Abstract
Aim:
To understand the impact of the niraparib individualized starting dose (ISD), compared with fixed starting dose (FSD), on the cost of hematologic adverse event (AE) management from a US payer perspective.
Methods:
The frequencies of grade ≥3 hematologic AEs that occurred in >1% of patients treated with niraparib were obtained from the primary analysis results of the phase III PRIMA/ENGOT-OV26/GOG-3012 trial. US unit costs for each grade ≥3 AE in the base case were obtained from the 2017 Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project database; unit costs were adjusted to 2020 US dollars. AE management costs per patient were calculated by multiplying AE unit cost by the frequency of each AE by niraparib starting dose. Because AEs were assumed to occur independently of one another, costs were added to derive the total cost.
Results:
For niraparib, the estimated AE management cost per patient was lower for the ISD than the FSD for all hematologic AEs (FSD vs ISD: thrombocytopenia, $4701.87 vs $1921.89; anemia, $2784.00 vs $1760.59; platelet count decreased, $2103.47 vs $922.51; neutropenia, $2112.50 vs $1369.56; neutrophil count decreased, $1285.87 vs $770.38). The total mean calculated AE management cost per patient was $12,987.71 with the FSD and $6744.93 with the ISD.
Conclusion:
For niraparib, the cost of managing hematologic AEs in the US was reduced by almost half with the ISD compared with the FSD. The cost reduction and improvements in safety associated with the niraparib ISD support its use in clinical practice.
Keywords: cost management, maintenance therapy, niraparib, ovarian cancer, PARP inhibitor
Plain language summary
How using an individualized dose of niraparib influences the cost of managing blood cell-related side effects.
What is this article about?
Patients with newly diagnosed advanced ovarian cancer that responds to platinum-based chemotherapy may receive niraparib maintenance therapy to extend the time before the cancer comes back or gets worse. The niraparib starting dose can be fixed (same amount for everyone) or individualized based on body weight and platelet count.
What were the results?
In this US-based analysis, using the niraparib individualized starting dose (ISD) reduced the cost of managing blood cell-related side effects compared with using the fixed starting dose (FSD).
What do the results mean?
Using the niraparib ISD reduced the cost of managing blood cell-related side effects compared with the FSD. Results of other past studies have also shown that using the niraparib ISD can reduce side effects and can treat the cancer just as effectively, when compared with the FSD. Along with the results of those past studies, the cost reductions observed in this study support using the niraparib ISD as maintenance therapy in patients with newly diagnosed advanced ovarian cancer that responds to platinum-based chemotherapy.
Shareable abstract
Researchers of the US-based analysis found that niraparib dosing based on patient body weight and platelet count was associated with nearly 50% lower costs for managing hematologic adverse events, compared with the fixed starting dose, for first-line maintenance treatment of patients with advanced epithelial ovarian cancer. These findings highlight the cost reduction and safety improvements associated with individualized starting doses of niraparib, further supporting its use in clinical practice.
Worldwide, ovarian cancer is the eighth leading cause of cancer-related deaths among women [1]. In the US, estimates indicate that there will be more than 19,600 new cases of ovarian cancer diagnosed in 2024 alone [2]. In addition to initial treatment with surgery and platinum-based chemotherapy, the treatment landscape for patients with advanced ovarian cancer has expanded to include maintenance treatment with poly(ADP)-ribose polymerase (PARP) inhibitors, alone or in combination with bevacizumab [3,4]. Maintenance treatment with the PARP inhibitor niraparib has been shown to improve progression-free survival in patients with newly diagnosed advanced epithelial ovarian cancer that responded to first-line platinum-based chemotherapy [5–7].
Consistent with other PARP inhibitors, niraparib is known to cause hematologic adverse events (AEs), particularly early during treatment [8–10]. In the ENGOT-OV16/NOVA trial (NCT01847274) of niraparib maintenance therapy in patients with platinum-sensitive recurrent epithelial ovarian cancer, all patients received a fixed starting dose (FSD) of 300 mg once daily [11]. The niraparib FSD was associated with grade ≥3 treatment-emergent AEs (TEAEs) of thrombocytopenia, anemia and neutropenia, with a high incidence of TEAEs resulting in dose reduction [11]. Results from subsequent analyses of the NOVA trial revealed that low baseline body weight (<77 kg) and platelet count (<150,000/μl) were associated with an increased incidence of grade ≥3 thrombocytopenia events, indicating that these patients could benefit from a lower starting dose of niraparib [12].
