Abstract
Background
Managing disseminated nontuberculous mycobacterial (NTM) infection in patients with neutralizing anti-interferon-γ autoantibodies (AIGAs) poses substantial challenges due to the lack of established treatment guidance and predictive tools for clinical outcomes. In this study, we investigated the utility of 18F-fluorodeoxyglucose (2-[18F]FDG) positron emission tomography (PET) in guiding treatment decisions, with a focus on its ability to predict rehospitalization outcomes.
Methods
We conducted a post hoc analysis of the first available 2-[18F]FDG PET scans of patients with AIGAs and disseminated NTM infection from a prospective observational multicenter cohort. Cox proportional hazards regression was used to determine predictors for disease-related rehospitalization within 1 year of the examination.
Results
Of the patients with AIGAs evaluated, 41.9% required rehospitalization within 1 year following the initial 2-[18F]FDG PET evaluation. Slowly growing mycobacteria were isolated in 64.5% of patients. Multivariable analysis identified splenic involvement (adjusted hazard ratio, 7.97; 95% CI, 2.34–27.16; P < .001) as a significant predictor of disease-related rehospitalization within 1 year following the examination. Moreover, mediastinal node involvement (adjusted odds ratio, 14.77; 95% CI, 1.01–216.76; P = .049) and axial skeleton involvement (adjusted odds ratio, 14.93; 95% CI, 1.11–201.43; P = .042) were significantly associated with the isolation of slowly growing mycobacteria.
Conclusions
2-[18F]FDG PET appears useful in initial evaluation of disease extent and microbiology in patients with AIGAs and disseminated NTM infection. Identifying splenic involvement through this modality may help recognize patients at increased risk of disease-related rehospitalization within 1 year. These findings suggest that 2-[18F]FDG PET could inform management decisions in this challenging population.
Keywords: anti-interferon-gamma autoantibodies, fluorodeoxyglucose F18, immunodeficiency, nontuberculous mycobacterial infection, PET
Patients with neutralizing anti-interferon-γ autoantibodies (AIGAs) are vulnerable to opportunistic infection, primarily nontuberculous mycobacteria (NTM) [1–3], which is traditionally categorized into rapidly and slowly growing mycobacteria (RGM and SGM, respectively) [3–6]. Challenges are often encountered in managing patients with AIGAs and concomitant NTM infection, especially in determining the optimal treatment strategies due to the absence of established standard treatment protocols [1, 6–9]. Recurrence and/or persistent infection despite antimicrobial therapy frequently occurs, yet reliable biomarkers for predicting clinical outcomes have remained elusive [1, 6–9]. Moreover, there is currently no guidance available to assess whether the benefits of adjunctive immunotherapy outweigh the potential risks of complications and new infections [1].
Additionally, delays in administrating targeted therapy often occur due to the prolonged time required for NTM culture and speciation [3–6]. Invasive procedures to obtain culture specimens from infected organs may not be feasible or may be overlooked due to other tentative diagnoses (eg, malignancy) associated with nonspecific clinical symptoms [3]. This is particularly problematic given the distinct antimicrobial regimens required for SGM and RGM [3, 6].
Positron emission tomography (PET) employing 18F-fluorodeoxyglucose (2-[18F]FDG) has emerged as one of the standard imaging tools for evaluating infectious and inflammatory diseases [10–12]. This noninvasive whole-body imaging technique provides effective and efficient assessment of disease extent and treatment response in mycobacterial infections in ordinary and immunocompromised conditions [13–17]. Since 2015, it has been a common practice in the participating hospitals in our study to employ 2-[18F]FDG PET in the clinical assessment of patients with disseminated NTM infections associated with AIGAs. Herein, we aimed to investigate whether the findings from 2-[18F]FDG PET could contribute to predict rehospitalization outcomes of NTM infection in patients with AIGAs, with the potential of informing treatment strategies. We also explored whether PET imaging might offer insights into differentiating between SGM and RGM infections, given the therapeutic implications.
MATERIALS AND METHODS
Patients
This study was approved by the institutional review board of National Taiwan University Hospital (201412163RIND and 202203047RINB). This investigation constitutes a post hoc analysis of participants from a prospectively established multicenter observational cohort that enrolled patients with unexplained opportunistic infections spanning from March 2015 to February 2022 [3]. Participants without detection of neutralizing AIGAs in plasma, evident NTM infection, or any available 2-[18F]FDG PET images were excluded from the analysis. Data pertinent to patient characteristics, underlying conditions, current medication, laboratory surveys at the time diagnosis (including serum AIGA levels), microbiological results, and first available 2-[18F]FDG PET images were collected for comparison. Disseminated infection was defined as the isolation of microorganisms from the blood, bone marrow, or ≥2 noncontiguous organs [6]. Primary outcome was the first hospitalization attributable to infection following the 2-[18F]FDG PET evaluation, aiming to establish correlations with disease progression. Secondary outcome was the presence of positive SGM culture from at least 1 clinical specimen of each participant throughout the treatment courses and follow-up periods. All participants provided written consent, and the study was conducted according to the Declaration of Helsinki.
Detection of Neutralizing AIGAs
Plasma was collected from fresh heparinized blood and stored at −80 °C until analysis with the protocol previously described [3]. A participant was considered to have biologically active AIGAs if an elevated concentration of AIGAs was detected in plasma, as measured through enzyme-linked immunosorbent assay, which could inhibit interferon-γ–induced STAT1 phosphorylation in monocytes. Data were collected with FACS Calibur (BD Biosciences), analyzed with FlowJo (Tree Star Inc), and graphed with Prism version 6 (GraphPad Software Inc).
