Abstract
Aim:
Percutaneous transluminal angioplasty (PTA) for peripheral artery disease (PAD) commonly leads to dissections which are associated with higher target lesion revascularization (TLR) rates. Clinical and economic consequences of dissection management in the femoropopliteal artery following PTA, and specifically the potential economic benefit of focal dissection repair using the novel Tack Endovascular System, remain unknown.
Methods:
A decision-analytic model was used to estimate 24-month clinical events, costs and quality-adjusted life year (QALY) gain for a Tack-supported versus status-quo PTA strategy. Patient and lesion characteristics and TLR rates were derived from the PTA cohort of the TOBA II clinical trial, an observational cohort, and literature. Cost–effectiveness was determined from a US payer and provider perspective separately for the non-severe (grade A or B), severe (grade C and higher) and the entire dissection cohort.
Results:
TLR rates were lower for the Tack-supported strategy compared with PTA (7.7 vs 27.4% in the non-severe, 13.9 vs 25.8% in the severe and 12.0 vs 26.3% in the entire dissection cohort). Cost and QALY differences were +$297/ + 0.0110 in the non-severe dissection cohort and -$1602/ + 0.0067 in the severe dissection cohort, resulting in an incremental cost–effectiveness ratio (ICER) of $25,622 in the non-severe cohort and dominance in the severe cohort and the entire cohort.
Conclusion:
Compared with a ‘status-quo’ approach, proactive focal stenting may lead to fewer reinterventions and improved quality of life. There appears to be a graded economic benefit of focal dissection treatment, being cost-effective in non-severe dissections and even cost saving in severe dissections.
Keywords: adverse effects, angioplasty, balloon, costs and cost analysis, dissection, peripheral arterial disease
Shareable abstract
This analysis explores the health-economic consequences of above-the-knee dissections and assesses the value of TACK treatment. TACK demonstrated to be a high-value intervention, with improved clinical outcomes and a cost-effective ICER.
Plain language summary
Clinical and economic implications of focal dissection following percutaneous transluminal angioplasty of the superficial femoral artery
What is this article about?
This analysis explores the clinical and economic implications of proactive focal stenting compared with standard treatment for dissections following percutaneous transluminal angioplasty, for dissections of different severity.
What were the results?
The cohort receiving proactive focal stenting was associated with lower target lesion revascularization rates (7.7 vs 27.4% in the non-severe, 13.9 vs 25.8% in the severe and 12.0 vs 26.3% in the entire dissection cohort). The resulting incremental cost–effectiveness ratio was $25,622/QALY in the non-severe dissection scenario, with spot stenting resulting in cost-savings in a severe cohort scenario.
What do the results mean?
The results of this analysis suggest spot stenting may contribute to improved clinical outcomes, while also being a cost-effective treatment option, regardless of dissection severity.
Endovascular revascularization is currently the primary recommended mode of treatment for lifestyle-disabling claudication due to hemodynamically significant femoropopliteal peripheral artery disease (PAD) after failing supervised exercise and guideline-directed medical therapy [1,2]. Although percutaneous transluminal angioplasty (PTA) is less invasive than surgical bypass revascularization, there are frequent manifestations of dissection events which may be underrecognized and yet have clinical impact [3]. First described and catalogued in coronary artery disease, dissection after femoropopliteal PTA has been shown to lead to worse acute and long-term outcomes [4–7].
A recent retrospective study analyzed the patterns of various dissections, treatment strategies and the increased risk of restenosis. At two years, untreated dissections of all grades demonstrated lower patency and higher clinically driven target lesion vascularization (TLR) rates, with significantly higher TLR rates with worse dissection severity [6,8,9]. Furthermore, data from recent studies have shown that dissection severity is frequently underestimated by the operator when compared with core lab-adjudicated findings [10].
While there has not been much disagreement that severe dissections need to be treated in order to secure the acute PTA result, uncertainty remains about the ideal treatment of non-severe (grade A and B) dissections. The observed increases in TLR rates with all grades of untreated dissection led to additional treatment costs, but the exact economic consequences have not been previously studied. The Tack Endovascular System (Intact Vascular, Inc. now a part of Philips Image Guided Therapy Corporation, MN, USA) is a newly developed treatment approach. It is designed specifically to treat dissections following PTA with either plain or drug-coated balloons (DCB) in peripheral arteries using a very short metallic implant with low outward radial force designed to impart minimal stress to the vessel wall and leave less metal behind compared with traditional stenting [11].
The objective of the present study was to analyze the economic consequences of untreated dissections in the United States and to estimate the potential impact of using the Tack system for dissection treatment compared with currently accepted treatment strategies.
Methods
Overview
A decision-analytic model was created to compute 24-month costs from a payer and provider (hospital, outpatient-based lab, ambulatory surgical center) perspective. For lower-grade dissections (A, B), we compared outcomes and costs of a cohort treated with PTA only to those treated with the Tack system in the TOBA II PTA subgroup. For higher-grade dissections (C through F), we assumed stent treatment as the standard approach to management compared with Tack treatment. For the full spectrum of dissections (A through F), an analysis was performed based on the same assumptions for the non-severe and severe dissection subgroups. The cost–effectiveness of the Tack-supported strategies versus status quo PTA was calculated based on estimates for a temporary reduction in health-related quality of life (HRQoL) associated with any TLR events. The focus on the TOBA II PTA subgroup was made for comparability to the historical control, which did not include any DCB data. Both the TOBA II study (NCT02522884) and the retrospective Fujihara et al. study, from which data for this analysis were obtained, were approved by the respective institutional review boards.