To improve patient safety and reduce the incidence of hematologic AEs, the starting dose of niraparib was adjusted in a portion of the patients included in the PRIMA/ENGOT-OV26/GOG-3012 trial (NCT02655016) of niraparib first-line maintenance [5]. The PRIMA protocol was amended partway through enrollment to introduce an individualized starting dose (ISD), in which patients with baseline body weight <77 kg or baseline platelet count <150,000/μl received 200 mg once daily and patients with baseline body weight ≥77 kg and baseline platelet count ≥150,000/μl received 300 mg once daily [5]. Results demonstrated that introduction of the ISD decreased the incidence of hematologic TEAEs while maintaining the progression-free survival benefit of niraparib compared with placebo [5,13]. Based on the results from the PRIMA trial, the niraparib ISD was approved for first-line maintenance therapy in the US [14]. However, the economic impact of the introduction of the niraparib ISD on the cost of hematologic AE management remains unexplored.
Methods
Evaluation of the niraparib ISD in the PRIMA/ENGOT-OV26/GOG-3012 trial
Niraparib first-line maintenance treatment was evaluated in the phase III randomized double-blind, placebo-controlled PRIMA trial in patients with newly diagnosed advanced epithelial ovarian cancer that responded to first-line platinum-based chemotherapy [5]. Detailed information on the study design and eligibility criteria have been described previously [5]. In the PRIMA trial, 2 different niraparib starting doses were used, the FSD and the ISD. At study start (July 2016), all patients received an FSD of 300 mg once daily. In November 2017, the protocol was amended so that newly enrolled patients received an ISD based on baseline body weight and platelet count (200 mg once daily in patients with baseline body weight <77 kg or baseline platelet count <150,000/μl and 300 mg once daily in all other patients) [5]. The primary analysis results from the PRIMA trial were published in 2019 and included an assessment of safety by niraparib starting dose [5]; these results are used for this analysis (clinical cut-off date, 17 May 2019).
Selection of hematologic AEs for assessment
This cost analysis included grade ≥3 hematologic AEs that occurred in >1% of patients in the niraparib arm in the PRIMA primary analysis results: thrombocytopenia, anemia, platelet count decreased, neutropenia and neutrophil count decreased [5]. Grade ≥3 events were selected for analysis because they were the most likely to require hospitalization and interventions such as transfusions for management because of their severity.
Frequencies of AEs
If a patient experienced ≥1 event within a given preferred term, that patient was counted only once for that term. Unrounded frequencies of grade ≥3 AEs for patients treated with the niraparib ISD and FSD were used for calculations.
Cost calculations
The unit costs in the US for the hospital-related management of each grade ≥3 AE in the base case were obtained from the 2017 Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) National (Nationwide) Inpatient Sample (NIS) database [15]. The all-payer HCUP NIS database is nationally representative and contains data from more than 7 million community hospital stays each year; the NIS database does not include rehabilitation and long-term acute care hospitals [16,17]. Unit costs were adjusted to 2020 US dollars, and AE management costs per patient were calculated by multiplying AE unit cost by the unrounded frequency of each AE by niraparib starting dose. Because AEs were assumed to occur independently of one another, their costs were added to derive the total cost.
Results
For niraparib, the estimated AE management cost per patient in the US was lower for the ISD than for the FSD for all hematologic AEs (Tables 1 & 2). The total mean calculated cost per patient was $12,987.71 with the FSD and $6744.93 with the ISD (Table 1). The percent reduction in the cost of hematologic AE management with the niraparib ISD compared with the FSD was also calculated (Figure 1). Thrombocytopenia had the greatest cost reduction (59.1%) of evaluated hematologic AEs. Implementing the niraparib ISD reduced the total cost of hematologic AE management by 48.1% as compared with the FSD.