Isolation of NTM From Clinical Specimens
The hospital laboratories followed established guidelines for processing clinical specimens for mycobacterial cultures, as previously described [18]. This involved inoculation onto the fluorometric BACTEC system (BACTEC MGIT 960 System; Becton Dickinson) and Middlebrook 7H11 selective agar with antimicrobial agents (BBL; Becton Dickinson). Acid-fast bacilli staining was initially performed upon specimen submission and repeated for positive cultures from the BACTEC system. The identification of NTM isolates utilized traditional biochemical methods, either independently or in conjunction with Bruker Biotyper matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
Image Acquisition and Interpretation
All patients received 5 to 6 MBq/kg of 2-[18F]FDG intravenously after a 6-hour fasting period. PET images were acquired 60 minutes after tracer injection, allowing for an adequate uptake period as recommended by the procedure guidelines [11]. Scans were performed by PET scanners equipped with computed tomography as part of the routine practice in the participating hospitals (Discovery 710 [GE Medical Systems]; Biograph mCT [Siemens Healthineers]). Semiquantitative measurements were calculated automatically by the built-in software (Syngo.via version 60; Siemens Healthineers) and included standardized uptake value (SUV; tissue concentration × body weight/injection dose), metabolic tumor volume (MTV; delineated with a threshold set at 40% of maximum SUV), and total lesion glycolysis (TLG; mean SUV × MTV) of each infection focus [11, 14]. The highest peak SUV of all foci in each participant was chosen to present the activeness of disease, the summation of MTV of all foci throughout the whole body (total MTV) to present the extent of disease, and the summation of TLG of all foci throughout the whole body (total TLG) to present the overall severity of disease considering the activeness and extent. Detailed assessment of the involvement of various organ parts is provided in Supplementary Table 1.
Statistical Analysis
Data were presented as number (percentage) in categorical variables and as mean ± SD or median (IQR) in continuous variables. Quantitative variables between patient groups were compared by t test for parametric data and Wilcoxon rank sum test for nonparametric data, while categorical variables were compared by chi-square test. Standard cutoff values for age and laboratory surveys in routine clinical practice were applied. Cutoff values without standardization, including the level of serum AIGAs and all PET semiquantitative measurements, were determined by the Youden method of the receiver operating characteristic curve analysis. The predictors for disease-related rehospitalization within 1 year after the 2-[18F]FDG PET evaluation were identified by Cox proportional hazard regression analysis with Firth penalized likelihood correction to minimize the analytic bias caused by rare events or complete separation of data. The robustness of these results was evaluated through multivariable and sensitivity analyses. To identify independent predictors, variables associated with the dependent variable on bivariable analysis (P < .4) were included into multivariable analysis, except those with multicollinearity with others (variance inflation factor >5), and were further selected in a stepwise backward manner to achieve the best value for Akaike information criterion. The rehospitalization-free survival analysis was conducted via the Kaplan-Meier method. The factors associated with the presence of SGM were identified by logistic regression analysis in the same manner. The significance level for statistical tests was set at 5%. All data were processed and analyzed in R (version 4.2.2, R Core Team, 2022) and RStudio (version 2022.07.2+576, RStudio Team, 2022).
RESULTS
Clinical Features of Patients With AIGAs and NTM Infection
A total of 31 patients were included for analysis, comprising 21 men and 10 women, with a mean age of 54.6 ± 11.1 years. Among them, 13 patients (41.9%) were rehospitalized for infection control due to disease progression or refractory illness within 1 year after the 2-[18F]FDG PET evaluation. Twenty patients (64.5%) yielded SGM in isolated microbiological specimens, whereas 11 (35.5%) yielded RGM alone. Table 1 and Supplementary Table 2 summarize their clinical characteristics, laboratory data, and sites of involvement revealed on 2-[18F]FDG PET.
Table 1.
