Abstract
This research explores the development of engineered oil–water microemulsions stabilized by a synergistic combination of polymer and surfactant to enhance stability and interfacial properties for improved enhanced oil recovery (EOR). Conventional surfactant-stabilized emulsions often suffer from phase instability and limited wettability alteration during water flooding and chemical injection, hindering the EOR efficiency. In contrast, our formulations incorporating polymers significantly increase the emulsion viscosity and resilience to temperature fluctuations, resulting in enhanced phase stability. Experimental investigations reveal that while the water-microemulsion interfacial tension (IFT) increases with salinity, the oil-microemulsion IFT decreases substantially, achieving an optimal IFT of 4.43 × 10–4 mN/m at balanced salinity levels. The microemulsions exhibit remarkable stability across varying temperatures, successfully transitioning between Winsor type II and III phases, which is critical for effective EOR applications. Notably, the addition of polymers enhances the viscosity of the surfactant-stabilized emulsion from 50 mPa·s at a shear rate of 10 s–1 to 300 mPa·s, significantly improving emulsion stability, as confirmed by measured zeta potential values of −31.1 mV for the surfactant system and −33.2 mV for the polymer-augmented surfactant system. These enhancements contribute to improved sweep efficiency during the oil recovery processes. Furthermore, the microemulsions effectively alter the sandstone wettability from oil-wet to water-wet, promoting better oil displacement. Core flooding experiments demonstrate that injecting one pore volume of the polymer-augmented surfactant-stabilized microemulsion results in an additional 20.58% oil recovery compared with conventional water flooding.
1. Introduction
Petroleum production engineering aims to economically optimize the production of oil and gas. Increasing new concepts and methods for extracting crude oil from well-established reservoirs are being drawn in by the combination of growing oil consumption and depleting supplies. Even after the completion of the natural drive and water-flooding process, about two-thirds of the original oil in place (OOIP) remains trapped in the reservoir.1 The industry is utilizing cutting-edge technologies in place of conventional techniques to enhance oil recovery due to the global shortage of crude oil and its increasing demand. Between 30 and 50% of the OOIP can be recovered by standard primary and secondary methods, which include water flooding, artificial lift, natural flow, and pressure maintenance.2 The chemical enhanced oil recovery (EOR) process employs surfactants to reduce interfacial tension (IFT), polymers to increase water viscosity for improved sweep efficiency, and alkalis to create in situ surfactants and enhance the effectiveness of other chemicals, either individually or in combination, to boost oil extraction efficiency.1 An increase in capillary number leads to a significant improvement in oil recovery by increasing the viscous force and decreasing the interfacial force.3 Though the use of polymer increases the viscous force, the use of surfactant reduces the IFT manyfold, leading to a significant increase in capillary number and subsequent improvement in oil recovery.4−6
Surfactants, molecules with both hydrophilic and hydrophobic groups, play a crucial role in EOR by altering the wettability of reservoir rocks and reducing IFT between oil and water.7,8 Their hydrophilicity is determined by the head and tail structures, such as hydrocarbon chain length and functional groups. Classified by the charge of the hydrophilic group, they include anionic, cationic, nonionic, and zwitterionic.9 These surfactants enhance oil recovery by improving the displacement of oil from the reservoir.10
Surfactant solutions are commonly used in EOR to mitigate IFTs between oil and water, but their lower viscosity relative to oil limits their effectiveness.