Table 1. . Estimated cost of hematologic AE management using the May 2019 data cut-off date.
| Hematologic AE | Frequency of grade ≥3 AE in patients treated with niraparib, n (%) |
AE unit cost† | Mean calculated cost per patient‡ |
||
|---|---|---|---|---|---|
| FSD (n = 315) | ISD (n = 169) | FSD | ISD | ||
| Thrombocytopenia | 114 (36.2) | 25 (14.8) | $12,992.00 | $4701.87 | $1921.89 |
| Anemia | 112 (35.6) | 38 (22.5) | $7830.00 | $2784.00 | $1760.59 |
| Platelet count decreased | 51 (16.2) | 12 (7.1) | $12,992.00 | $2103.47 | $922.51 |
| Neutropenia | 46 (14.6) | 16 (9.5) | $14,466.00 | $2112.50 | $1369.56 |
| Neutrophil count decreased | 28 (8.9) | 9 (5.3) | $14,466.00 | $1285.87 | $770.38 |
| Total cost | $12,987.71 | $6744.93 | |||
Cost in the US adjusted to 2020 US dollars.
Costs calculated using the unrounded frequency of grade ≥3 AEs.
AE: Adverse event; FSD: Fixed starting dose; ISD: Individualized starting dose.
Table 2. . Estimated cost of hematologic AE management using November 2021 data cut-off date.
| Hematologic AE | Frequency of grade ≥3 AE in patients treated with niraparib, n (%) |
AE unit cost† | Mean calculated cost per patient‡ |
||
|---|---|---|---|---|---|
| FSD (n = 315) | ISD (n = 169) | FSD | ISD | ||
| Thrombocytopenia§ | 155 (49.2) | 37 (21.9) | $12,992.00 | $6392.82 | $2844.40 |
| Anemia | 114 (36.2) | 39 (23.1) | $7830.00 | $2833.71 | $1806.92 |
| Neutropenia¶ | 78 (24.8) | 25 (14.8) | $14,466.00 | $3582.06 | $2139.94 |
| Total cost | $12,808.66 | $6791.27 | |||
Cost in the US adjusted to 2020 US dollars.
Costs calculated using the unrounded frequency of grade ≥3 AEs.
Includes platelet count decreased.
Includes neutrophil count decreased.
AE: Adverse event; FSD: Fixed starting dose; ISD: Individualized starting dose.
Figure 1. . Hematologic AE management cost reduction with the niraparib individualized starting dose.
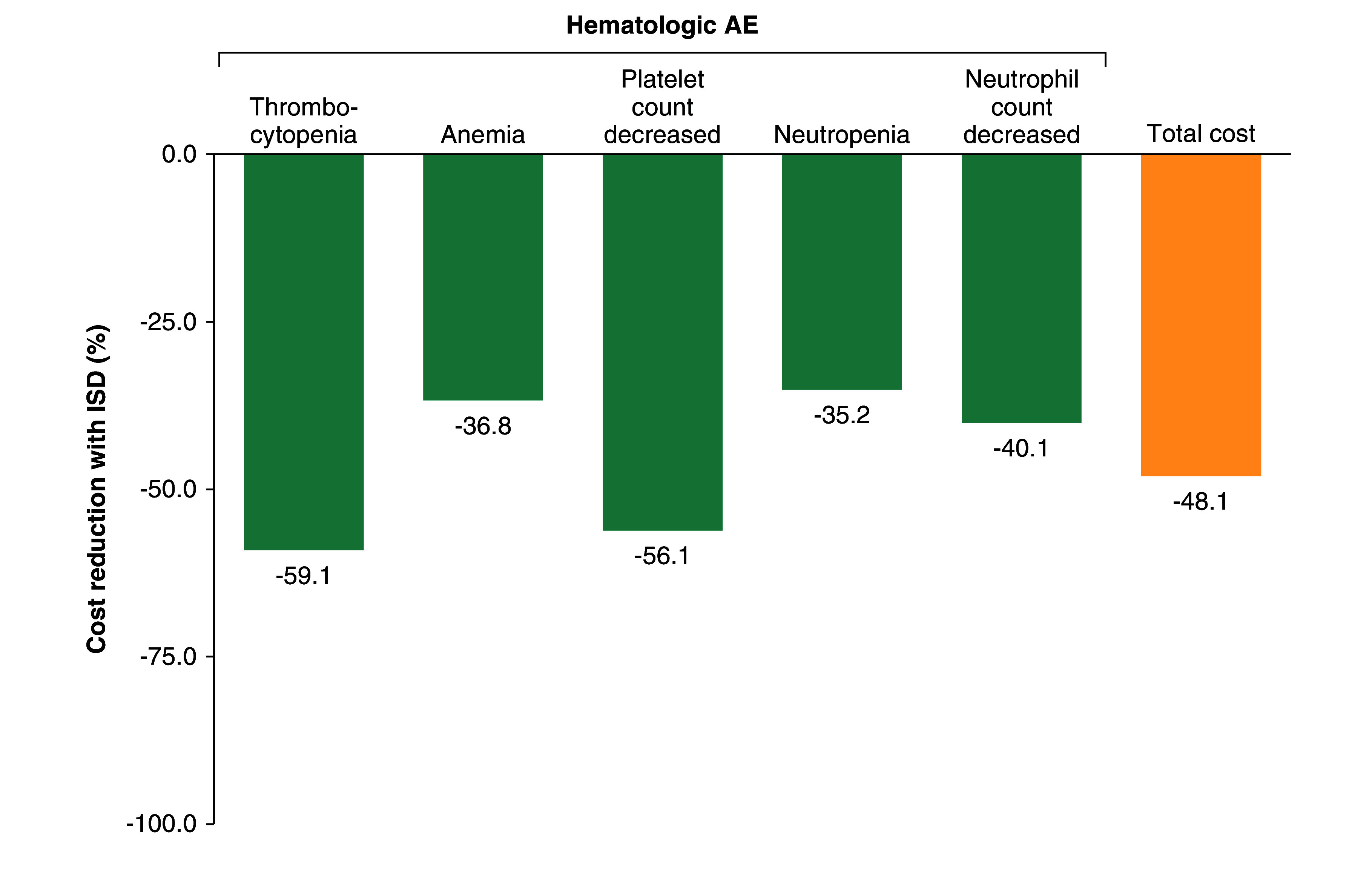
Percent reduction in the cost of hematologic AE management with the niraparib ISD. AE: Adverse event; ISD: Individualized starting dose.