Clinical Characteristics, Laboratory Survey, and Findings on 2-[18F]FDG PET Between Patients Who Required Disease-Related Rehospitalization and Those Who Did Not
| Rehospitalization | ||||
|---|---|---|---|---|
| All (n = 31) | Yes (n = 13) | No (n = 18) | P Value | |
| Onset age, y | 54.55 ± 11.10 | 55.00 ± 8.99 | 54.22 ± 12.65 | .851 |
| Sex: male | 21 (67.7) | 9 (69.2) | 12 (66.7) | .882 |
| Underlying condition | ||||
| Diabetes mellitus | 5 (16.1) | 2 (15.4) | 3 (16.7) | .925 |
| Chronic lung disease | 1 (3.2) | 0 (0) | 1 (5.6) | .395 |
| Malignancy | 3 (9.7) | 2 (15.4) | 1 (5.6) | .369 |
| Immunosuppressant use | ||||
| Steroid | 9 (29.0) | 4 (30.8) | 5 (27.8) | .859 |
| Nonsteroid | 3 (9.7) | 1 (7.7) | 2 (11.1) | .873 |
| NTM identified | ||||
| Isolation of SGM | 20 (64.5) | 10 (76.9) | 10 (55.6) | .227 |
| M kansasii | 11 (35.5) | 6 (46.2) | 5 (27.8) | .291 |
| MAC | 9 (29.0) | 3 (23.1) | 6 (33.3) | .535 |
| Othersa | 3 (9.7) | 1 (7.7) | 2 (11.1) | .751 |
| Isolation of RGM | 13 (41.9) | 7 (53.8) | 6 (33.3) | .253 |
| M abscessus | 9 (29.0) | 4 (11.5) | 5 (27.8) | .856 |
| M fortuitum | 2 (6.5) | 2 (15.4) | 0 (0) | .085 |
| Othersb | 2 (6.5) | 1 (7.7) | 1 (5.6) | .811 |
| ≥2 NTM species | 9 (29.0) | 5 (38.5) | 4 (22.2) | .522 |
| Laboratory data at disease onset | ||||
| White blood cell, k/μL | 18.03 ± 8.90 | 18.13 ± 8.31 | 17.96 ± 9.54 | .960 |
| Absolute neutrophil count, k/μL | 14.49 ± 8.31 | 14.32 ± 7.83 | 14.61 ± 8.87 | .925 |
| Lymphocyte count, k/μL | 2.08 ± .97 | 2.04 ± .96 | 2.10 ± 1.01 | .864 |
| C-reactive protein, mg/dL | 10.55 [10.63] | 10.55 [14.53] | 10.37 [10.42] | .603 |
| AIGA, μg/mL | 584.46 [761.83] | 598.84 [861.28] | 404.20 [793.10] | .496 |
| Involving site on 2-[18F]FDG PET | ||||
| Time from diagnosis to PET, mo | 0.6 [13.6] | 0.3 [5.6] | 2.6 [18.2] | .262 |
| ≥3 organs | 17 (54.8) | 8 (61.5) | 9 (50.0) | .531 |
| Both sides of the diaphragm | 20 (64.5) | 9 (69.2) | 11 (61.1) | .646 |
| Lungs | 22 (71.0) | 10 (76.9) | 12 (66.7) | .541 |
| Lymph nodes | 27 (87.1) | 12 (92.3) | 15 (83.3) | .469 |
| Deep-seated | 21 (67.7) | 10 (76.9) | 11 (61.1) | .361 |
| Superficial | 20 (64.5) | 10 (76.9) | 10 (55.6) | .227 |
| Across the diaphragm | 12 (38.7) | 7 (53.8) | 5 (27.8) | .148 |
| Deep and superficialc | 8 (25.8) | 6 (46.2) | 2 (11.1) | .030d |
| Tonsils, asymmetric | 14 (45.2) | 5 (38.5) | 9 (50.0) | .531 |
| Spleen | 5 (16.1) | 5 (38.5) | 0 (0) | .005d |
| Bones | 15 (48.4) | 6 (46.2) | 9 (50.0) | .835 |
| Axial skeletons | 13 (41.9) | 6 (46.2) | 7 (38.9) | .691 |
| Appendicular skeletons alone | 2 (6.5) | 0 (0) | 2 (11.1) | .222 |
| Other organse | 9 (29.0) | 3 (23.1) | 6 (33.3) | .541 |
Data are expressed as mean ± SD, median [IQR], or No. (%) of patients.
Abbreviations: 2-[18F]FDG PET, 18F-fluorodeoxyglucose positron emission tomography; AIGA, anti-interferon-γ autoantibody; MAC, M avium complex; NTM, nontuberculous mycobacteria; RGM, rapid growing mycobacteria; SGM, slowly growing mycobacteria.
aOther SGM includes M gordonae (1), M paragordonae (1), and M wolinskyi (1).
bOther RGM includes M massiliense (1) and M porcinum (1).
cRefers to disease involving deep-seated and superficial nodes across the diaphragm.
d P < .05. Quantitative variables: t test (parametric), Wilcoxon rank sum (nonparametric). Categorical variables: chi-square test.
eInclude skin, muscles, breasts, thyroid glands, liver, and cecum.