Patient characteristics & other input parameters
The proportion of TLRs at 24 months were derived separately for the non-severe and severe dissection groups. For the dissection analysis, clinical outcomes for the Tack strategy were based on the TOBA II PTA cohort (n = 90) [11]. For the comparator PTA strategy, two groups of data were used according to the NHLBI grade of dissection severity.
For the non-severe dissection cohorts (grades A and B), data were obtained from the TOBA II PTA subgroup and the matched PTA-only subgroup of the Fujihara et al. data [6,11]. The Fujihara study was chosen as it is the only larger-scale study to-date reporting dissection severity-specific outcomes, and for which patient-level data could be made available. The matched subgroup (n = 39) included patients with A or B dissections with mean lesion length <120 mm and no Rutherford 5 or 6 symptoms. Table 1 provides an overview of the detailed cohort and lesion characteristics. Cohort characteristics differed in the proportions of patients with dyslipidemia and coronary artery disease, both of which were lower in the Fujihara cohort, but were comparable across age, gender, hypertension and current smoker status. Lesion characteristics were closely comparable, with an average lesion length of 60 mm, residual vessel diameter (RVD) of 5.5 mm, and comparable proportion of Rutherford 2 or 3 lesions higher than 90% in both cohorts, while the proportion of chronic total occlusion differed, with 8.9% reported in the TOBA PTA cohort, and zero in the Fujihara subcohort. Dissection severity was core lab-adjudicated in the TOBA II study, but not the Fujihara et al. study. Therefore, for the current study, dissection severity, by grade, was derived from the core lab-adjudicated data from the TOBA II PTA cohort, with 30.3% falling into the non-severe (A, B) group and 69.7% into the severe dissection (C, D, E, F) group.
Table 1. . Cohort and lesion characteristics of TOBA II percutaneous transluminal angioplasty cohort and matched percutaneous transluminal angioplasty cohort.
| Parameter | TOBA II PTA cohort [11] (n = 90) | Matched PTA cohort [6] (n = 39) | p-value |
|---|---|---|---|
| Age (years) | 70.2 | 73.4 | 0.0578 |
| Male | 70.0% | 82.0% | 0.1307 |
| Hypertension | 92.2% | 92.3% | 0.9869 |
| Diabetes | 48.9% | 46.1% | 0.7781 |
| Dyslipidemia | 84.4% | 53.8% | 0.0012† |
| Current smoker | 30.0% | 33.3% | 0.7140 |
| Coronary artery disease | 65.9% | 43.6% | 0.02185† |
| Lesion length (mm) | 59.8 | 60.4 | 0.9224 |
| RVD (mm) | 5.5 | 5.5 | 0.9258 |
| Chronic Total Occlusions | 8.9% | 0.0% | 0.0041† |
| Rutherford 2 or 3 | 97.8% | 92.3% | 0.2398 |
p-values calculated using Welch Two Sample t-test, with variables where statistically significant difference (p < 0.05).
PTA: Percutaneous transluminal angioplasty; RVD: Reference vessel diameter.
For severe dissections, TOBA II PTA data were used for Tack-treated patients. For the control group, separate TLR data were identified as follows: for patients treated with bare metal stents (BMS), 24-month TLR was obtained from a recent literature search combined with a meta-analytic pooling approach (weighted mean lesion length of 86.4 mm for the BMS group) [12]. For drug eluting stent (DES) treatment, data were obtained from that same study and were primarily based on Zilver PTX randomized controlled trial and single-arm studies (weighted mean lesion length of 90.3 mm for the DES group) [13,14]. For PTA alone treatment, data were obtained from the Fujihara et al. PTA-only subgroup [6]. Except for sensitivity analyses, it was assumed all grade C and higher dissections would be treated with stents, with 85% of these procedures using BMS and 15% DES.
For estimation of reimbursement amount spent on reinterventions, assumptions were made about the expected percentages of treatment modality used in reinterventions, by index treatment strategy. These estimates were derived based on the clinical co-authors' experience in contemporary clinical practice. See Supplementary Materials for details.
Model structure, perspectives & analysis
The decision-analytic model considered the index procedure and up to one reintervention, over a 24-month analysis horizon. This time horizon, in line with earlier health-economic studies of SFA endovascular interventions [12–15], was chosen to reflect the expected additional clinical benefit of the studied therapies. Cohort mortality was considered and implemented based on age- and gender-specific lifetable data for the US, adjusted to match two-year survival observed in the TOBA II study [16,17]. The model structure is shown in Figure 1 and key model inputs are detailed in Table 2.
Figure 1. . Model structure.