Emulsification is an important mechanism of EOR. Analysis of produced water and crude oil, even during water flooding, reveals in situ emulsification by natural surfactants and migrated fine particles within the reservoir.11−13 However, these natural emulsions are often unstable and unpredictable, leading to an inconsistent recovery. Injecting engineered microemulsions, designed for stability and tailored interfacial properties, can address this challenge by offering predictable emulsification effects that improve sweep efficiency and displacement.14,15 Surfactant flooding in EOR is significantly improved by finely tuned microemulsion formulations, which achieve ultralow IFT compared to simple surfactant systems.16−18
Microemulsions, being thermodynamically stable mixtures of oil, water, and surfactants, can be tailored to adapt to varying reservoir conditions such as temperature, pressure, and salinity.18−20 Their unique phase behavior enhances solubilization and displacement efficiency, creating continuous oil pathways within the reservoir. This fine-tuning and adaptability of microemulsions make them highly effective in capturing and mobilizing trapped oil, thereby significantly enhancing the overall oil recovery. Microemulsion flooding presents a promising alternative due to its higher viscosity and ability to maintain a low IFT, thereby improving oil displacement efficiency. However, achieving satisfactory results with low-concentration surfactant flooding is impeded by potential adsorption onto reservoir rocks. A more effective strategy involves employing surfactants at higher concentrations, where understanding the phase behavior of microemulsion systems becomes crucial. The hydrophilic–lipophilic balance (HLB) values of surfactants dictate their effectiveness as emulsifiers for either oil-in-water (o/w) or water-in-oil (w/o) emulsions. The values of HLB of surfactants in the range of 8–18 indicate their tendency to form an o/w emulsion. Salinity significantly influences microemulsion type and phase volumes, with low salinity favoring water-external phases and higher salinity promoting oil-external microemulsions.21,22 Further, to improve the stability of the microemulsion under harsh conditions, polymers or polymeric materials may be used to maintain stability and improvement of viscoelastic properties microemulsions, being thermodynamically stable mixtures with smaller droplet sizes, prevent phase separation and, when combined with temperature-resistant polymers, improve the solution’s viscoelasticity, ensuring better mobility control and sweep efficiency in the reservoir. Polymers contribute to lowering the IFT between oil and water phases. Some studies highlighted that the presence of branched hydrocarbon nonionic surfactants in combination with polymers can lead to ultralow IFT values, facilitating the migration of oil droplets toward the microemulsion interface. This reduction in IFT is essential for maximizing oil recovery as it promotes better displacement efficiency.23−25 Schabes et al.26 in their study reported that the presence of PHPA increases droplet diameter from approximately 115 nm (surfactant) to 196 nm (surfactant + polymer) due to its ability to adsorb at the oil–water interface and create a thicker interfacial layer. This results in larger droplets that are less prone to coalescence, thus enhancing the stability of the microemulsion. Polymers can also enhance electrostatic stabilization within the microemulsions. De Gennes27 discussed how charged polymers increase the zeta potential of microemulsions, leading to stronger electrostatic repulsion between droplets. This repulsion reduces the extent of aggregation and phase separation, contributing to long-term stability. For instance, zeta potential measurements indicated an increase in its value from −31.1 mV for surfactant-only systems to −33.2 mV when PHPA was added, demonstrating improved stability through enhanced electrostatic forces. Polymers, often hydrophilic, adsorb to form viscoelastic layers that enhance microemulsion stability by preventing droplet coalescence and increasing viscosity, which improves mobility control.28,29 Combined surfactant–polymer interactions exhibit synergistic effects, resulting in improved stability, reduced IFT, and effective wettability alteration.30 Factors such as salinity, ionic strength, temperature, and molecular structure of surfactants and polymers impact adsorption dynamics and interfacial properties. High salinity, for example, may enhance ionic surfactant adsorption while reducing nonionic surfactant effectiveness. Temperature changes influence the polymer viscosity and adsorption. The resulting modifications to surface tension, zeta potential, and interfacial viscosity enhance oil displacement by altering capillary forces and stabilizing the microemulsion.31
Despite the known benefits of emulsification in EOR by reducing IFT and improving sweep efficiency, challenges persist in achieving emulsion formulations that remain stable and effective under varied reservoir temperature and salinity conditions.32,33 Conventional surfactant-stabilized emulsions often exhibit phase instability, low viscosity, and limited wettability alteration, restricting their capacity for effective oil mobilization and displacement.34,35 Polymer-augmented surfactant-stabilized microemulsions offer enhanced viscosity, increasing sweep efficiency and mobility control compared to surfactant-only formulations.36,37 They exhibit greater phase stability under varying reservoir conditions, withstand temperature fluctuations, and demonstrate effective wettability alteration, shifting surfaces from oil-wet to water-wet. Polymers also synergize with surfactants, enhancing efficiency and reducing the amount needed for stabilization. Additionally, some polymers form networks that trap droplets, further improving the stability. These actions collectively contribute to a more stable microemulsion system. Unfortunately, only a few works have reported on the role of polymers in enhancing the stability, viscosity, and interfacial strength of surfactant-stabilized emulsions, particularly in applications such as EOR.38,39
The interactions of surfactants and polymers at the oil–water interface in microemulsions are critical for optimizing their stability and performance, particularly in EOR applications. In terms of phase behavior modification, the addition of polymers significantly alters the phase behavior of oil–water–surfactant systems. Polymer addition can stabilize Winsor type III microemulsions, which are characterized by the presence of a middle phase that is thermodynamically stable over time. This stability is crucial for EOR as it allows for the efficient solubilization of oil within the microemulsion phase, enhancing recovery rates.40−42
This study addresses these limitations by developing oil–water microemulsions stabilized through a synergistic combination of polymer and surfactant. The innovation lies in the addition of polymer, which significantly enhances emulsion viscosity and stability, creating a formulation that is resilient to temperature fluctuations and capable of transitioning between Winsor type II and III phases. Detailed investigations were conducted into their phase behavior, stability, and microstructure to evaluate their stability and potential application in EOR. The primary goal is to enhance the volumetric sweep efficiency and microscopic displacement efficiency to improve oil recovery. A core flooding experiment using the formulated microemulsion was performed to determine the percentage recovery of the OOIP.
