Abstract
Nitric oxide (NO) has a central role in host defense against intracellular microbes. HLA-B27 has been shown to directly modulate host-microbe interaction in vitro, leading to the impaired elimination of Salmonella in human monocytic U937 cells. Here, we studied whether impaired elimination of Salmonella would result from differences in NO production between HLA-B27- and HLA-A2-transfected U937 cells. Both human monocytic transfectants produced NO equally well and killed Salmonella via NO-independent mechanisms.
Nitric oxide (NO) is generally produced in large quantities by activated macrophages during host defense responses (16), and it has activity against many bacteria, viruses, and protozoa (2). NO is especially important in the primary response against intracellular microbes, but it may also play a role in the development of microbial latency (7). However, most studies concerning the antimicrobial function of NO have been performed with mouse macrophages. It remains controversial whether human monocytes are able to induce high-output NO formation (4, 19). Despite some negative results (27), human mononuclear cells have been shown to induce NO production in response to infection with different microbes, including Mycobacterium (5, 9, 17), Leishmania (26), Trypanosoma (14), and human immunodeficiency virus type 1 (1). All these studies have been performed with highly differentiated macrophage-like cells, and in most cases, NO production correlated with diminished surveillance of microbes. The regulation of NO synthesis in human monocytes/macrophages is probably different from that in mouse macrophages (7), and optimal conditions for the stimulation of NO production by human mononuclear cells in vitro are currently unknown (27). Recently, alpha interferon (IFN-α) has been shown to bring about inducible nitric oxide synthase (iNOS) expression in normal human peripheral blood monocytes (20), whereas IFN-γ, together with lipopolysaccharide (LPS), has been known for years to be an efficient inducer of iNOS in rodent macrophages (28).
Reactive arthritis develops after particular gastrointestinal (Salmonella, Shigella, Yersinia, and Campylobacter) or urogenital (Chlamydia) infections in susceptible, mostly HLA-B27-positive, individuals. The strong association between HLA-B27 and the seronegative spondyloarthropathies has been known for decades, but it is still unclear how HLA-B27 plays a role in predisposition to these conditions (10). We previously reported that the expression of HLA-B27 in human monocytic cells (11) and mouse fibroblasts (25) modifies the host-microbe interaction, leading to the enhanced survival of Salmonella within HLA-B27-transfected cells in vitro. The reasons underlying the impaired elimination of Salmonella within HLA-B27-transfected human monocytic cells remain unknown. Interestingly, in HLA-B27-transfected mouse fibroblasts, the killing defect was associated with the diminished production of NO (25). The purpose of this study was to examine whether HLA-B27-positive human monocytic U937 cells differ in their NO-synthetic capacity from the control HLA-A2-transfectants and whether endogenous NO production affects the survival of Salmonella.
HLA-B27- and HLA-A2-transfected U937 cells synthesize iNOS mRNA equally well.
To examine the ability of transfected human monocytic U937 cells to induce NO synthesis, we studied the expression of iNOS mRNA with the experimental setup described previously (11). The human monocytic cell line U937 (American Type Culture Collection, Rockville, Md.) was transfected with either DNA containing the gene or cDNA of HLA-B+ 2705 or DNA containing the HLA-A2 gene, as described previously (11). Cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (PAA; Labor- und Forschungsgesellschaft GmbH, Linz, Austria), 1.8 mmol of l-glutamine per liter, and 50 μg of gentamicin per ml (both from Biological Industries, Kibbutz Beit-Haemek Herennek, Israel) at 37°C in a humidified 5% CO2 atmosphere. The prestimulation of transfected U937 cells with phorbol myristate acetate (PMA) and the infection of PMA-stimulated cells with Salmonella was performed as described previously (11). The strain of Salmonella enteritidis used was originally isolated from a patient with Salmonella-triggered reactive arthritis and was cultured in Luria-Bertani broth to obtain bacteria in the logarithmic phase of growth as described previously (11). PMA-stimulated U937 cells (0.7 × 106 to 1 × 106/ml) were cocultured with salmonellae (3.5 × 106 to 5 × 106/ml) in a medium containing heat-inactivated 10% human AB serum (The Finnish Red Cross, Helsinki, Finland) without antibiotics for 1 h at 37°C, washed, overlaid with fresh medium containing gentamicin (50 μg/ml), and cultured for up to 1 week. Noninfected cells, pretreated with PMA, were similarly cultured and harvested in parallel with the Salmonella-infected cells.