Features on 2-[18F]FDG PET and Risk Factors Associated With Disease-Related Rehospitalization
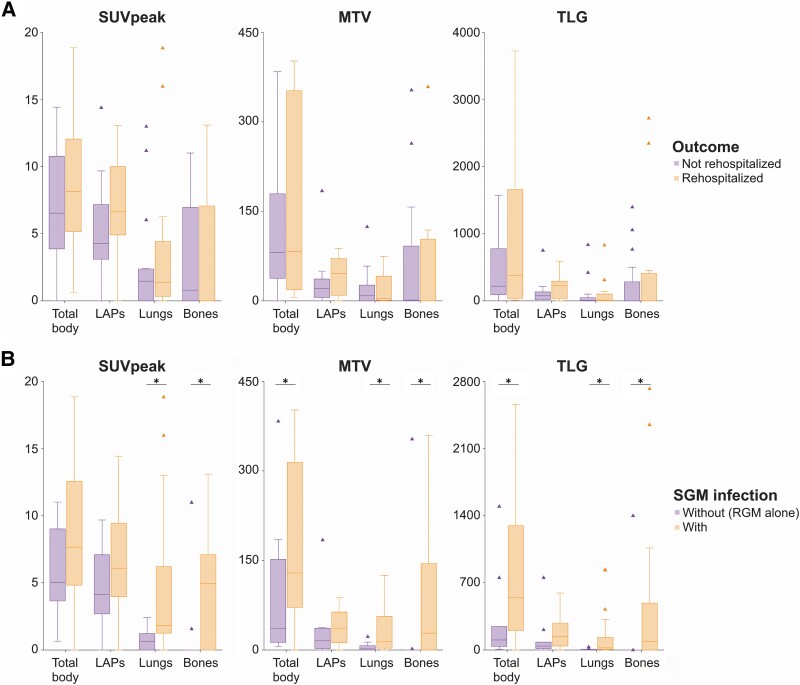
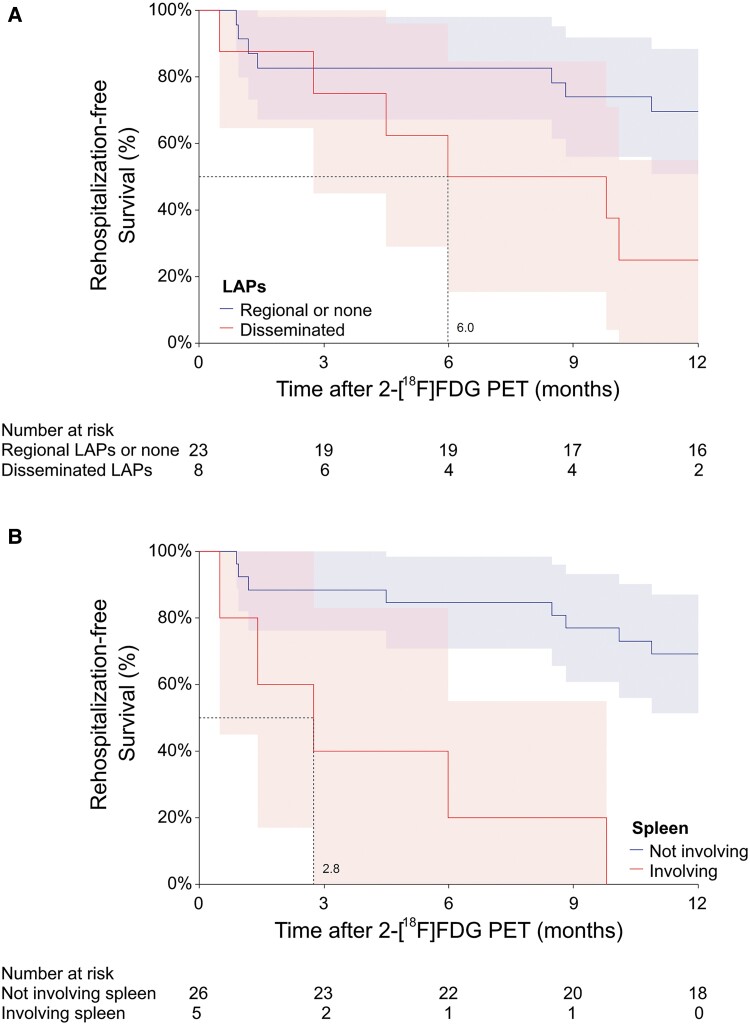
In 19 (61.3%) of the 31 patients, 2-[18F]FDG PET identified more infectious foci beyond routine clinical investigations (ie, physical examination, radiographic imaging, and laboratory tests; Supplementary Table 3). Semiquantitative measurements of disease activity or extent on initial 2-[18F]FDG PET images showed no significant differences between patients with disease-related rehospitalization and those without (Figure 1A, Table 1). However, patients with extensive lymphadenopathies involving deep-seated and superficial nodes across the diaphragm on 2-[18F]FDG PET (6/13, 46.2%) were more likely to require rehospitalization for disease control than those without (2/18, 11.1%, P = .030), with a median time to rehospitalization of 6.0 months (Figure 2A). Rehospitalization occurred in all patients with splenic involvement on 2-[18F]FDG PET. The median time to rehospitalization was 2.8 months for patients with splenic involvement, as opposed to >12 months for those without (P < .001; Figure 2B).
Figure 1.
Comparisons of semiquantitative measurements of various body sites on 2-[18F]FDG PET: A, between patients with AIGAs who required rehospitalization within 1 year after the evaluation and those who did not; B, between those with and without SGM infection. Data are presented as median (line), IQR (box), 95% CI (error bars), and outlier (triangle). 2-[18F]FDG PET, 18F-fluorodeoxyglucose positron emission tomography; AIGA, anti-interferon-γ autoantibody; LAP, lymphadenopathy; MTV, metabolic tumor volume; RGM, rapidly growing mycobacteria; SGM, slowly growing mycobacteria; SUVpeak, peak standardized uptake value; TLG, total lesion glycolysis. *P < .05.
Figure 2.
Kaplan-Meier survival analysis of disease-related rehospitalization within 1 year after 2-[18F]FDG PET evaluation shows a significantly lower median rehospitalization-free survival: A, 6.0 months in patients with disseminated LAPs (P = .027); B, 2.8 months in patients with splenic involvement (P < .001). Data are presented as percentage (line) and 95% CI (shaded area). Dotted line indicates the time to achieve 50% rehospitalization-free survival. 2-[18F]FDG PET, 18F-fluorodeoxyglucose positron emission tomography; LAP, lymphadenopathy.
After variable selection, a multivariable analysis encompassing underlying condition, laboratory survey at diagnosis, disease patterns, and semiquantitative measurements revealed by 2-[18F]FDG PET showed that splenic involvement was the sole independent predictor for disease-related rehospitalization within 1 year after the evaluation (adjusted hazard ratio, 7.64; 95% CI, 1.02–57.16; P = .048; Table 2). This association proved to be robust, accounting for different clinical factors by sensitivity analysis (Supplementary Table 4). Supplementary Figure 1 illustrates representative initial 2-[18F]FDG PET images from 2 patients, 1 each with favorable and unfavorable outcomes.