Confirmatory calculations were performed using grouped terms for thrombocytopenia (thrombocytopenia and platelet count decreased), anemia (anemia, hemoglobin decreased, red blood cell decreased, hematocrit decreased and anemia macrocytic) and neutropenia (neutropenia, neutrophil count decreased, febrile neutropenia and neutropenic sepsis) using published findings from 17 November 2021, clinical cut-off date, with 3.5 years of follow-up [6]. Results were similar, with AE management cost reductions of 55.5%, 36.2% and 40.3% observed with the ISD compared with the FSD for thrombocytopenia, anemia and neutropenia, respectively (Table 2).
Discussion
In patients with advanced ovarian cancer, PARP inhibitor maintenance therapy represents an important treatment option after a response to first-line treatment [3,4]. The safety profiles of the 3 PARP inhibitors approved for use in the US vary, but as a class, PARP inhibitors have been associated with the hematologic AE of anemia [18]. Niraparib is also associated with increased incidences of thrombocytopenia and neutropenia [10,18]. In the PRIMA trial, introduction of the niraparib ISD reduced the incidence of any-grade and grade ≥3 events of thrombocytopenia, anemia and neutropenia [5,13]. A detailed assessment of the safety and tolerability of the niraparib ISD in the PRIMA trial found that first events of thrombocytopenia, anemia and neutropenia occurred early during treatment, had a short duration (≈2 weeks) and resolved in ≥90% of patients [19]. On the basis of the ISD findings in the PRIMA trial, the ISD is the current globally approved dosing for niraparib first-line maintenance [14,20,21].
In this analysis, the use of the niraparib ISD reduced the cost of managing severe hematologic AEs compared with the FSD. Reductions were observed for all hematologic AEs examined, and the total cost reduction as compared with the FSD was more than 48%. The reduced cost associated with the ISD is notable and supports the use of the ISD from a US payer perspective.