The expression of iNOS mRNA by transfected U937 cells was studied by reverse-transcriptase PCR (RT-PCR) with primers specific for human β-actin (5′-GAA ATC GTG CGT GAC ATT AAG GAG-3′ and 5′-ATA CTC CTG CTT GCT GAT CCA CAT-3′) (8) and iNOS (5′-ATG CCA GAT GGC AGC ATC AGA-3′ and 5′-ACT TCC TCC AGG ATG TTG TA-3′) (26). Details of mRNA extraction and cDNA synthesis were described previously (8). Initial denaturation at 94°C for 5 min and final extension at 72°C for 5 min were used for both primer pairs. The cycling conditions were 94°C for 1 min, 60°C for 30 s, and 72°C for 1 min for 30 cycles (β-actin) and 94°C for 1 min, 62°C for 1 min, and 72°C for 2 min for 35 cycles (iNOS) with a thermal reactor (TouchDown; Hybaid, Middlesex, United Kingdom). The resulting PCR products were analyzed by electrophoresis on a 1.5% agarose gel, stained with ethidium bromide, and compared to each other by determining the relative optical densities of the photographed iNOS and β-actin bands with an MCID-M4 program (version 3.0 beta 1.3; Imaging Research Inc., St. Catharines, Ontario, Canada).
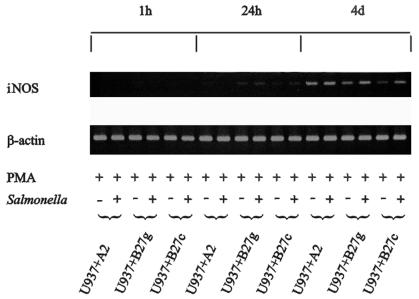
No iNOS mRNA expression was detectable by a sensitive RT-PCR assay at 1 h postinfection, but the weak induction of iNOS mRNA was detected in PMA-stimulated cells infected with Salmonella at 24 h of incubation (Fig. 1). The expression of iNOS mRNA was strongest at 4 days of incubation. At this time point, PMA-stimulated cells infected with Salmonella yielded a higher expression of iNOS mRNA than cells stimulated with PMA alone. There were no apparent differences in iNOS mRNA expression between HLA-B27- and HLA-A2-transfected U937 cells. Four independent samples of Salmonella-infected cells were studied by RT-PCR with similar results.
FIG. 1.
Induction of iNOS mRNA expression in human monocytic cells by PMA stimulation and Salmonella infection. The expression of iNOS mRNA in U937 cells transfected with either DNA containing the gene for HLA-A2 (U937+A2) or HLA-B27 (U937+B27g) or cDNA encoding HLA-B27 (U937+B27c) was studied by RT-PCR. Representative results from one of two similar experiments are shown.
HLA-B27- and HLA-A2-transfected U937 cells do not differ in their capacity to synthesize iNOS protein.
The expression of iNOS protein by the transfected U937 cells was studied by the Western blotting method. Cell lysates were prepared with 1% Nonidet P-40 lysis buffer containing a cocktail of protease inhibitors essentially as described previously (18). The proteins were resolved onto sodium dodecyl sulfate-polyacrylamide gels (5 to 12.5% acrylamide) and transferred onto nitrocellulose membranes (Hybond-ECL; Amersham, Little Chalfont, United Kingdom). A monoclonal antibody (MAb) against mouse iNOS (Transduction Laboratories, Lexington, Ky.) that is known to cross-react with human iNOS (27) and the enhanced chemiluminescence technique were used to detect iNOS protein. A subclass-matched (immunoglobulin G2a) MAb against chicken c-kit, KIT-2C75 (23), was used as a negative control. As a positive control, mouse J774.A1 cells (American Type Culture Collection) stimulated for 24 h with 200 IU of mouse recombinant IFN-γ (Sigma) per ml and 100 ng of S. enteritidis LPS (Sigma) per ml were used. The same cells without stimulation served as a negative control.