Table 2.
Cox Proportional Hazard Analysis of Predictors for Rehospitalization Within 1 Year After 2-[18F]FDG PET Evaluation
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Onset age ≥60 y | 0.92 (.29-2.95) | .891 | ||
| Male sex | 0.98 (.31–3.14) | .972 | ||
| Diabetes mellitus | 1.01 (.24–4.18) | .989 | ||
| Chronic lung disease | 0.87 (.05–16.27) | .924 | ||
| Malignancy | 2.54 (.61–10.56) | .200 | 0.48 (.07–3.60) | .479 |
| Steroid use | 1.02 (.32–3.25) | .979 | ||
| NTM identified | ||||
| Isolation of SGM | 1.86 (.53–6.56) | .335 | ||
| Laboratory survey at disease onset | ||||
| White blood cell ≥11, k/μL | 0.78 (.22–2.75) | .703 | ||
| Absolute neutrophil count ≥7, k/μL | 0.87 (.21–3.59) | .843 | ||
| Lymphocyte count ≥3, k/μL | 1.20 (.29–4.95) | .804 | ||
| C-reactive protein ≥10, mg/dL | 1.65 (.51–5.31) | .399 | 1.70 (.46–6.31) | .428 |
| AIGA ≥550, μg/mL | 1.74 (.57–5.30) | .330 | ||
| 2-[18F]FDG PET findings | ||||
| Involving ≥3 organs | 1.48 (.48–4.50) | .492 | ||
| Both sides of the diaphragm | 1.02 (.32–3.26) | .977 | ||
| Lung | 1.20 (.34–4.21) | .779 | ||
| Lymph nodes | 1.64 (.28–9.54) | .583 | ||
| Deep-seated | 1.71 (.49–6.01) | .405 | ||
| Superficial | 2.03 (.58–7.13) | .272 | ||
| Across the diaphragm | 2.02 (.68–6.05) | .207 | ||
| Deep and superficiala | 3.28 (1.09–9.89) | .035b | 1.90 (.45–8.06) | .385 |
| Asymmetric tonsils | 0.70 (.23–2.12) | .523 | ||
| Spleen | 7.97 (2.34–27.16) | <.001a | 7.64 (1.02–57.16) | .048b |
| Bones | 0.98 (.33–2.91) | .970 | ||
| Axial skeletons | 1.37 (.46–4.08) | .572 | ||
| Semiquantitative measurements | ||||
| SUVpeak ≥7.3 | 1.53 (.51–4.56) | .444 | ||
| Total MTV ≥240 | 2.50 (.81–7.66) | .109 | 1.80 (.30–10.73) | .517 |
| Total TLG ≥350 | 1.71 (.57–5.10) | .337 | 0.41 (.06–3.03) | .385 |
Multivariable analysis included variables associated with the dependent variable (rehospitalization) on bivariable analysis (P < .4), except those with multicollinearity (variance inflation factor >5), and select variables by stepwise backward selection to achieve the best value of Akaike information criterion.
Abbreviations: 2-[18F]FDG PET, 18F-fluorodeoxyglucose positron emission tomography; AIGA, anti-interferon-γ autoantibody; HR, hazard ratio; MTV, metabolic tumor volume; NTM, nontuberculous mycobacteria; SGM, slowly growing mycobacteria; SUVpeak, peak standardized uptake value; TLG, total lesion glycolysis.
aRefers to disease involving deep-seated and superficial nodes across the diaphragm.
b P < .05.
Features on 2-[18F]FDG PET and Risk Factors Associated With SGM Infection
A more extensive disease, involving multiple organs (≥3) or both sides of the diaphragm, was observed more frequently in patients with SGM infection than those without (Supplementary Table 2). Additionally, there were significantly higher values for total MTV (median, 128.81 vs 25.65; P = .038) and total TLG (median, 545.83 vs 50.53; P = .025; Figure 1B) in patients infected with SGM. More frequent mediastinal nodal involvement was observed in patients infected with SGM (85.0% vs 36.4%, P = .006), with no significant differences observed in nodal involvement at other body sites (Supplementary Table 3). Skeletal involvement, especially in axial skeletons (60.0% vs 9.1%, P = .007), was more frequently observed in patients infected with SGM than those without. Splenic involvement was exclusively observed in those with SGM infection (25.0%).
In logistic regression analysis, patients with a more extensive disease pattern revealed by 2-[18F]FDG PET had a higher likelihood of being infected with SGM, particularly those involving mediastinal nodes (adjusted odds ratio [OR], 14.77; 95% CI, 1.01–216.76; P = .049) and axial skeletons (adjusted OR, 14.93; 95% CI, 1.11–201.43; P = .042; Table 3). The robustness of these associations was confirmed by sensitivity analysis, accounting for different clinical factors (Supplementary Table 5). A substantially elevated serum C-reactive protein level (>10 mg/dL) at disease onset was the sole risk factor for SGM infection among all clinical factors and laboratory surveys (OR, 6.84; 95% CI, 1.41–33.22; P = .017). This association was less significant in multivariable analysis combining other 2-[18F]FDG PET findings.
Table 3.