Limitations
Several limitations must be considered when interpreting these findings. First, the PRIMA trial was a global, phase III clinical trial with specific eligibility criteria. Accordingly, the niraparib FSD and ISD results for hematologic AEs may not be representative of the experience of patients with advanced ovarian cancer in the USA treated in everyday clinical practice. The small sample size (n = 169) for the niraparib ISD should also be considered. Additionally, the HCUP database is designed to capture costs for hospital-related care and does not capture costs for care provided in physician offices or outside laboratories. Similar to other large, nationwide databases, the HCUP database is also subject to data entry errors and incomplete reporting. Last, it is important to note that these are estimated costs, and the actual costs of managing grade ≥3 hematologic AEs may vary depending on circumstances.
Conclusion
For niraparib, the cost of managing severe (ie, grade ≥3) hematologic AEs in the US was reduced for the ISD as compared with the FSD. The cost reduction and improvements in safety associated with the niraparib ISD support its use in clinical practice.
Summary points
Based on the results from PRIMA/ENGOT-OV26/GOG-3012 trial (NCT02655016), the niraparib individualized starting dose (ISD) was approved for first-line maintenance therapy in the US. However, the economic impact of the niraparib ISD on the cost of hematologic adverse event (AE) management remains unexplored.
Grade ≥3 hematologic AEs that occurred in >1% of patients treated with niraparib in the PRIMA primary analysis (thrombocytopenia, anemia, platelet count decreased, neutropenia and neutrophil count decreased) were selected for inclusion in the cost analysis.
This US-based analysis used the 2017 Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project National Inpatient Sample database to obtain unit costs for each grade ≥3 AE, adjusted to 2020 US dollars.
AEs were assumed to occur independently of one another, so costs were added to derive the total cost.
For niraparib, the estimated AE management cost per patient in the US was lower for the ISD than for the fixed starting dose (FSD) for all hematologic AEs.
The total mean calculated cost per patient was $12,987.71 with the FSD and $6744.93 with the ISD.
Among the evaluated hematologic AEs, thrombocytopenia had the greatest cost reduction (59.1%).
Niraparib ISD reduced the total cost of hematologic AE management by 48.1% as compared with the FSD.
These findings highlight the cost reduction and safety improvements associated with the ISDs of niraparib.
Footnotes
Author contributions
Conceptualization, resources, data curation, writing – review and editing: WS Graybill, I Vergote, B Pothuri, M Anttila, DM O'Malley, D Lorusso, A Haggerty, M Fabbro, JK Chan, F Heitz, L Willmott, I Bruchim, Y Zhuo, P Estévez-García, BJ Monk, H Denys, A Knudsen, AV Tinker, L Manso Sánchez, D Provencher, MP Barretina-Ginesta, A González-Martín. Conceptualization, formal analysis, data curation, writing – review and editing: J Hartman. Conceptualization, writing – review and editing: DV Booth.
Financial disclosure
The PRIMA/ENGOT-OV26/GOG-3012 trial (NCT02655016) trial and this analysis (OneCDP 213359) were funded by GSK. GSK participated in the design and conduct of the study; the analysis and interpretation of the data; the preparation, review and approval of the manuscript; and the decision to submit the manuscript for publication. The authors have received no other financial and/or material support for this research or the creation of this work apart from that disclosed.