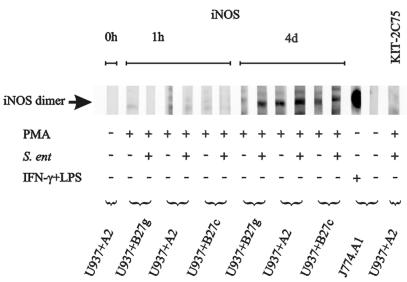
The iNOS protein was detected in cell lysates as a 130-kDa band (Fig. 2). iNOS protein was nearly absent 1 h after infection but became clearly detectable at 4 days of incubation. No apparent differences in iNOS expression were detected between different transfectants. These data are in accordance with the iNOS mRNA results. Nonetheless, human monocytic U937 cells expressed much less iNOS protein than stimulated mouse macrophages as assessed by the intensity of bands. Infection of the PMA-stimulated U937 cells with Salmonella did not clearly upregulate the expression of iNOS protein.
FIG. 2.
Induction of iNOS protein synthesis of human monocytic cells by PMA stimulation and Salmonella infection. The expression of iNOS protein in U937 cells (2 × 106) transfected with either DNA containing the gene for HLA-A2 (U937+A2) or HLA-B27 (U937+B27g) or cDNA encoding HLA-B27 (U937+B27c) was studied by Western blotting. Mouse macrophages (J774.A1) (106) were used as a positive control after stimulation with IFN-γ and LPS. J774.A1 cells without any stimulation and a subclass-matched control MAb, KIT-2C75, were used as negative controls.
HLA-B27- and HLA-A2-transfected U937 cells produce NOx equally well.
The production of NO was studied by measuring the accumulation of its stable metabolites NOx (NO2− and NO3−) in the cell-free supernatants of U937 cells. Iscove’s modified Dulbecco’s medium (Life Technologies, Paisley, Scotland) was used instead of RPMI 1640 medium in experiments when NOx concentrations were determined. NOx concentrations were measured after reduction of nitrate to nitrite with commercial NO2 and NO3 assays (Dojindo, Tokyo, Japan, or R&D Systems, Minneapolis, Minn.) and determination of nitrite concentrations by following the manufacturers’ instructions. The absorbances were measured in 96-well microculture plates by using a Victor multilabel counter (Wallac Oy, Turku, Finland) at a wavelength of 540 nm. NOx concentration in the culture medium was taken as a baseline. The experiments were repeated at least twice.
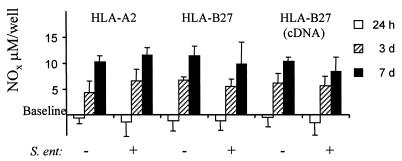
The NOx levels were usually very low after 1 h of incubation. However, NOx production was mainly induced in both PMA-stimulated cells and PMA-stimulated plus Salmonella-infected U937 cells after 3 to 7 days of incubation (Fig. 3). No statistically significant difference in the formation of NO metabolites between the transfectants was observed. Nonstimulated U937 cells did not produce NOx either spontaneously or after infection with Salmonella (data not shown). Infection of PMA-stimulated U937 cells with Salmonella did not alter the quantity or the kinetics of NO production. The production of only low amounts of nitrite from the expression of iNOS in freshly isolated human mononuclear cells has been previously observed (27). Additionally, a remarkable interindividual variation in nitrite production in highly differentiated human macrophages during human immunodeficiency virus infection has been observed (1). In this study, the nitrite and nitrate levels produced by human monocytic cells were much lower than those produced by stimulated mouse macrophages (15). Although the expression of iNOS mRNA by human monocytic cells had already started after 24 h of incubation, iNOS protein was detected only after 3 to 7 days of incubation, which coincides with maximal iNOS mRNA expression and nitrite production. Overall, these data indicate that human monocytic cells are capable of inducing NO synthesis after the differentiation of cells with PMA.
FIG. 3.