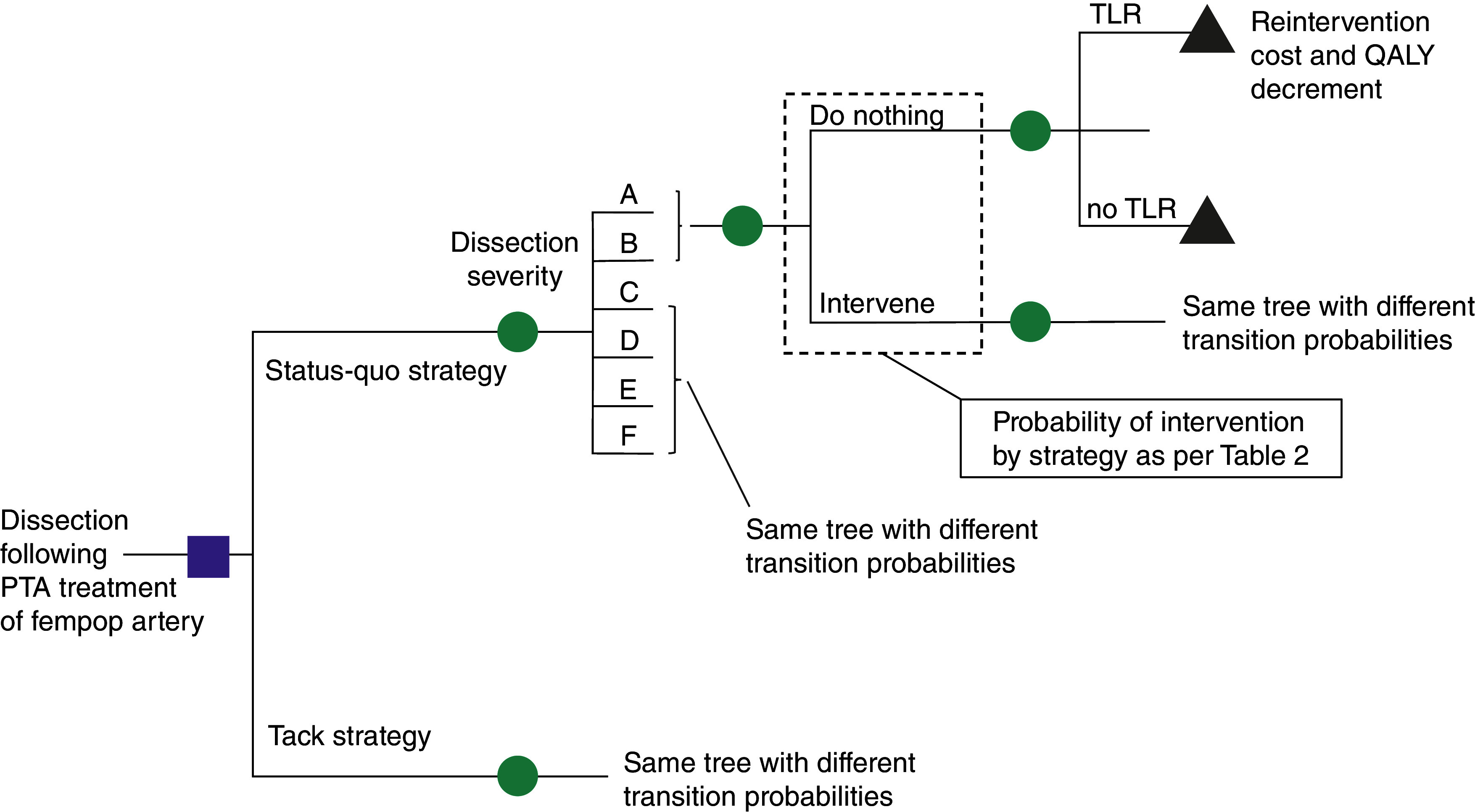
Depicted is a simplified version of the decision-analytic model, in this case a decision tree. Patients start in the model after a PTA for femoropopliteal (fem/pop) disease complicated by a dissection. The status quo strategy involves treatment of the dissection with a bare metal or drug-eluting stent or doing nothing (the probabilities of these interventions vary by dissection severity and are mentioned in Table 2. The combination of dissection severity and nature of the intervention or non-intervention determines the likelihood of a TLR which leads to additional costs for the reintervention and a quality-adjusted life year decrement.
PTA: Percutaneous transluminal angioplasty; QALY: Quality-adjusted life year; TLR: Target lesion revascularization.
Table 2. . Key input parameters.
| 24-month TLR rates | Value | Source (year) | Ref. |
|---|---|---|---|
| PTA – grade A/B dissections | 27.4% | Fujihara et al. (2017) PTA sub cohort <120 mm lesion length, no Rutherford 5 or 6. See supplementary materials. | [6] |
| PTA – grade C/D/E/F dissections | 82.6% | Fujihara et al. (2017) PTA sub cohort <120 mm lesion length, no Rutherford 5 or 6 (shown for reference only, not used in model calculation, as stent treatment was assumed for all severe dissections) | [6] |
| PTA + Tack – grade A/B dissections | 7.7% | Brodmann et al. (2023) TOBA II PTA sub cohort. See supplementary materials. | [16] |
| PTA + Tack – grade C/D/E/F dissections | 13.9% | Brodmann et al. (2023) TOBA II PTA sub cohort. See supplementary materials. | [16] |
| BMS | 26.9% | Katsanos et al. (2016), Table 2 | [12] |
| DES | 19.4% | ZILVER-PTX and ZILVER-PTX SAS (12-month data) | [13,14] |
| Strategy and reintervention probabilities | |||
| Non-severe dissections – status quo | 100% left untreated | Assumption for analysis | |
| Severe dissections – status quo | 100% stent-treated (85% BMS, 15% DES) | Assumption for analysis | |
| Non-severe dissections – Tack-supported | 100% Tack treatment | Assumption for analysis | |
| Severe dissections – Tack-supported | 100% Tack treatment | Assumption for analysis | |
| Reimbursement | |||
| Inpatient DRG 252 | $22,933 | IPPS FY (2023) | |
| Percent | 48.4% | Based on latest CMS data (2021) MEDPAR | |
| Inpatient DRG 253 | $18,342 | IPPS FY (2023) | |
| Percent | 37.0% | Based on latest CMS data (2021) MEDPAR | |
| Inpatient DRG 254 | $12,543 | IPPS FY (2023) | |
| Percent | 14.6% | Based on latest CMS data (2021) MEDPAR | |
| Resulting weighted DRG inpatient | $19,720 | Computed from inputs above | |
| Outpatient APC 5192 (PTA) | $5215 | HOPPS CY (2023) | |
| Outpatient APC 5193 (stent, or isolated atherectomy) (applies to stent[s] and to Tack) | $10,615 | HOPPS CY (2023) | |
| Outpatient APC 5194 (atherectomy + stent) (applies to stent[s] and to Tack) | $17,178 | HOPPS CY (2023) | |
| OBL (PTA) | $2987 | CY (2023) payment rate | |
| OBL (Stent(s), or isolated atherectomy) (applies to stent[s] and to Tack) | $8337 | CY (2023) payment rate | |
| OBL (atherectomy + stent[s]) (applies to stent[s] and to Tack) | $11,473 | Final CY (2023) OPPS payment rate | |
| Percent of procedures outpatient (vs inpatient) | 53.8% | Medicare Phys. Supplier Proc. Summary (CPT) (2020) | |
| Percent of outpatient procedures performed in OBL | 34.6% | Medicare Phys. Supplier Proc. Summary (CPT) (2019) | |
| Device prices | |||
| PTA | $516 | Estimate based on 2022 market research data – POBA catheter, fempop indication | |
| DCB | $1590 | Estimate based on 2022 market research data | |
| BMS | $840 | Estimate based on 2022 market research data – BMS, fempop indication | |
| DES | $2085 | Estimate based on 2022 market research data – DES, fempop indication | |
| Tack | $1945 | Current list price, as communicated by the manufacturer | |
| Scoring balloon | $1352 | Estimate based on 2022 market research data – specialty balloon | |
| Atherectomy | $2210 | Estimate based on 2022 market research data – atherectomy device, total (rotational/directional/laser) | |
| Device utilization – see appendix | |||
| Distribution of dissections | |||
| Type A | 11.2% | TOBA II PTA cohort, core lab-reported | |
| Type B | 19.1% | TOBA II PTA cohort, core lab-reported | |