Competing interests disclosure
WS Graybill reports consulting and speaker fees from GSK. I Vergote reports institutional payments for corporate-sponsored research from Amgen and Roche and contracted research from Genmab and Oncoinvent AS; institutional consulting fee payments from Amgen (Europe) GmbH, AstraZeneca, Carrick Therapeutics, Clovis Oncology, Deciphera Pharmaceuticals, Elevar Therapeutics, F. Hoffmann–La Roche, Genmab, GSK, ImmunoGen, Mersana Therapeutics, Millennium Pharmaceuticals, MSD, Novocure, Octimet Oncology, Oncoinvent AS, Sotio, Verastem Oncology and Zentalis; consulting fees from Deciphera Pharmaceuticals, Jazz Pharmaceuticals and Oncoinvent AS; honoraria payment from Agenus, Aksebio, AstraZeneca, Bristol Myers Squibb, Deciphera Pharmaceuticals, Eisai, F. Hoffmann–La Roche, Genmab, GSK, ImmunoGen, Jazz Pharmaceuticals, Karyopharm Therapeutics, MSD, Novocure, Novartis, Oncoinvent AS, Seagen and Sotio; institutional travel support from Amgen, AstraZeneca, MSD, Roche and Tesaro; and advisory board fees from Agenus, AstraZeneca, Bristol Myers Squibb, Deciphera Pharmaceuticals (2021), Eisai, F. Hoffmann–La Roche, Genmab, GSK, ImmunoGen, MSD, Novocure, Novartis, Seagen (2021) and Sotio. B Pothuri reports institutional grant support from AstraZeneca, Celsion, Clovis Oncology, Duality Bio, Eisai, Genentech/Roche, ImmunoGen, Karyopharm Therapeutics, Merck, Mersana Therapeutics, Onconova, Seagen, Sutro Biopharma, Takeda Pharmaceuticals, Tesaro/GSK, Toray and VBL Therapeutics; consulting fees from AstraZeneca, BioNtech, Clovis Oncology, Eisai, GOG Foundation, Lily, Merck, Mersana Therapeutics, Onconova, Seagen, Sutro Biopharma, Tesaro/GSK and Toray; and travel support from GSK and BioNtech. M Anttila has nothing to disclose. DM O'Malley reports personal fees from Ambry, Arquer Diagnostics, Celsion Corp, Corcept Therapeutics, Elevar, INXMED, MSA, Myriad Genetics, Novartis, Roche Diagnostics, Rubis, Sorrento, Takeda, Tarveda and Toray; personal fees and funding for clinical research from AbbVie, Agenus, Amgen, AstraZeneca, Clovis Oncology, Eisai, Genentech/Roche, GOG Foundation, ImmunoGen, Iovance, Janssen/J&J, Merck, Mersana Therapeutics, Novocure, Regeneron, SDP Oncology (BBI), Seagen and Tesaro/GSK; and funding for clinical research from Ajinomoto, Array Biopharma, Bristol Myers Squibb, Cerulean Pharma, EMD Serono, Ergomed, Genmab, INC Research, inVentiv Health Clinical, Ludwig Cancer Research, New Mexico Cancer Care Alliance, PRA Intl, Serono, Stemcentrx, TRACON Pharmaceuticals, VentiRx and Yale University. D Lorusso reports consulting fees from Amgen, AstraZeneca, Clovis Oncology, Genmab, GSK, ImmunoGen, MSD, PharmaMar and Seagen; honoraria from AstraZeneca, Clovis Oncology, GSK, MSD and PharmaMar; payment for expert testimony from Clovis Oncology; support for attending meetings and/or travel from AstraZeneca, Clovis Oncology, GSK, PharmaMar and Roche; personal advisory board fees from AstraZeneca, Clovis Oncology, Corcept, Genmab, GSK, ImmunoGen, Merck Serono, MSD, Oncoinvent, PharmaMar, Seagen and Sutro; membership on board of directors for GCIG (no compensation); medical writing support from Clovis Oncology, GSK, MSD and PharmaMar; and institutional funding for work in clinical trials from AstraZeneca, Clovis Oncology, Genmab, GSK, ImmunoGen, Incyte, MSD, Novartis, PharmaMar, Roche and Seagen. A Haggerty reports research/grant funding from Clovis Oncology and GSK and honoraria from AstraZeneca. M Fabbro has nothing to disclose. JK Chan reports research, consulting and speakers' bureau fees from AbbVie, Acerta, Aravive, AstraZeneca, Clovis Oncology, Eisai, GSK, Merck and Roche. F Heitz reports honoraria and consulting fees from AstraZeneca, GSK, Roche and Tesaro. L Willmott reports speakers' bureau fees from AstraZeneca, Eisai, ImmunoGen, Merck and Seagen and advisory board fees from AstraZeneca, ImmunoGen and Seagen. I Bruchim has no disclosures. Y Zhuo has no disclosures. P Estévez-García reports consulting or advisory role fees from AstraZeneca, Clovis Oncology, GSK, MSD, PharmaMar and Roche and travel support from