NOx production of human monocytic cells after PMA stimulation and Salmonella infection. The accumulation of stable metabolites of NO (NOx) in the cell-free supernatants of U937 cells transfected with either DNA containing the gene for HLA-A2 or HLA-B27 or cDNA encoding HLA-B27 is shown. The cells were pretreated with PMA and infected with S. enteritidis or left uninfected. The concentration of NOx in fresh culture medium was taken as a baseline. Values are means ± standard deviations of the cumulative NOx values of two independent experiments with triplicate wells. S. ent, S. enteritidis.
The administration of NOS inhibitors does not affect the survival of Salmonella.
Although we did not detect any differences in iNOS expression or NOx production between different transfectants that could explain the killing defect in HLA-B27-transfected U937 cells, further data was gathered by experiments with specific NOS inhibitors. Therefore, we inhibited NO synthesis with 1 mM aminoguanidine (AG) (Sigma) or 0.1 to 2 mM NG-monomethyl-l-arginine (l-NMMA) (Sigma), an l-arginine analogue. NOS inhibitors were added to culture media immediately after the removal of extracellular Salmonella by washing. After 3 days of incubation, the culture media were changed, and new aliquots of NOS inhibitors were added to the fresh media. The number of viable intracellular microbes was measured by culturing and was expressed as CFU as described previously (11). A statistical comparison between HLA-A2- and HLA-B27-transfected U937 cells was performed with an unpaired two-tailed Student’s t test.
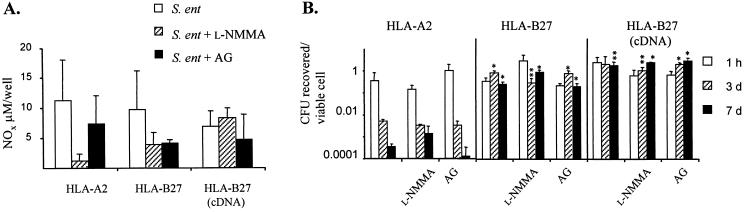
Both l-NMMA and AG caused inhibition of the production of NOx, although the inhibition was not complete (Fig. 4A). However, the decrease in NO production did not correlate with the killing of Salmonella by transfected U937 cells (Fig. 4B). The U937 cells transfected with genomic DNA encoding HLA-B27 (B27g) or cDNA encoding HLA-B27 (B27c) contained more viable intracellular Salmonella than did HLA-A2 transfectants (A2) after 3 days (A2 versus B27g, P < 0.01) and 7 days (A2 versus B27g, P < 0.01; A2 versus B27c, P < 0.05) of incubation without any NOS inhibition. The administration of NOS inhibitors did not noticeably affect the survival of bacteria in any of the transfectants. In general, the blocking effect of l-NMMA on the formation of nitrite is not complete (12). In addition, l-NMMA is not highly selective for iNOS, since it can also block constitutive NOS isoforms (13); therefore, a more iNOS-selective inhibitor, AG, was included in the study. Results obtained with both NOS inhibitors were essentially similar. However, we cannot formally exclude the possibility that other ways to produce nitrite, in addition to an iNOS-dependent pathway, also exist in these cells.
FIG. 4.
NO-independent killing of Salmonella in U937 cells. (A) Effect of NOS inhibitors, 1 mM AG and 0.1 mM l-NMMA, on NO production in Salmonella-infected U937 cells transfected with either DNA containing the gene for HLA-A2 or HLA-B27 or cDNA encoding HLA-B27 [HLA-B27 (cDNA)]. The accumulation of stable metabolites of NO (NOx) in the cell-free supernatants of U937 cells was analyzed, and the NOx concentration in fresh culture medium was taken as a baseline. (B) The number of viable S. enteritidis bacteria per viable U937 cell in the presence or absence of NOS inhibitors is shown on a logarithmic scale. Asterisks indicate P values determined by an unpaired two-tailed Student’s t test when HLA-A2 was compared to HLA-B27 (genomic DNA or cDNA): ∗, P < 0.01; ∗∗, P < 0.05. Values are the means ± standard deviations (triplicate wells) of one representative experiment of two (AG) or four (l-NMMA) experiments with similar results. S. ent, S. enteritidis.
Killing of Salmonella by U937 cells is NO independent.