| Type C | 46.1% | TOBA II PTA cohort, core lab-reported | |
| Type D | 23.6% | TOBA II PTA cohort, core lab-reported | |
| Type E | 0.0% | TOBA II PTA cohort, core lab-reported | |
| Type F | 0.3% | TOBA II PTA cohort, core lab-reported | |
| QALY decrement | |||
| QALY decrement per TLR | -0.059 | Salisbury et al. (2016) | [18] |
BMS: Bare metal stent; CMS: Centers for Medicare and Medicaid Services; CPT: Current procedural terminology; CY: Calendar year; DCB: Drug-coated balloon; DES: Drug-eluting stent; DRG: Diagnosis-related group; HOPPS: Hospital Outpatient Prospective Payment System; OBL: Office-based lab; OPPS: Outpatient Prospective Payment System; PTA: Percutaneous transluminal angioplasty; QALY: Quality-adjusted life year; SAS: Single arm study; TLR: Target lesion revascularization.
Strategy-specific results were compared separately for non-severe dissections and for the global cohort (non-severe and severe dissections). Costs at 24 months (in 2023 US dollars) and differences in quality-adjusted life years (QALYs) gained were computed based on a previously calculated QALY decrement of 0.059 associated with each TLR and assumed no difference in survival or HRQoL otherwise [18]. Other outcomes calculated included TLRs avoided, and – based on cost difference and QALY gain – an incremental cost effectiveness ratio (ICER, in USD per QALY gained). In addition, numbers needed to treat (NNT) to avoid one TLR event and costs per TLR avoided at 24 months were calculated.
Analyses were conducted from a payer and from a provider perspective, with the first considering the two-year analysis horizon and the latter the index procedure only. The payer perspective was based on US Medicare rates, with national average reimbursement amounts considered as a representative proxy for true costs. Tack procedures are reimbursed as stent procedures, so the same reimbursement amounts apply as in BMS or DES procedures. Given that provider costs might vary substantially, the provider perspective was defined as the margin between the device costs and the reimbursement amounts; these margins include all other costs aside from the ones for the device (including, but not limited to, personnel costs, operating room costs, other disposal costs, and, if applicable, costs for a post-procedural observation) [12]. Device costs were determined based on recent market reports on average selling prices and the most recent available list price of the Tack system.
For determination of cost–effectiveness, current recommendations for willingness-to-pay thresholds endorsed by the American College of Cardiology/American Heart Association were applied, with incremental cost–effectiveness ratios (ICER) below $150,000 per QALY gained considered of value and ICERs below $50,000 per QALY gained denoting a high value intervention. Costs and outcomes were discounted at 3% per annum [19].
Model validation & scenario analyses
Internal model validation was conducted as follows: First, the model was independently reviewed by one of the co-authors for accuracy and consistency. Second, projected clinical event rates at 24 months were checked for each subgroup to ensure proper implementation and agreement with clinical input parameters. Third, the decision tree was validated for consistency by inspecting each branch of the model for proper implementation of probabilities and associated costs. Fourth, extreme parameter inputs (such as only considering one type of cost) were explored to ensure proper model response as expected.
In light of the non-randomized nature of the underlying clinical data, several scenario analyses were conducted to explore robustness of the cost–effectiveness findings. These included threshold analyses to explore the effect of variation in comparator strategy performance (TLR rates of PTA only, BMS and DES) onto the cost–effectiveness of the Tack strategy.
Statistical analyses
The analysis model was implemented in Microsoft Excel. Analyses of MEDPAR claims data and of cohort and lesion characteristics were conducted in JMP 17 (JMP Inc., JMP Statistical Discovery LLC, NC, USA). Differences in the mean values of cohort and lesion characteristics were evaluated using the Welch Two Sample t-test and evaluated at a threshold of p < 0.05.