AstraZeneca, GSK, MSD and PharmaMar. BJ Monk reports consulting fees from Agenus, Akeso Biopharma, Amgen, Aravive, Bayer, Elevar, EMD Merck, Genmab/Seagen, GOG Foundation, Gradalis, ImmunoGen, Iovance, Karyopharm Therapeutics, MacroGenics, Mersana Therapeutics, Myriad, Novartis, Novocure, Pfizer, Puma, Regeneron, Sorrento, US Oncology Research and VBL Therapeutics and speakers' bureau honoraria from AstraZeneca, Clovis Oncology, Eisai, Merck, Roche/Genentech and Tesaro/GSK. H Denys reports an institutional research grant from Gilead; consulting fees from Gilead and GSK; honoraria from Amgen, AstraZeneca, Daiichi Sankyo, Eli Lilly, Gilead, GSK, Leo Pharma, MSD and Roche; travel support from AstraZeneca, Gilead, GSK, MSD, Pfizer, PharmaMar, Roche and Teva; and participation on advisory boards for AstraZeneca, Eli Lilly, Gilead, GSK, Menarini Group, MSD and Pfizer. A Knudsen has no disclosures. AV Tinker reports honoraria from AstraZeneca, Eisai, GSK and Merck and travel support from GSK. L Manso Sánchez has nothing to disclose. D Provencher reports honoraria and educational grants from AstraZeneca, Eisai, GSK and Merck. MP Barretina-Ginesta reports consulting or advisory role fees from AstraZeneca, Clovis Oncology, GSK, MSD, PharmaMar and Roche and travel support from AstraZeneca, GSK, MSD, PharmaMar and Roche. J Hartman and DV Booth are employed by the study sponsor (GSK). A González-Martín reports consulting fees from Alkermes, Amgen, AstraZeneca, Clovis Oncology, Daichii Sankyo, Genmab, GSK, Hedera DX, Illumina, ImmunoGen, Incyte, Kartos Therapeutics, Karyopharm Therapeutics, Mersana Therapeutics, MSD, Novartis, Novocure, Oncoinvent, Pharma&, PharmaMar, Roche, Seagen SOTIO, Sutro, Takeda and Tubulis; grants or contracts from Asociación Española Contra el Cáncer, GSK, Instituto de Salud Carlos III and Roche; payment or honoraria for lectures, presentations, or speakers' bureau from AstraZeneca, Clovis Oncology, GSK, ImmunoGen, Mersana Therapeutics, MSD, Novocure, PharmaMar, Roche, Takeda, Seagen and Zai Labs; and travel support from AstraZeneca, Clovis Oncology, GSK, ImmunoGen, Mersana Therapeutics, MSD, Novocure, PharmaMar, Roche and Takeda.
Writing disclosure
Medical writing and editorial support were coordinated by S Cuozzo, CMPP, of GSK and provided by BC Taylor, PhD, CMPP, and MC Wiggin of Ashfield MedComms, an Inizio company.
Ethical conduct of research
No direct patient contact was made, no individual patient-level data were collected and all results were presented solely as aggregate analyses. Therefore, no ethics committee or institutional review board approval was required.
Data sharing statement
Please refer to GSK weblink to access GSK's data sharing policies and as applicable seek anonymized subject-level data via the link https://www.gsk-studyregister.com/en/.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J. Clin. 74(1), 12–49 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Tew WP, Lacchetti C, Kohn EC. PARP Inhibitors in the Management of Ovarian Cancer Guideline Expert Panel. Poly(ADP-ribose) polymerase inhibitors in the management of ovarian cancer: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 40(33), 3878–3881 (2022). [DOI] [PubMed] [Google Scholar]
- 4.González-Martín A, Harter P, Leary A et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 34(10), 833–848 (2023). [DOI] [PubMed] [Google Scholar]
- 5.González-Martín A, Pothuri B, Vergote I et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 381(25), 2391–2402 (2019). [DOI] [PubMed] [Google Scholar]; • Primary study results that demonstrated first-line maintenance treatment with niraparib improved progression-free survival in patients with newly diagnosed advanced epithelial ovarian cancer that responded to first-line platinum-based chemotherapy. The trial protocol was amended to introduce an individualized starting dose, in which patients with baseline body weight <77 kg and baseline platelet count <150,000/μl received 200 mg once daily and patients with baseline body weight ≥77 kg or baseline platelet count ≥150,000/μl received 300 mg once daily.