Logistic Regression Analysis of Factors Associated With the Isolation of SGM vs RGM Alone in Patients With AIGAs
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| Onset age ≥60 y | 1.65 (.36–7.55) | .518 | ||
| Male sex | 1.34 (.30–5.94) | .701 | ||
| Diabetes mellitus | 0.76 (.13–4.62) | .766 | ||
| Chronic lung disease | 0.17 (.01–4.56) | .292 | ||
| Malignancy | 0.29 (.03–2.55) | .266 | ||
| Steroid use | 1.09 (.23–5.13) | .915 | ||
| Laboratory data at disease onset | ||||
| White blood cell ≥11, k/μL | 1.51 (.30–7.66) | .619 | ||
| Absolute neutrophil count ≥7, k/μL | 0.52 (.07–3.88) | .527 | ||
| Lymphocyte count ≥3, k/μL | 0.33 (.05–2.02) | .229 | ||
| C-reactive protein ≥10, mg/dL | 6.84 (1.41–33.22) | .017a | 17.33 (.88–342.36) | .061 |
| AIGA ≥550, μg/mL | 1.43 (.34–5.94) | .622 | ||
| 2-[18F]FDG PET findings | ||||
| Involving ≥3 organs | 5.42 (1.15–25.55) | .033a | 0.22 (.01–3.76) | .296 |
| Both sides of the diaphragm | 4.33 (.93–20.24) | .062 | ||
| Lungs | 3.10 (.66–14.49) | .150 | ||
| Lymph nodes | 1.95 (.29–13.28) | .496 | ||
| Deep-seated | 8.33 (1.62–42.87) | .011a | ||
| Mediastinal | 8.33 (1.62–42.87) | .011a | 14.77 (1.01–216.76) | .049a |
| Superficial | 1.89 (.44–8.17) | .395 | 6.66 (.49–90.72) | .155 |
| Across the diaphragm | 2.01 (.44–9.11) | .367 | ||
| Deep and superficialb | 3.89 (.56–26.78) | .168 | ||
| Asymmetric tonsils | 0.33 (.08–1.45) | .143 | 0.20 (.03–1.42) | .107 |
| Spleen | 8.16 (.41–162.92) | .169 | ||
| Bones | 6.84 (1.31–35.78) | .023a | ||
| Axial skeletons | 10.29 (1.51–70.21) | .017a | 14.93 (1.11–201.43) | .042a |
| Semiquantitative measurements | ||||
| SUVpeak ≥6.1 | 2.64 (.61–11.41) | .195 | 0.16 (.01–3.52) | .245 |
| Total MTV ≥70 | 8.90 (1.76–45.16) | .008a | ||
| Total TLG ≥190 | 6.11 (1.28–29.26) | .023a | ||
Multivariable analysis included variables associated with the dependent variable (isolation of SGM) on bivariable analysis (P < .4), except those with multicollinearity (variance inflation factor >5), and select variables by stepwise backward selection to achieve the best value of Akaike information criterion.
Abbreviations: 2-[18F]FDG PET, 18F-fluorodeoxyglucose positron emission tomography; AIGA, anti-interferon-γ autoantibody; MTV, metabolic tumor volume; OR, odds ratio; SGM, slowly growing mycobacteria; SUVpeak, peak standardized uptake value; TLG, total lesion glycolysis.
a P < .05.
bRefers to disease involving deep-seated and superficial nodes across the diaphragm.
DISCUSSION
Our study demonstrated that utilizing 2-[18F]FDG PET is instrumental in assessing disease extent and severity of NTM infection in the presence of AIGAs. Splenic involvement independently predicted rehospitalization within 1 year after the 2-[18F]FDG PET evaluation, suggesting the need for a more aggressive therapeutic and disease-monitoring approach in cases with splenic involvement to mitigate the risk of early reinfection or disease progression. Additionally, the independent association observed between the involvement of mediastinal nodes and axial skeletons and the isolation of SGM may offer pragmatic insights for guiding the selection of empiric antimycobacterial regimens in cases where a definitive microbiological report is not yet available.
A thorough survey for disease extent and severity is essential in the management of NTM infection [2, 3, 5, 6, 9]. Nonetheless, deep-seated infectious foci may cause little or no symptoms, resulting in underawareness, especially in patients with impaired consciousness [2, 3, 6]. 2-[18F]FDG, a glucose analogue, highly accumulates in activated granulocytes and macrophages by means of the upregulation of glucose transporter, thereby highlighting active inflammatory foci [10–12]. Whole-body PET imaging employing 2-[18F]FDG may complement routine clinical investigation, in which additional survey for possible extrapulmonary involvement is typically conducted only when suspicious symptoms are present [4]. In our study, 61.3% of patients were revealed to have additional foci per 2-[18F]FDG PET, aligning the result of Sood et al in their cohort of pulmonary tuberculosis [17].
