): 1388–1397, 10.1002/cncr.29932. [DOI] [PubMed] [Google Scholar]
- 37. Rajappa S. K., Maheshwari U., Jaipuria J., et al., “Number of Positive Nodes ‐ Current Relevance in Determining Prognosis of Oral Cavity Cancer After the Recent AJCC Staging Update,” Oral Oncology 90 (2019): 1–5, 10.1016/j.oraloncology.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 38. National Comprehensive Cancer Network , “NCCN clinical practice guidelines in oncology: head and neck cancers: Version1 ed,” 2023, https://www.nccn.org/professionals/physician_gls/head_and_neck_cancer.pdf. [DOI] [PubMed]
- 39. Zumsteg Z. S., Luu M., Kim S., et al., “Quantitative Lymph Node Burden as a ‘very‐High‐risk’ Factor Identifying Head and Neck Cancer Patients Benefiting From Postoperative Chemoradiation,” Annals of Oncology 30, no. 1 (2019): 76–84, 10.1093/annonc/mdy490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spiotto M. T., Jefferson G. D., Wenig B., Markiewicz M. R., Weichselbaum R. R., and Koshy M., “Survival Outcomes for Postoperative Chemoradiation in Intermediate‐Risk Oral Tongue Cancers,” Head & Neck 39, no. 12 (2017): 2537–2548, 10.1002/hed.24932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data described in this study have been deposited at the Sun Yat‐Sen University Cancer Center for future reference (www.researchdata.org.cn) and are available upon reasonable request.


