Abstract
1. Three phosphodiesterases that are capable of hydrolysing 3':5'-cyclic nucleotides were purified from potato tubers. 2. The phosphodiesterases were fractionated by (NH4)2SO4 precipitation and CM-cellulose chromatography. The phosphodiesterases were resolved from each other and further purified by gel filtration in high- and low-ionic-strength conditions. 3. All three enzymes lacked significant nucleotidase activity. 4. Enzymes I and II had mol. wts. 240,000 and 80,000 respectively, determined by gel filtration, whereas enzyme III showed anomalous behaviour on gel filtration, behaving as a high- or low-molecular-weight protein in high- or low-ionic-strength buffers respectively. 5. All enzymes hydrolysed 2':3'-cyclic nucleotides as well as 3':5'-cyclic nucleotides. The enzymes also had nucleotide pyrophosphatase activity, hydrolysing NAD+ and UDP-glucose to various extents. Enzymes I and II hydrolyse cyclic nucleotides at a greater rate than NAD+, whereas enzyme III hydrolyses NAD+ at a much greater rate than cyclic nucleotides. All three enzymes hydrolysed the artificial substrate bis-(p-nitro-phenyl) phosphate. 6. The enzymes do not require the addition of bivalent cations for activity. 7. Both enzymes I and II have optimum activity at pH6 with 3':5'-cyclic AMP and bis-(p-nitrophenyl) phosphate as substrates. The products of 3':5'-cyclic AMP hydrolysis were 3'-AMP and 5'-AMP, the ratio of the two products being different for each enzyme and varying with pH. 8. Theophylline inhibits enzymes I and II slightly, but other methyl xanthines have little effect. Enzymes I and II were competitively inhibited by many nucleotides containing phosphomonoester and phosphodiester bonds, as well as by Pi. 9. The possible significance of these phosphodiesterases in cyclic nucleotide metabolism in higher plants is discussed.
Full text
PDF

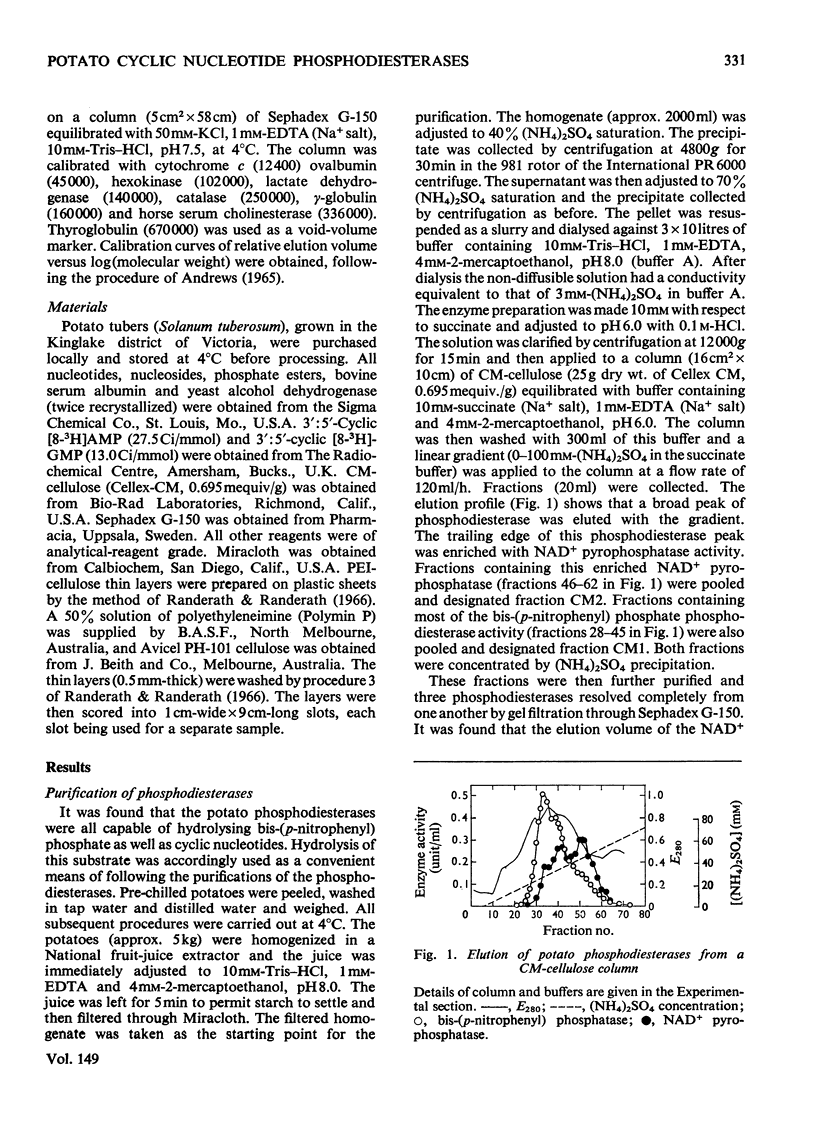
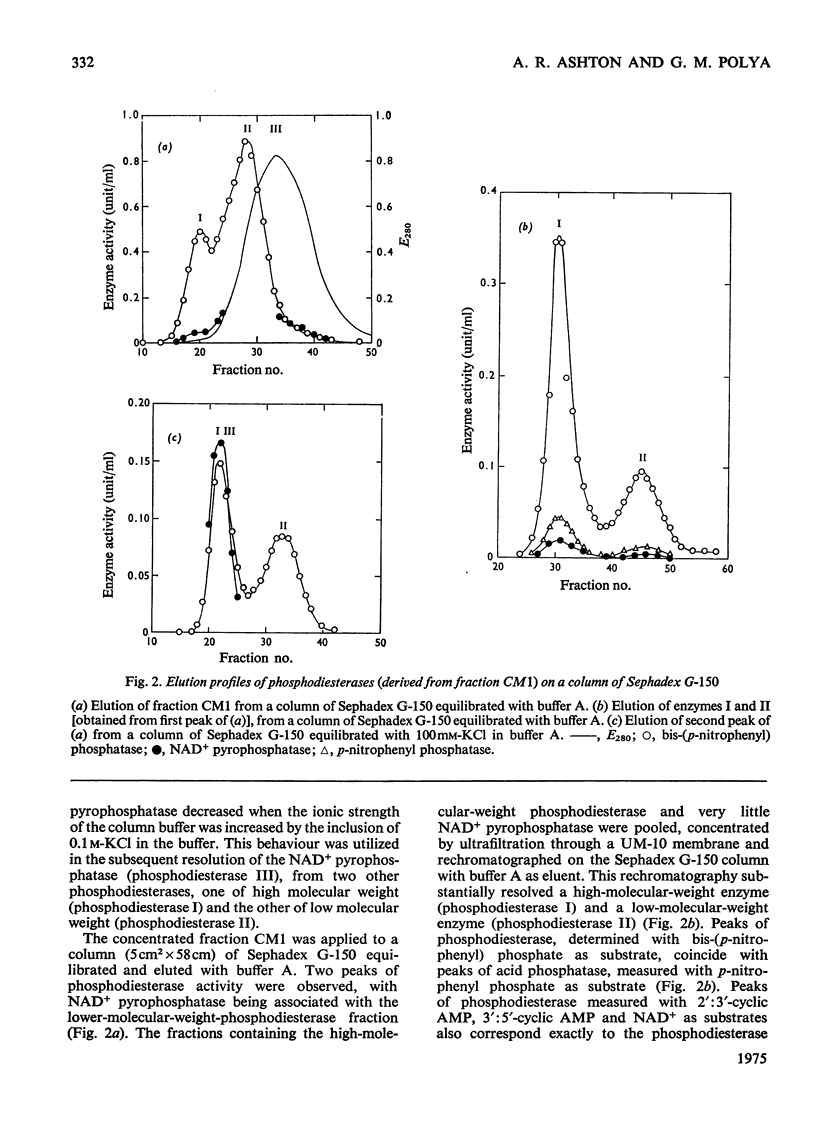
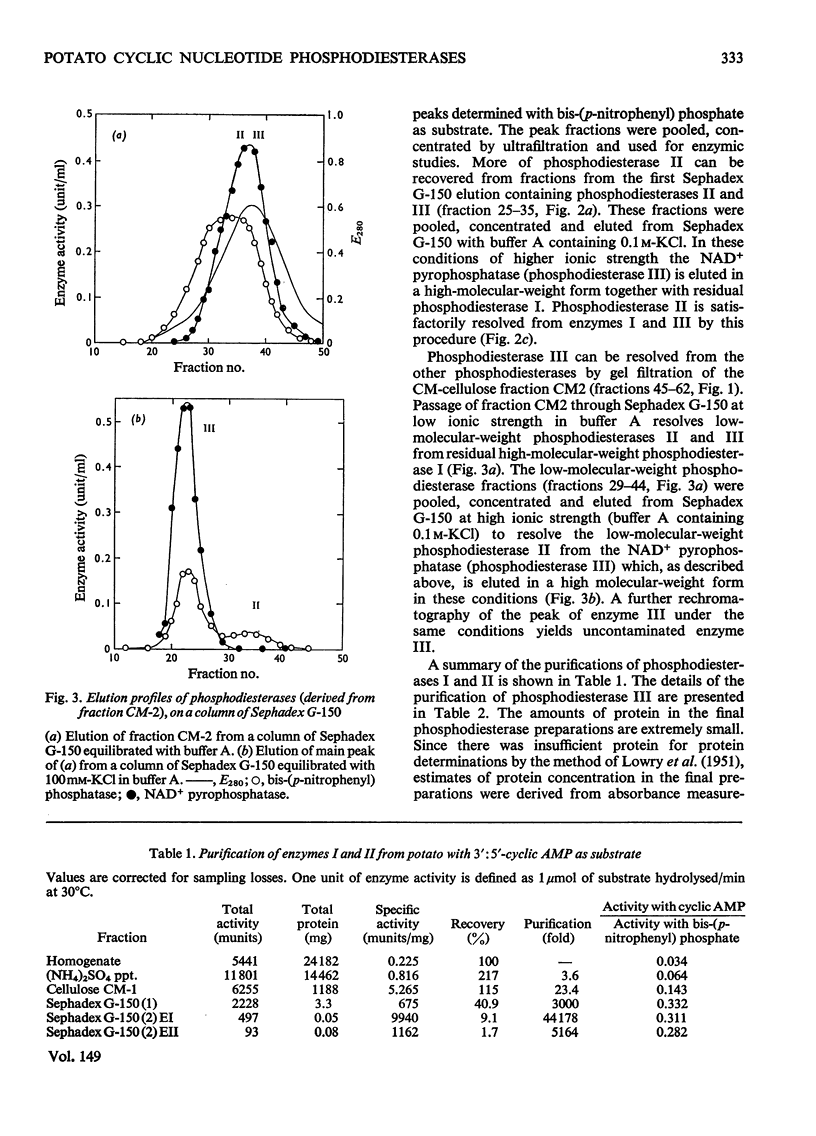
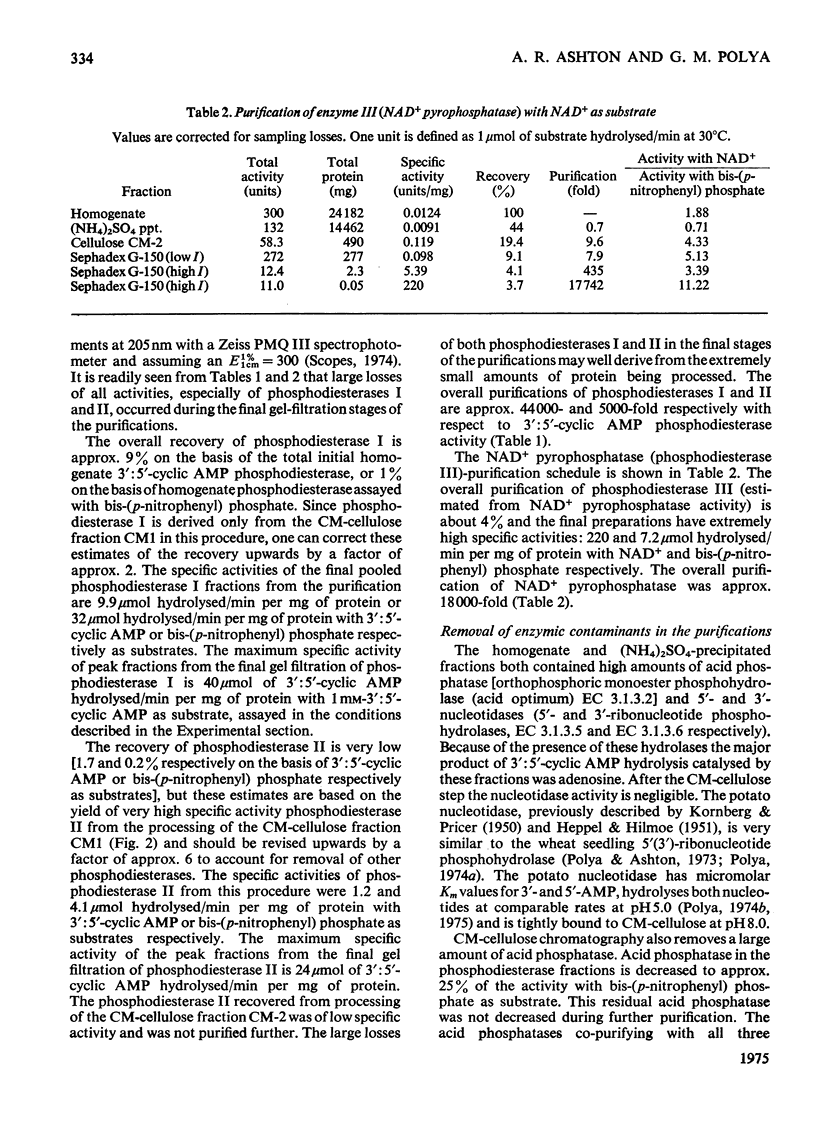



Selected References
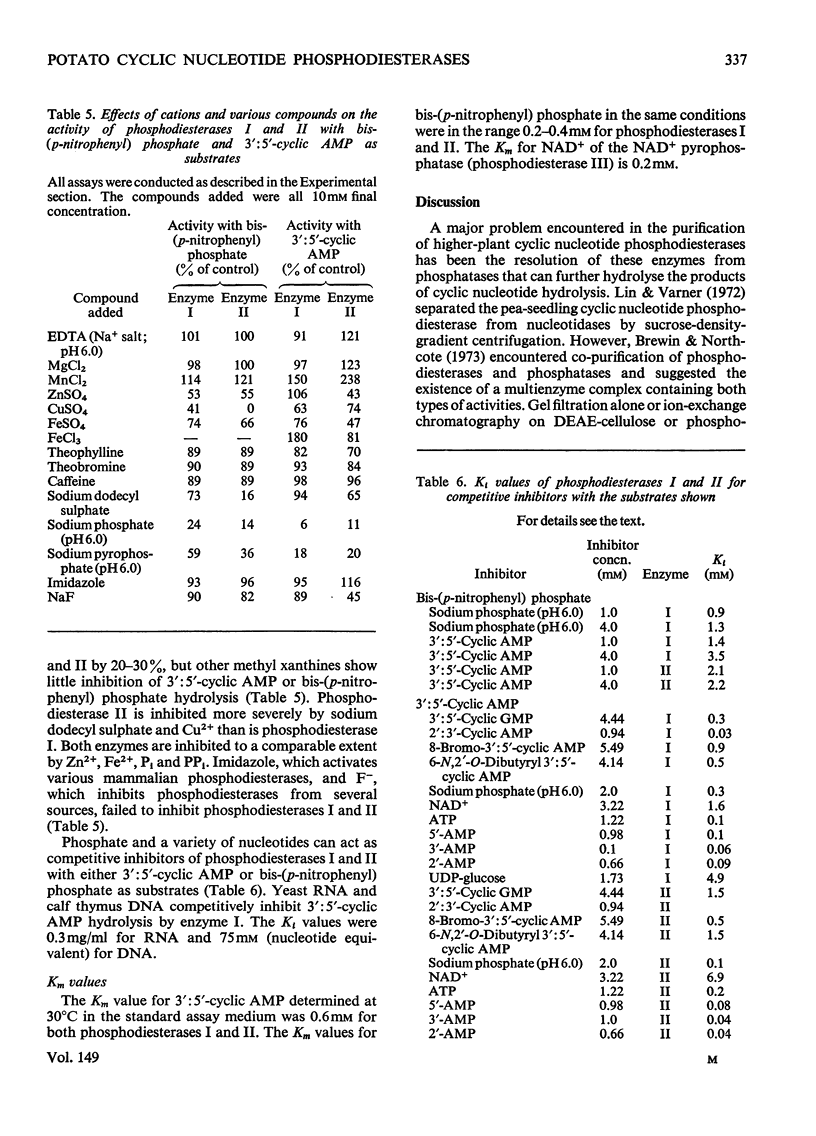
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
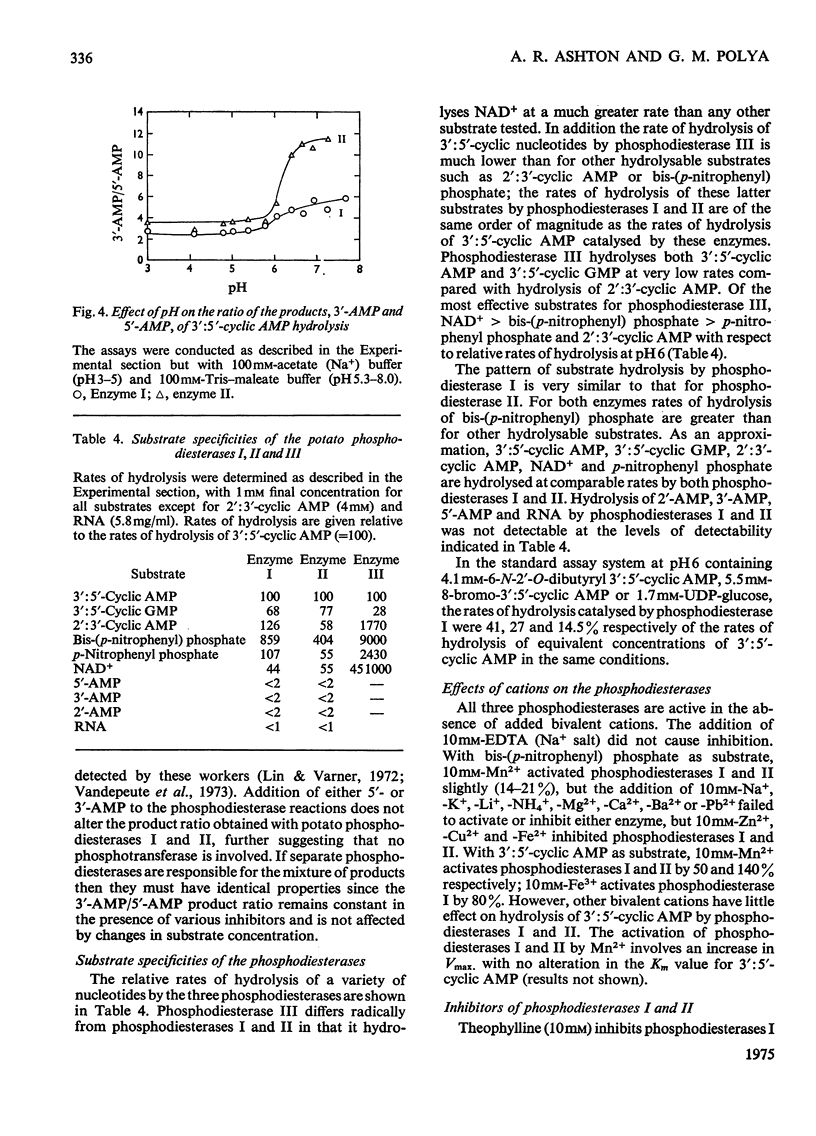