- 6.González-Martín A, Pothuri B, Vergote I et al. Progression-free survival and safety at 3.5 years of follow-up: results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer. Eur. J. Cancer 189, 112908 (2023). [DOI] [PubMed] [Google Scholar]; • Study results showed that niraparib maintained clinically significant improvements in progression-free survival with 3.5 years of follow-up in patients with newly diagnosed advanced ovarian cancer at high risk of progression irrespective of homologous recombination deficiency status.
- 7.Li N, Zhu J, Yin R et al. Treatment with niraparib maintenance therapy in patients with newly diagnosed advanced ovarian cancer: a phase 3 randomized clinical trial. JAMA Oncol. 9(9), 1230–1237 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagkali A, Mamais I, Michalinos A, Agouridis AP. Safety profile of niraparib as maintenance therapy for ovarian cancer: a systematic review and meta-analysis. Curr. Oncol. 29(1), 321–336 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Results of this systematic review and meta-analysis showed that the niraparib safety profile is consistent with that of other poly(ADP-ribose) polymerase inhibitors, including the fact that hematologic adverse events may occur.
- 9.Cheng H, Yang J, Liu H, Xiang Y. Poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors as maintenance therapy in women with newly diagnosed ovarian cancer: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 304(2), 285–296 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monk BJ, González-Martín A, Buckley L et al. Safety and management of niraparib monotherapy in ovarian cancer clinical trials. Int. J. Gynecol. Cancer. 33(6), 971–981 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirza MR, Monk BJ, Herrstedt J et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 375(22), 2154–2164 (2016). [DOI] [PubMed] [Google Scholar]; • The results of this study of niraparib maintenance therapy in patients with platinum-sensitive recurrent epithelial ovarian cancer showed that a fixed starting dose of 300 mg of niraparib once daily was associated with grade ≥3 treatment-emergent adverse events of thrombocytopenia, anemia and neutropenia.
- 12.Berek JS, Matulonis UA, Peen U et al. Safety and dose modification for patients receiving niraparib. Ann. Oncol. 29(8),1784–1792 (2018). [DOI] [PubMed] [Google Scholar]; • This study's results showed that low baseline body weight (<77 kg) and platelet count (<150,000/μl) were associated with an increased incidence of grade ≥3 thrombocytopenia events, indicating that these patients may benefit from a lower starting dose of niraparib.
- 13.Mirza MR, González-Martín A, Graybill WS et al. Prospective evaluation of the tolerability and efficacy of niraparib dosing based on baseline body weight and platelet count: results from the PRIMA/ENGOT-OV26/GOG-3012 trial. Cancer 129(12),1846–1855 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Zejula [prescribing information]. GSK; (2023) (Accessed 10 May 2024). https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/214876s000lbl.pdf [Google Scholar]
- 15.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUPnet). (Accessed 25 July 2023). https://datatools.ahrq.gov/hcupnet/
- 16.Healthcare Cost and Utilization Project. HCUP Fact Sheet 2022. (2022). https://hcup-us.ahrq.gov/news/exhibit_booth/HCUPFactSheet.pdf
- 17.Agency for Healthcare Research and Quality. HCUP Overview. Healthcare Cost and Utilization Project (HCUP). (2022) (Accessed 7 May 2024). https://hcup-us.ahrq.gov/overview.jsp [PubMed]; • Describes the 2017 Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project National Inpatient Sample database used to obtain the unit costs for each grade ≥3 adverse event.
- 18.Friedlander M, Lee C, Tew W. Managing adverse effects associated with poly(ADP-ribose) polymerase inhibitors in ovarian cancer: a synthesis of clinical trial and real-world data. Am. Soc. Clin. Oncol. Educ. Book. 43, e390876 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Vulsteke C, Chambers SK, Jesús Rubio Pérez M et al. Tolerability of the niraparib individualized starting dose in the PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib first-line maintenance therapy. Eur. J. Cancer 208, 114157 (2024). [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence. Niraparib for maintenance treatment of advanced ovarian, fallopian tube and peritoneal cancer after response to first-line platinum-based chemotherapy. (2021) (Accessed 10 May 2024). https://www.nice.org.uk/guidance/ta673/documents/final-appraisal-determination-document
- 21.Zejula [summary of product characteristics]. (2023) (Accessed 11February 2024). https://www.ema.europa.eu/en/documents/product-information/zejula-epar-product-information_en.pdf