This study represents the first analysis demonstrating the time to the first rehospitalization after imaging evaluation as a measure of disease progression. In routine clinical settings, physicians often rely on serum biomarkers to assess disease status [4, 7, 8, 19]. Previous studies have reported conflicting findings in using the level of neutralizing serum AIGAs as a biomarker for current disease status [20, 21]. Other researchers have observed that only changes in intrapatient autoantibody titers are indicative of predicting disease activity, whereas interpatient absolute values may not predict specific outcomes [22]. Although our results showed that a higher level of AIGAs correlated with rehospitalization, this relationship did not reach statistical significance. Similarly, the disease extent and activity levels measured by the initial 2-[18F]FDG PET showed insignificant correlations with disease-related rehospitalization. Instead, the disease pattern revealed by 2-[18F]FDG PET was found to be the key predictor of disease-related rehospitalization. Specifically, patients with extensive lymphadenopathies involving deep-seated and superficial nodes across the diaphragm tended to require rehospitalization. Moreover, splenic involvement was identified as an independent predictor of early disease progression requiring rehospitalization within 1 year after 2-[18F]FDG PET evaluation, a novel finding not previously reported. As the body's largest secondary lymphoid organ, the spleen plays a central role in mounting innate and adaptive immune responses. Its failure to effectively contain the infection may indicate an overwhelmed immune response. Additionally, splenic involvement may signal extensive disease burden, marked by dissemination across multiple organ systems and active systemic inflammation—all of which suggest an elevated risk for recurrent or refractory infection.
Lymphadenopathies were the most frequently observed site of infection in our cohort of patients with AIGAs, irrespective of the causative NTM species involved, which is consistent with findings from previous studies [3, 5, 8, 23]. Notably, the present study is the first to pinpoint mediastinal nodes as the most significant group of lymph nodes indicating SGM infection, with an OR of 8.33. Previous studies also reported higher incidence of bone infection in patients with SGM infection than those without [3, 5]. Our results further highlight this trend and identify a novel correlation between axial skeleton involvement and SGM infection.
This cohort study had some limitations. Referral bias was inevitable since 2-[18F]FDG PET was performed at the discretion of primary physicians, leading to a small sample size and a varied diagnosis-to-PET period (Table 1, Supplementary Table 3). However, given the long-term nature of antimycobacterial therapy and protracted clinical course in patients with AIGAs, the timing of PET may have a limited impact on the overall findings. Other critical factors, such as disease severity, the appropriateness of the antimycobacterial regimen, and the patient's immune response at the time of PET imaging, likely played a significant role as well in influencing the PET results and the risk of rehospitalization. While assessing these factors can be complex and challenging, PET provides objective data on disease burden and immune response at a specific time point, independent of treatment stage.
Additionally, since not all infection sites could be cultured, especially if invasive procedures were required, we may have missed cases of SGM infection in participants allocated to the RGM-alone group. Further studies with a larger patient cohort and a prospective design are warranted to validate our results and establish the role of 2-[18F]FDG PET in treatment guidance. Nonetheless, these limitations do not obscure our results, which provide valuable diagnostic and prognostic insights into real-world practice, highlighting the advantage of 2-[18F]FDG PET in enabling visualization of disease extent and severity in this unique clinical context.
In conclusion, the utilization of 2-[18F]FDG PET aids in predicting clinical outcomes and categorizing NTM infection in patients with AIGAs through a noninvasive approach. This information enables tailored interventions, such as aggressive therapeutic and monitoring strategies, and the consideration of adjunctive immunotherapy, particularly with splenic involvement. Overall, the findings from 2-[18F]FDG PET imaging offer insights into the underlying pathogenesis of NTM infection in patients with AIGAs and advance the development of standardized care protocols for managing this complex disease entity.
Supplementary Material
Contributor Information
Pei-Ju Chuang, Department of Nuclear Medicine, National Taiwan University Hospital, Yunlin Branch, Yunlin, Taiwan.
Wei-Cheng Lan, Department of Nuclear Medicine, National Taiwan University Hospital, Yunlin Branch, Yunlin, Taiwan.
Mei-Fang Cheng, Department of Nuclear Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Chun-Kai Huang, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Tzu-Chan Hong, Department of Medicine, National Taiwan University Cancer Center, Taipei, Taiwan.
Chi-Ying Lin, Department of Internal Medicine, National Taiwan University Hospital, Yunlin Branch, Yunlin, Taiwan.
Yu-Shan Huang, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Pao-Yu Chen, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Un-In Wu, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan; Department of Medicine, National Taiwan University Cancer Center, Taipei, Taiwan.
Jann-Tay Wang, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Wang-Huei Sheng, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Yee-Chun Chen, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Shan-Chwen Chang, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the Taiwan Clinical Trials Consortium of Infectious Diseases (MOST 105-2325-B-002-026, MOST 106-2321-B-002-031, MOST 107-2321-B-037) for their support in patient enrollment of the initial cohort.
Author contributions. All authors contributed to the study conception and design, leading by C.-K. H., C.-Y. L., and U.-I. W. Material preparation and data collection were performed by C.-K. H., T.-C. H., C.-Y. L., Y.-S. H., P.-Y. C., U.-I. W., J.-T. W., W.-H. S., and Y.-C. Chen. Image processing and semiquantitative analysis were carried out by W.-C. L., P.-J. C., and M.-F. C. Data analysis was designed and performed by P.-J. C. under the assistance of M.-F. C., T.-C. H., and U.-I. W. The first draft of the manuscript was written by P.-J. C. and U.-I. W., and all other authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Consent to participate and to publish. Informed consent regarding study participation and publication was obtained from all participants included in the study.
Data availability. The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of National Taiwan University Hospital (201412163RIND and 202203047RINB).
Financial support. This work was supported in part by the National Taiwan University Hospital Yunlin Branch (NTUHYL112.N011); and the National Science and Technology Council, R.O.C. (110-2314-B-002-247, 111-2314-B-002-145).