- Anderson W. B., Pastan I. The cyclic AMP receptor of Escherichia coli: immunological studies in extracts of Escherichia coli and other organisms. Biochim Biophys Acta. 1973 Oct 5;320(3):577–587. doi: 10.1016/0304-4165(73)90137-2. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleman M. M., Thompson W. J., Russell T. R. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1973;3:65–98. [PubMed] [Google Scholar]
- Bideon G. M. Purification and characterization of a cyclic nucleotide-regulated 5'-nucleotidase from potatoe. Biochim Biophys Acta. 1975 Apr 19;384(2):443–457. doi: 10.1016/0005-2744(75)90045-5. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E. Cellular responses to cyclic AMP. Prog Biophys Mol Biol. 1973;26:409–461. doi: 10.1016/0079-6107(73)90023-0. [DOI] [PubMed] [Google Scholar]
- Brewin N. J., Northcote D. H. Partial purification of a cyclic AMP phosphodiesterase from soybean callus. Isolation of a non-dialysable inhibitor. Biochim Biophys Acta. 1973 Aug 17;320(1):104–122. doi: 10.1016/0304-4165(73)90171-2. [DOI] [PubMed] [Google Scholar]
- Giannattasio M., Mandato E., Macchia V. Content of 3',5' cyclic AMP and cyclic AMP phosphodiesterase in dormant and activated tissues of Jerusalem artichoke tubers. Biochem Biophys Res Commun. 1974 Mar 25;57(2):365–371. doi: 10.1016/0006-291x(74)90939-5. [DOI] [PubMed] [Google Scholar]
- Goren E. N., Rosen O. M. Purification and properties of a cyclic nucleotide phosphodiesterase from bovine heart. Arch Biochem Biophys. 1972 Nov;153(1):384–397. doi: 10.1016/0003-9861(72)90459-6. [DOI] [PubMed] [Google Scholar]
- HEPPEL L. A., HILMORE R. J. Purification and properties of 5-nucleotidase. J Biol Chem. 1951 Feb;188(2):665–676. [PubMed] [Google Scholar]
- Hamagishi Y., Yoshida H. Phosphodiesterase-phosphomonoesterases from Fusarium moniliforme. V. Mode of action on various nucleotides. J Biochem. 1974 Jul;76(1):81–89. doi: 10.1093/oxfordjournals.jbchem.a130563. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr On the structure of triphosphopyridine nucleotide. J Biol Chem. 1950 Oct;186(2):557–567. [PubMed] [Google Scholar]
- Keates R. A. Cyclic nucleotide-independent protein kinase from pea shoots. Biochem Biophys Res Commun. 