Results
Model validation confirmed consistent implementation and proper projection for the two treatment strategies across the studied dissection severity groups. The model-projected two-year TLR rates obtained for the different strategies and dissection groups were as follows: for non-severe dissections, 27.4% for the PTA-only strategy and 7.7% for the Tack-supported strategy, per two-year Kaplan-Meier estimate [16]. For severe dissections, 26.9% and 19.4% for BMS and DES, respectively and 13.9% for the Tack-supported strategy [16]. See Figures S.1.1 & S.1.2 in the supplementary materials for the full freedom from TLR and CD-TLR Kaplan Meier curves. Two-year survival of the modeled cohort was 94.8%, in line with the clinical data from TOBA II reporting 11 deaths in 213 patients [16].
In the model, these led to 2-year TLR estimates of 27.4% versus 7.7% (risk difference: -19.7%) for PTA only versus PTA and Tack use in non-severe dissections, 25.8% versus 13.9% (risk difference: -11.9%) in severe dissections and 26.3% versus 12.0% (risk difference: -14.3%) when considering the entire dissection cohort.
For the non-severe dissection cohort, total 24-month payer costs were $297 higher for the Tack-supported strategy ($15,381 vs $15,084). This total 24-month cost difference resulted from the net of the increased index procedure cost in the Tack-supported strategy balanced by subsequent cost savings with Tack based on reduced need for reinterventions. For the severe dissection cohort, 24-month costs were $1602 lower ($16,164 vs $17,766) for the Tack-supported strategy based on same index reimbursement cost and a reduction in 24-month TLR rates using Tack. For the entire dissection cohort, costs were $1026 lower with the Tack-supported strategy ($15,927 vs $16,953). See Figure 2.
Figure 2. . 24-month total costs and projected target lesion revascularizations for the percutaneous transluminal angioplasty ‘status quo’ versus Tack-supported strategies and projected 24-month incremental QALY gain with Tack.
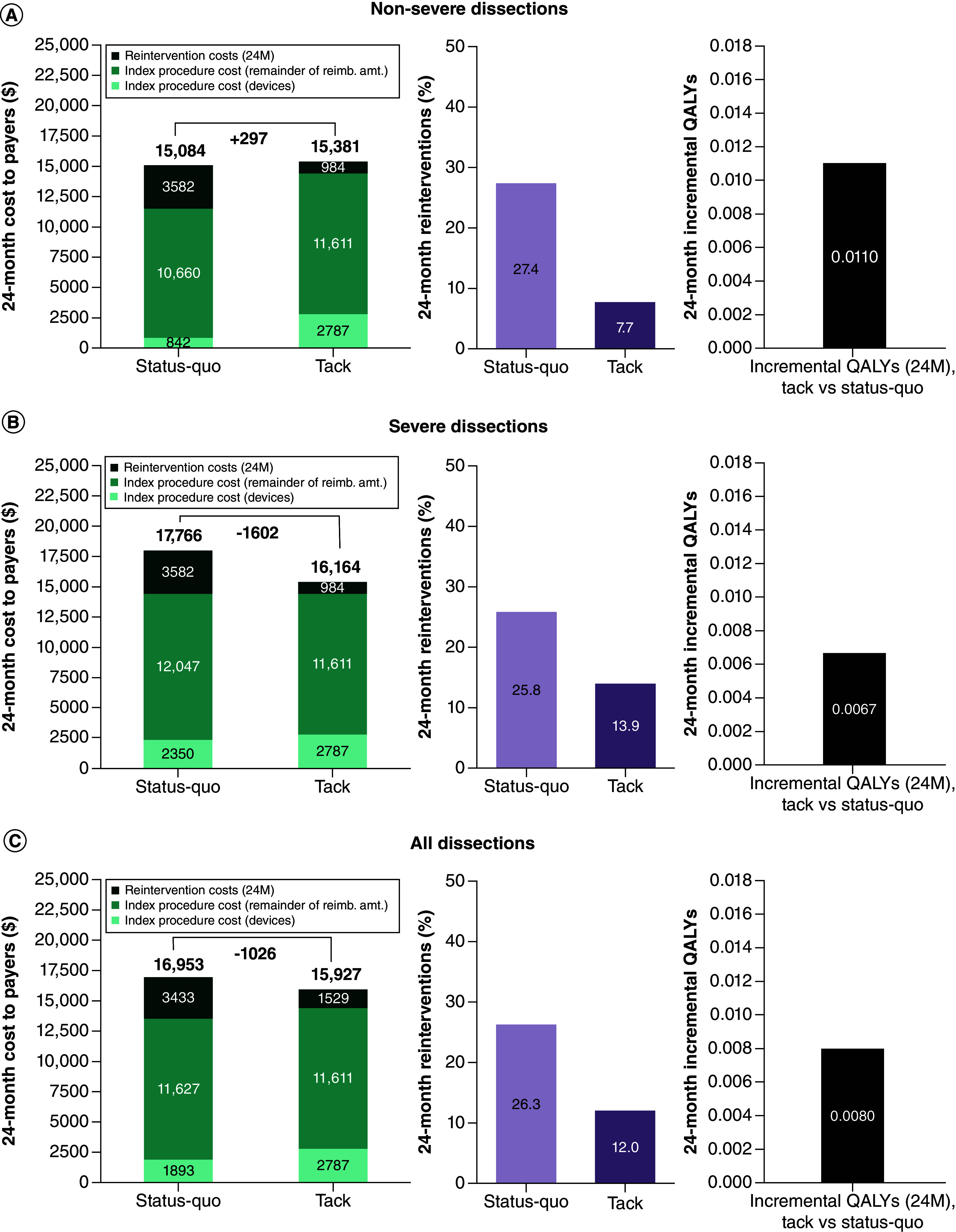
(A) Non-severe dissection cohort. (B) Severe dissection cohort. (C) Total dissection cohort.