References
- 1. Qiu Y, Fang G, Ye F, et al. Pathogen spectrum and immunotherapy in patients with anti-IFN-gamma autoantibodies: a multicenter retrospective study and systematic review. Front Immunol 2022; 13:1051673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shih HP, Ding JY, Yeh CF, Chi CY, Ku CL. Anti-interferon-gamma autoantibody-associated immunodeficiency. Curr Opin Immunol 2021; 72:206–14. [DOI] [PubMed] [Google Scholar]
- 3. Wu UI, Wang JT, Sheng WH, et al. Incorrect diagnoses in patients with neutralizing anti-interferon-gamma-autoantibodies. Clin Microbiol Infect 2020; 26:1684.e1–6. [DOI] [PubMed] [Google Scholar]
- 4. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis 2020; 71:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hase I, Morimoto K, Sakagami T, Ishii Y, van Ingen J. Patient ethnicity and causative species determine the manifestations of anti-interferon-gamma autoantibody-associated nontuberculous mycobacterial disease: a review. Diagn Microbiol Infect Dis 2017; 88:308–15. [DOI] [PubMed] [Google Scholar]
- 6. Matono T, Suzuki S, Yamate R, Nakamura K, Sakagami T. Diagnostic and therapeutic challenges in disseminated Mycobacterium colombiense infection caused by interferon-gamma neutralizing autoantibodies. Open Forum Infect Dis 2023; 10:ofad035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Angkasekwinai N, Suputtamongkol Y, Phoompoung P, et al. Clinical outcome and laboratory markers for predicting disease activity in patients with disseminated opportunistic infections associated with anti-interferon-gamma autoantibodies. PLoS One 2019; 14:e0215581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chi CY, Lin CH, Ho MW, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-gamma autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine (Baltimore) 2016; 95:e3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuan CT, Wang JT, Sheng WH, et al. Lymphadenopathy associated with neutralizing anti-interferon-gamma autoantibodies could have monoclonal T-cell proliferation indistinguishable from malignant lymphoma and treatable by antibiotics: a clinicopathologic study. Am J Surg Pathol 2021; 45:1138–50. [DOI] [PubMed] [Google Scholar]
- 10. Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol 2013; 2013:623036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jamar F, Buscombe J, Chiti A, et al. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med 2013; 54:647–58. [DOI] [PubMed] [Google Scholar]
- 12. Kung BT, Seraj SM, Zadeh MZ, et al. An update on the role of (18)F-FDG-PET/CT in major infectious and inflammatory diseases. Am J Nucl Med Mol Imaging 2019; 9:255–73. [PMC free article] [PubMed] [Google Scholar]
- 13. Lawal IO, Fourie BP, Mathebula M, et al. (18)F-FDG PET/CT as a noninvasive biomarker for assessing adequacy of treatment and predicting relapse in patients treated for pulmonary tuberculosis. J Nucl Med 2020; 61:412–7. [DOI] [PubMed] [Google Scholar]
- 14. Malherbe ST, Chen RY, Dupont P, et al. Quantitative 18F-FDG PET-CT scan characteristics correlate with tuberculosis treatment response. EJNMMI Res 2020; 10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Namkoong H, Fujiwara H, Ishii M, et al. Immune reconstitution inflammatory syndrome due to Mycobacterium avium complex successfully followed up using 18 F-fluorodeoxyglucose positron emission tomography-computed tomography in a patient with human immunodeficiency virus infection: a case report. BMC Med Imaging 2015; 15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sathekge M, Maes A, Van de Wiele C. FDG-PET imaging in HIV infection and tuberculosis. Semin Nucl Med 2013; 43:349–66. [DOI] [PubMed] [Google Scholar]
- 17. Sood A, Mittal BR, Modi M, et al. 18F-FDG PET/CT in tuberculosis: can interim PET/CT predict the clinical outcome of the patients? Clin Nucl Med 2020; 45:276–82. [DOI] [PubMed] [Google Scholar]
- 18. Lee MR, Cheng A, Huang YT, et al. Performance assessment of the DR TBDR/NTM IVD kit for direct detection of Mycobacterium tuberculosis isolates, including rifampin-resistant isolates, and nontuberculous mycobacteria. J Clin Microbiol 2012; 50:3398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jhun BW, Moon SM, Jeon K, et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: a 15-year follow-up study. Eur Respir J 2020; 55:1900798. [DOI] [PubMed] [Google Scholar]
- 20. Xie YL, Rosen LB, Sereti I, et al. Severe paradoxical reaction during treatment of disseminated tuberculosis in a patient with neutralizing anti-IFNgamma autoantibodies. Clin Infect Dis 2016; 62:770–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshizawa K, Aoki A, Shima K, et al. Serum anti-interferon-gamma autoantibody titer as a potential biomarker of disseminated non-tuberculous mycobacterial infection. J Clin Immunol 2020; 40:399–405. [DOI] [PubMed] [Google Scholar]
- 22. Ochoa S, Ding L, Kreuzburg S, Treat J, Holland SM, Zerbe CS. Daratumumab (anti-CD38) for treatment of disseminated nontuberculous mycobacteria in a patient with anti-interferon-gamma autoantibodies. Clin Infect Dis 2021; 72:2206–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen D, Chen Y, Yang S, et al. The additional value of 18F-FDG PET/CT imaging in guiding the treatment strategy of non-tuberculous mycobacterial patients. Respir Res 2024; 25:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.