1973 Sep 18;54(2):655–661. doi: 10.1016/0006-291x(73)91473-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanzani G. A., Giannattasio M., Manzocchi L. A., Bollini R., Soffientini A. N., Macchia V. The influence of cyclic GMP on polypeptide synthesis in a cell-free system derived from wheat embryos. Biochem Biophys Res Commun. 1974 May 7;58(1):172–177. doi: 10.1016/0006-291x(74)90907-3. [DOI] [PubMed] [Google Scholar]
- Lin P. P. Cyclic nucleotides in higher plants? Adv Cyclic Nucleotide Res. 1974;4(0):439–460. [PubMed] [Google Scholar]
- Lin P. P., Varner J. E. Cyclic nucleotide phosphodiesterase in pea seedlings. Biochim Biophys Acta. 1972 Aug 28;276(2):454–474. doi: 10.1016/0005-2744(72)91007-8. [DOI] [PubMed] [Google Scholar]
- Nielsen L. D., Monard D., Rickenberg H. V. Cyclic 3',5'-adenosine monophosphate phosphodiesterase of Escherichia coli. J Bacteriol. 1973 Nov;116(2):857–866. doi: 10.1128/jb.116.2.857-866.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya G. M. Regulation of a plant 5'(3')-ribonucleotide phosphohydrolase by cyclic nucleotides and pyrimidine, purine, and cytokinin ribosides. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1299–1303. doi: 10.1073/pnas.71.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XV. Preparation, properties and applications of paper-like PEI-cellulose sheets. J Chromatogr. 1966 Apr;22(1):110–117. doi: 10.1016/s0021-9673(01)97076-1. [DOI] [PubMed] [Google Scholar]
- Raymond P., Narayanan A., Pradet A. Evidence for the presence of 3', 5'-cyclic AMP in plant tissues. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1115–1121. doi: 10.1016/0006-291x(73)90580-9. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Shimoyama M., Kawai M., Tanigawa Y., Ueda I. Evidence for and some properties of a 3', 5'-cyclic AMP phosphodiesterase inhibitor in potato. Biochem Biophys Res Commun. 1972 Apr 14;47(1):59–65. doi: 10.1016/s0006-291x(72)80010-x. [DOI] [PubMed] [Google Scholar]
- Vandepeute J., Huffaker R. C., Alvarez R. Cyclic nucleotide phosphodiesterase activity in barley seeds. Plant Physiol. 1973 Sep;52(3):278–282. doi: 10.1104/pp.52.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H. Acid phosphatases from Fusarium moniliforme. 3. Mode of action of acid phosphatase II on bis-p-nitrophenyl phosphate. J Biochem. 1973 Jan;73(1):23–29. [PubMed] [Google Scholar]