24M: Twenty-four months; QALY: Quality-adjusted life year.
From the provider perspective, the resulting index procedure margin with Tack was $951 higher in the non-severe dissection cohort ($11,611 vs $10,660), $436 lower in the severe dissection cohort ($11,611 vs $12,047) and $16 lower in the entire dissection cohort ($11,611 vs $11,627). See Table 3.
Table 3. . 24-month costs for the percutaneous transluminal angioplasty ‘status quo’ and Tack-supported strategies – non-severe dissection cohort, severe dissection cohort and entire dissection cohort.
| Non-severe dissection cohort (A, B) | Status quo strategy | Tack-supported strategy | Difference | |
|---|---|---|---|---|
| Reimbursement/cost to payers | Index | $11,502 | $14,398 | $2896 |
| Reintervention | $3582 | $984 | -$2598 | |
| Total | $15,084 | $15,381 | $297 | |
| Device costs | Index | $842 | $2787 | $1945 |
| Reintervention | $556 | $198 | -$358 | |
| Total | $1398 | $2984 | $1587 | |
| Margin | Index | $10,660 | $11,611 | $951 |
| Severe dissection cohort (C, D, E, F) | Status quo strategy | Tack-supported strategy | Difference | |
| Reimbursement/cost to payers | Index | $14,398 | $14,398 | $0 |
| Reintervention | $3369 | $1767 | -$1602 | |
| Total | $17,766 | $16,164 | -$1602 | |
| Device costs | Index | $2350 | $2787 | $436 |
| Reintervention | $807 | $364 | -$443 | |
| Total | $3157 | $3150 | -$7 | |
| Margin | Index | $12,047 | $11,611 | -$436 |
| Entire dissection cohort (A, B, C, D, E, F) | Status quo strategy | Tack-supported strategy | Difference | |
| Reimbursement/cost to payers | Index | $13,520 | $14,398 | $877 |
| Reintervention | $3433 | $1529 | -$1904 | |
| Total | $16,953 | $15,927 | -$1026 | |
| Device costs | Index | $1893 | $2787 | $893 |
| Reintervention | $731 | $313 | -$417 | |
| Total | $2624 | $3100 | $476 | |
| Margin | Index | $11,627 | $11,611 | -$16 |
Results stratified into reimbursement/costs to payer, device costs, index procedure provider margins (defined as reimbursement minus device costs). Discounting and general population mortality applied.
The Tack-supported treatment strategy was associated with a calculated QALY gain at 2 years of 0.0110 QALYs in the non-severe dissection cohort, of 0.0067 QALYs in the severe dissection cohort, and of 0.0080 QALYs in the entire cohort (Figure 2). In conjunction with the calculated incremental costs at 24-months, this resulted in an incremental cost–effectiveness ratio (ICER) of $25,622 per QALY gained for the non-severe dissection cohort, rendering the Tack strategy cost-effective; both the severe dissection cohort and the entire cohort were associated with cost savings, at overall improved projected QALYs, leading to health–economic dominance.
The NNT to avoid one TLR over 24-months with the Tack strategy was 5.1 in the non-severe dissection cohort, 8.4 in the severe dissection cohort and 7.0 in the global cohort. Costs per TLR avoided were $1512 in the non-severe cohort and cost savings per TLR avoided in both the severe dissection cohort and the entire cohort.
In sensitivity analysis, the Tack strategy remained cost-effective (at willingness-to-pay of $150,000 per QALY gained) in the non-severe dissection cohort as long as the 24-month TLR rate of the PTA control group was not lower than 20.8% (compared with the observed TLR estimate of 27.4%). In the severe dissection cohort, the Tack strategy remained cost-effective as long as the Tack 24-month TLR was the same or better than the control cohort receiving BMS or DES stent treatment, suggesting BMS and DES performance was no more than 50% improved compared with their respective 24-month base case TLR performance of 26.9% and 19.4%, respectively.
Discussion
The present study demonstrates that untreated dissections after PTA convey a substantial clinical and economic burden. While most severe dissections are treated, non-severe dissections left untreated were found to lead to TLRs in approximately 30% of cases at 2 years of follow-up. The study found that the initial upfront investment in a proactive treatment strategy involving focal dissection repair with the Tack system may lead to savings that almost amount to the Tack device cost after 24 months, while improving index procedure margins for providers. These outcome improvements may be achieved at a favorable NNT around five in the non-severe dissection group and seven in the entire cohort.
Our findings suggest it is necessary to carefully consider treatment choices for dissections, and to appreciate the need for proper dissection identification, especially in non-severe A and B dissections that frequently remain unrecognized and untreated.
From a technology assessment perspective, the research conducted for this study highlighted the very limited amount of data available to provide insight into reintervention rates for different dissection severities, prompting the need for future studies that report dissection grade-specific outcomes. The Fujihara et al. data used for this analysis, to the authors' knowledge, is the largest dataset to report outcomes by dissection severity [6]. The data required for the current analysis, dissection-grade specific outcomes for a PTA only cohort, were only available by conducting a post-hoc analysis of patient-level data from that study. Similarly, there is very limited – if any – formal evidence available about stent performance in dissections, even though it can be argued that published outcomes of stent trials include a substantive proportion of dissection lesions, and that these data might be reasonably reflective of outcomes that can be expected in stented dissections.
Based on referenced willingness-to-pay thresholds for the United States, this study's findings suggest Tack-supported treatment might be a high-value intervention that is cost-effective in non-severe dissections and even cost saving in the treatment of severe dissections. These economic consequences are heavily driven by current reimbursement levels, which provide 60% higher reimbursement for procedures involving stents, as opposed to balloon only. As such, the incremental costs to payers of converting a balloon only to a stented or Tack-treated index procedure are sizable and lead to the observed improvement in provider margins. In other words, at least theoretically, future reimbursements might strike a balance that provides the improved clinical outcomes at more or less cost neutrality to both payers and providers/facilities.