Although the HLA-B27-transfected U937 cells killed Salmonella less efficiently than did the HLA-A2-transfected controls, the two cell lines did not differ in their rates of NO synthesis. The amounts of iNOS protein and NOx were notably lower in human monocytic cells than in mouse fibroblasts (24), and therefore it is more difficult to observe differences between different human transfectants with the methods used here. The possible differences in the regulation of NO synthesis between murine and human cells make comparisons even more difficult. The importance of NO for the elimination of Salmonella typhimurium (3, 22) and S. enteritidis (25) has been demonstrated with mouse macrophages and fibroblasts. However, a recent report describing mouse macrophages with genetic deficiencies in iNOS or in the 91-kDa subunit of respiratory burst oxidase suggests that oxidative respiratory burst could be more important than NO in killing Salmonella (21). Nonetheless, bacterial enteritis is a potent stimulus for NO synthesis (6), and both NO and its derivative, peroxynitrite, are likely to be important in the more complex in vivo system.
In this report we show that the more Salmonella-permissive HLA-B27-transfected U937 cells did not differ in their NO-producing capacity from the less Salmonella-permissive HLA-A2-transfected cells. In fact, the differences in the killing capacities of the HLA-B27 and the HLA-A2 cells were observed several days before maximal NO production was even induced. Additionally, the survival of Salmonella was not affected by partially blocking the iNOS-dependent NO synthesis or by treatment with NO donor (data not shown). In light of these results, the killing of Salmonella by human monocytic U937 cells in vitro appears to be NO independent, and consequently, the impaired elimination of Salmonella in HLA-B27-transfected human monocytic cells is not associated with ineffective NO synthesis. It remains to be seen how these in vitro results relate to the more complex and interactive situation in vivo. Nevertheless, these results will help in focusing on other antimicrobial mechanisms in future investigations.
Acknowledgments
We acknowledge Heikki Arvilommi for valuable and critical comments during the preparation of the manuscript and David Smith for revising the language. Tiina Lähde, Tuula Lehtonen, and Erkki Nieminen are warmly thanked for their skillful technical assistance.
This work was supported by grants from the European Commission Biomed 2 Programme, the Academy of Finland, the Finnish Cultural Foundation, the Sigrid Jusélius Foundation, the Turku University Foundation, and the Yrjö Jahnsson Foundation.
REFERENCES
- 1.Bukrinsky M I, Nottet H S L M, Schmidtmayerova H, Dubrovsky L, Flanagan C R, Mullins M E, Lipton S A, Gendelman H E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Groote M A, Fang F C. NO inhibitions: antimicrobial properties of nitric oxide. Clin Infect Dis. 1995;21:162–165. doi: 10.1093/clinids/21.supplement_2.s162. [DOI] [PubMed] [Google Scholar]
- 3.De Groote M A, Testerman T, Xu Y, Stauffer G, Fang F C. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium. Science. 1996;272:414–417. doi: 10.1126/science.272.5260.414. [DOI] [PubMed] [Google Scholar]
- 4.Denis M. Human monocytes/macrophages: NO or no NO? J Leukoc Biol. 1994;55:682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 5.Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991;49:380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- 6.Dykhuizen R S, Masson J, McKnight G, Mowat A N, Smith C C, Smith L M, Benjamin N. Plasma nitrate concentration in infective gastroenteritis and inflammatory bowel disease. Gut. 1996;39:393–395. doi: 10.1136/gut.39.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang F C. NO contest: nitric oxide plays complex roles in infection. ASM News. 1997;63:668–673. [Google Scholar]
- 8.He Q, Tran Minh N N, Edelman K, Viljanen M K, Arvilommi H, Mertsola J. Cytokine mRNA expression and proliferative responses induced by pertussis toxin, filamentous hemagglutinin, and pertactin of Bordetella pertussis in the peripheral blood mononuclear cells of infected and immunized schoolchildren and adults. Infect Immun. 1998;66:3796–3801. doi: 10.1128/iai.66.8.3796-3801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagannath C, Actor J K, Hunter R L., Jr Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide. 1998;2:174–186. doi: 10.1006/niox.1998.9999. [DOI] [PubMed] [Google Scholar]
- 10.Khare S D, Luthra H S, David C S. HLA-B27 and other predisposing factors in spondyloarthropathies. Curr Opin Rheumatol. 1998;10:282–291. doi: 10.1097/00002281-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Laitio P, Virtala M, Salmi M, Pelliniemi L J, Yu D T Y, Granfors K. HLA-B27 modulates intracellular survival of Salmonella enteritidis on human monocytic cells. Eur J Immunol. 1997;27:1331–1338. doi: 10.1002/eji.1830270606. [DOI] [PubMed] [Google Scholar]
- 12.Leibovich S J, Polverini P J, Fong T W, Harlow L A, Koch A E. Production of angiogenic activity by human monocytes requires an l-arginine/nitric oxide-synthase-dependent effector mechanism. Proc Natl Acad Sci USA. 1994;91:4190–4194. doi: 10.1073/pnas.91.10.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 14.Munoz-Fernández M A, Fernández M A, Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-α and IFN-γ through a nitric oxide-dependent mechanism. Immunol Lett. 1992;33:35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- 15.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 16.Nathan C F, Hibbs J B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 17.Nozaki Y, Hasegawa Y, Ichiyama S, Nakashima I, Shimokata K. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect Immun. 1997;65:3644–3647. doi: 10.1128/iai.65.9.3644-3647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salmi M, Jalkanen S. Different forms of human vascular adhesion protein-1 (VAP-1) in blood vessels in vivo and in cultured endothelial cells: implications for lymphocyte-endothelial cell adhesion models. Eur J Immunol. 1995;25:2803–2812. doi: 10.1002/eji.1830251014. [DOI] [PubMed] [Google Scholar]
- 19.Schoedon G, Schneemann M, Walter R, Blau N, Hofer S, Schaffner A. Nitric oxide and infection: another view. Clin Infect Dis. 1995;21:152–157. doi: 10.1093/clinids/21.supplement_2.s152. [DOI] [PubMed] [Google Scholar]
- 20.Sharara A I, Perkins D J, Misukonis M A, Chan S U, Dominitz J A, Weinberg J B. Interferon (IFN)-α activation of human blood mononuclear cells in vitro and in vivo for nitric oxide synthase (NOS) type 2 mRNA and protein expression: possible relationship of induced NOS2 to the anti-hepatitis C effects of IFN-α in vivo. J Exp Med. 1997;186:1495–1502. doi: 10.1084/jem.186.9.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiloh M U, Ruan J, Nathan C. Evaluation of bacterial survival and phagocyte function with a fluorescence-based microplate assay. Infect Immun. 1997;65:3193–3198. doi: 10.1128/iai.65.8.3193-3198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vainio O, Dunon D, Aïssi F, Dangy J-P, McNagny K M, Imhof B A. HEMCAM, an adhesion molecule expressed by c-kit+ hemopoietic progenitors. J Cell Biol. 1996;135:1655–1668. doi: 10.1083/jcb.135.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virtala, M., Q. He, M. Saarinen, M. Ikeda, T. Holmström, D. T. Y. Yu, M. Viljanen, H. Arvilommi, and K. Granfors. Submitted for publication.
- 25.Virtala M, Kirveskari J, Granfors K. HLA-B27 modulates the survival of Salmonella enteritidis in transfected L cells, possibly by impaired nitric oxide production. Infect Immun. 1997;65:4236–4242. doi: 10.1128/iai.65.10.4236-4242.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vouldoukis I, Riveros-Moreno V, Dugas B, Ouaaz F, Bécherel P, Debré P, Moncada S, Mossalayi M D. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the FcɛRII/CD23 surface antigen. Proc Natl Acad Sci USA. 1995;92:7804–7808. doi: 10.1073/pnas.92.17.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberg J B, Misukonis M A, Shami P J, Mason S N, Sauls D L, Dittman W A, Wood E R, Smith G K, McDonald B, Bachus K E, Haney A F, Granger D L. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 28.Xie Q-W, Cho H J, Calaycay J, Mumford R A, Swiderek K M, Lee T D, Ding A, Troso T, Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]