The current analysis used only the plain balloon (POBA) subcohort of the TOBA II study. In that study, the POBA subcohort had fewer reintervention events than the DCB subcohort which used the Lutonix drug-coated balloon (DCB) (Becton, Dickinson and Company, NJ, USA). The reason for limiting inclusion to the POBA subgroup was twofold: first, the only available comparator data involved plain balloons, as opposed to DCB. As such, the inclusion of DCB-Tack subjects would have introduced a potential confounder. Second, the higher TLR rates observed in the TOBA II DCB cohort are contrary to the findings of the more recent, larger TOBA III study, which studied Tack use in a 100% urea-excipient based DCB cohort. Based on a recent presentation of 12-month data, Tack TLR rates in that more recent study were 2.5% in the standard lesion (<150 mm lesion length) and 3.2% in the long lesion cohort [20].
The present study is subject to several limitations. First, in the absence of controlled studies or evidence that would allow for indirect treatment comparison, the analysis relied on a combination of primary data collected in the recent TOBA II study and data from other clinical studies to provide comparator performance for health–economic purposes. The subset of the Fujihara et al. data closely matched the cohort and lesion characteristics of the TOBA II PTA subgroup, with the exception of the proportion of dyslipidemia, coronary artery disease and chronic total occlusion, which were lower in the Fujihara data [6,11]. While lesion length in the SFA stent data overall was reasonably comparable, the use of SFA stent data without knowledge about the percentage of dissections in those trials is a limitation. However, it can be argued that the TLR estimate identified from a prior meta-analysis of SFA stent studies likely includes a reasonable proportion of dissections that were treated, and that outcomes between a stented dissection versus non-dissection lesion might not differ materially [12]. Overall, these limitations underscore the need to strongly consider reporting of dissection severity-specific outcomes in future clinical studies, including consideration of the potential effect of prolonged balloon inflation prior to stent placement. The current analysis addressed these uncertainties by conducting threshold analyses which found Tack to remain cost-effective as long as comparator performance was no more than 28% improved relative to its base case assumption in the non-severe dissection group, and no more than 50% improved relative to its base case assumption in the severe dissection cohort, suggesting the findings of the current study are reasonably robust. Second, since the subject of this analysis was a health–economic research question, no formal hypothesis testing was performed to establish non-inferiority among the strategies. Third, this exploratory analysis relied on current reimbursement rates to estimate costs to payers. While this is appropriate for calculation of the intended payer perspective, future studies would benefit from detailed cost collection alongside a trial. The same limitation, by extension, applies to the estimation of the incremental cost–effectiveness ratios, which again consider the payer perspective as opposed to a societal perspective. Fourth, in light of no expected mortality difference between the studied cohorts, the computed QALY gain was calculated solely based on a previously measured QALY decrement associated with TLRs observed in a prior endovascular SFA trial [18]. Fifth, only up to one reintervention was considered in the current analysis, owed to non-availability of repeat reintervention data from the underlying studies. However, this can be expected to be a conservative approach with regard to cost–effectiveness estimations of the studied Tack treatment. Finally, the current provider analysis was limited to reimbursement revenue and device costs. Future studies will benefit from additional consideration of potential differences in resource utilization such as procedure duration or personnel use. Such considerations will help facilities quantify their margin impact even more accurately.
Conclusion
In conclusion, patients who experience dissections during PTA – especially if left untreated – are subject to suboptimal anatomic and clinical outcomes, resulting in subsequent treatments with associated substantial economic burden. Proper treatment for non-severe dissections needs to be carefully considered and might involve stents and novel treatments such as the Tack system which, while adding the largest clinical value in non-severe dissections currently left unrecognized and untreated, might offer good clinical and health–economic value across the full spectrum of dissections.
Summary points
Dissections after percutaneous angioplasty continue to present a clinical challenge and are associated with inferior outcomes, especially if left untreated.
In this analysis, the clinical and economic consequences of current dissection treatment and the potential benefit of focal treatment using a novel spot stenting device were assessed.
A Markov model was utilized to project costs and outcomes over 24 months, from both the perspective of a payer and provider.
The primary analysis outcomes were the incremental cost–effectiveness ratio (ICER) and target lesion revascularization (TLR) rate. ICERs were evaluated against established willingness-to-pay thresholds of $50,000 and $150,000 per QALY gained.
For the non-severe dissection cohort, incremental costs and QALYs were $297 and 0.0110, with a resulting ICER of $25,622 per QALY gained. While in the severe dissection cohort, incremental costs and QALYs were -$1602 and 0.0067, resulting in spot stenting being the dominant strategy (results in cost-savings).
The resulting 24-month TLR rates were 7.7 versus 27.4% in the non-severe, 13.9 versus 25.8% in the severe and 12.0 versus 26.3% in the entire dissection cohort, for Tack and the comparator strategy, respectively.
Findings confirm a sizable clinical and economic burden exists, and suggest spot stenting may meaningfully improve long-term patency, regardless of dissection severity.
These improvements might be achieved at overall cost savings or acceptable cost increase that renders spot stenting a cost-effective intervention.
Supplementary Material
Acknowledgments
We would like to thank the authors of the prior observational study (Drs. Fujihara, Takahara, Sasaki, Nanto, Utsunomiya, Iida and Yokoi) for providing access to the study's patient-level data. Also, we would like to thank M Jaff for his thoughtful advice and suggestions in support of the development of this analysis.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2024-0055
Author contributions
JB Pietzsch: conceptualization, methodology, investigation, software, formal analysis, data curation, writing – original draft, supervision. BP Geisler: conceptualization, methodology, software, formal analysis, data curation, writing – original draft, supervision. AM Garner: investigation, resources, writing – review and editing. AM Ryschon: validation, data curation, writing – review and editing. WA Gray: conceptualization, methodology, writing – review and editing. M Fujihara: conceptualization, methodology, data curation, resources, writing – review and editing. PA Schneider: conceptualization, methodology, writing – review and editing, supervision.
Financial disclosure
Funding for this study was provided by Intact Vascular Inc. (health–economic consulting services paid to Wing Tech Inc.). Wing Tech Inc. (JB Pietzsch, A Garner, A Ryschon and – during his employment – BP Geisler) provided health–economic consulting services to Intact Vascular, Inc. PA Schneider: consultant to Intact Vascular. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Gerhard-Herman MD, Gornik HL, Barrett C et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 69, 1465–1508 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Aboyans V, Ricco JB, Bartelink MEL et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 39, 763–816 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Tepe G, Schnorr B, Albrecht T et al. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. 8, 102–108 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Rogers JH, Lasala JM. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J. Invasive Cardiol. 16, 493–499 (2004). [PubMed] [Google Scholar]
- 5.Huber MS, Mooney JF, Madison J, Mooney MR. Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am. J. Cardiol. 68, 467–471 (1991). [DOI] [PubMed] [Google Scholar]
- 6.Fujihara M, Takahara M, Sasaki S et al. Angiographic dissection patterns and patency outcomes after balloon angioplasty for superficial femoral artery disease. J. Endovasc. Ther. 24, 367–375 (2017). [DOI] [PubMed] [Google Scholar]; •• Informed the comparator (percutaneous transluminal angioplasty [PTA]-only subgroup) data.
- 7.Kobayashi N, Hirano K, Yamawaki M et al. Simple classification and clinical outcomes of angiographic dissection after balloon angioplasty for femoropopliteal disease. J. Vasc. Surg. 67, 1151–1158 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Tepe G, Zeller T, Schnorr B et al. High-grade, non-flow-limiting dissections do not negatively impact long-term outcome after paclitaxel-coated balloon angioplasty: an additional analysis from the THUNDER study. J. Endovasc. Ther. 20, 792–800 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Armstrong EJ, Brodmann M, Deaton DH et al. Dissections after infrainguinal percutaneous transluminal angioplasty: a systematic review and current state of clinical evidence. J. Endovasc. Ther. 26, 479–489 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Bosiers M, Scheinert D, Hendriks JM et al. Results from the Tack Optimized Balloon Angioplasty (TOBA) study demonstrate the benefits of minimal metal implants for dissection repair after angioplasty. J. Vasc. Surg. 64, 109–116 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Gray WA, Cardenas JA, Brodmann M et al. Treating post-angioplasty dissection in the femoropopliteal arteries using the tack endovascular system: 12-month results from the TOBA II Study. JACC Cardiovasc. Interv. 12, 2375–2384 (2019). [DOI] [PubMed] [Google Scholar]; •• Provided 12-month clinical data for the Tack PTA cohort.
- 12.Katsanos K, Geisler BP, Garner AM, Zayed H, Cleveland T, Pietzsch JB. Economic analysis of endovascular drug-eluting treatments for femoropopliteal artery disease in the UK. BMJ Open. 6, e011245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dake MD, Scheinert D, Tepe G et al. Nitinol stents with polymer-free paclitaxel coating for lesions in the superficial femoral and popliteal arteries above the knee: twelve-month safety and effectiveness results from the Zilver PTX single-arm clinical study. J. Endovasc. Ther. 18, 613–623 (2011). [DOI] [PubMed] [Google Scholar]; •• Provided drug eluting stent (DES)-related clinical data.
- 14.Dake MD, Ansel GM, Jaff MR et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J. Am. Coll. Cardiol. 61, 2417–2427 (2013). [DOI] [PubMed] [Google Scholar]; •• Provided DES-related clinical data.
- 15.Pietzsch JB, Geisler BP, Garner AM, Zeller T, Jaff MR. Economic analysis of endovascular interventions for femoropopliteal arterial disease: a systematic review and budget impact model for the United States and Germany. Catheter Cardiovasc. Interv. 84, 546–554 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Brodmann M, Werner M, Sood A, Gray WA. Treating post-angioplasty dissection in the femoropopliteal arteries using the tack endovascular system: tack optimized balloon angioplasty II 24-month results. Vascular. 32(4), 850–857 (2023). [DOI] [PubMed] [Google Scholar]; •• Provided Tack clinical data.
- 17.Arias E, Jiaquan X, Kochanek K. United States Life Tables. (2021) (Accessed: 8 November 2024): https://stacks.cdc.gov/view/cdc/132418 [PubMed]
- 18.Salisbury AC, Li H, Vilain KR et al. Cost–effectiveness of endovascular femoropopliteal intervention using drug-coated balloons versus standard percutaneous transluminal angioplasty: results from the IN.PACT SFA II Trial. JACC Cardiovasc. Interv. 9, 2343–2352 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Sanders GD, Neumann PJ, Basu A et al. Recommendations for conduct, methodological practices, and reporting of cost–effectiveness analyses: second panel on cost–effectiveness in health and medicine. JAMA 316, 1093–1103 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Brodmann M. TOBA III: 1-year outcomes from a single-arm study of focal dissection repair after drug-coated balloon angioplasty of superficial femoral and proximal popliteal arteries. Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA: (2019). [Google Scholar]; •• Provided Tack clinical data.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