2. Methodology
2.1. Materials
TX-100, as a nonionic surfactant, was purchased from Sisco Research Laboratories, India. It contains a hydrophilic chain of 9–10 ethylene oxide units and an aromatic ring. It has a HLB value of 13.4. The HLB value of any surfactant determines its degree of hydrophilicity or lipophilicity.43 It is highly effective for EOR due to its ability to reduce IFT and alter rock wettability from oil-wet to water-wet, improving oil mobilization and displacement efficiency. Additionally, it is environmentally friendly, cost-effective, thermally stable, and compatible with various salinity levels and other chemicals, making it a versatile choice for EOR applications. TCI Chemicals supplied additional chemicals, including NaCl, methanol, diethyl ether, KOH, and NaOH. PUSHER 1000, a partially hydrolyzed polyacrylamide (PHPA) polymer with an average molecular weight of 2 × 107 g/mol, was obtained from SNF, India, for stabilizing microemulsions alongside a surfactant. It offers significant advantages in EOR by increasing water viscosity, improving mobility control, and enhancing oil recovery rates. It reduces water permeability in reservoirs, leading to more effective oil displacement, and demonstrates temperature and salinity tolerance for various reservoir conditions. Crude oil with a gravity of 33.5°API was obtained from the Cambay basin in India for wettability alteration and core flooding studies.
2.2. Core Mineralogy
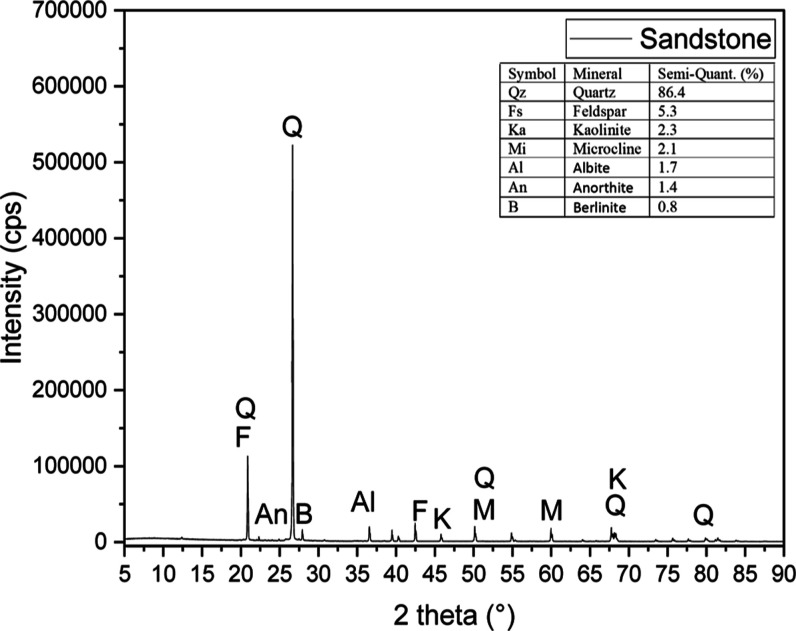
Sandstone Berea core was used throughout the study, and the mineralogical composition was analyzed by XRD analysis, as shown in Figure 1. The XRD analysis indicates that the quartz is the major component of the rock, with peaks represented at 21.01, 26.78, 36.69, 50.28, 68.45, and 80.0°. The peaks at 50 and 60° represent Microcline components in the rock. Feldspar has a peak at 43°. The peaks at 25, 26.89, 37.56, and 67.78° represent anorthite, berlinite, albite, and kaolinite, respectively. The compositional analysis of different clay minerals is shown in the figure.
Figure 1.
XRD analysis of sandstone rock.
2.3. Surface Tension Measurements
The surface tension of TX-100 surfactant solutions was measured using a Kruss tensiometer (K20 Easy Dyne), following the Du Nouy ring method. The platinum ring was carefully washed with acetone and dried over a flame after each measurement to remove impurities and ensure reliable readings. The surface tension is calculated using eq 1
| 1 |
where θ is the contact angle, F is the maximum force, and L is the wetted length of the ring, which is the total of the inner and outer circumferences. In the presence of a surfactant, molecules align at the interface with hydrophilic heads in the water and hydrophobic tails in the air, reducing the surface tension. As the concentration increases, surfactant monomers at the interface saturate, forming micelles in the bulk phase. The critical micelle concentration (CMC) marks the point at which the surfactant exhibits optimal surface activity.
2.4. Surfactant and Polymeric Surfactant Microemulsion Formulations
The surfactant stabilized microemulsion was prepared by using a 1:1 volume ratio of surfactant solution of different concentrations and synthetic oil. The mixture was shaken continuously by hand after regular intervals of time as a low-energy method of formulation. The characterization of this surfactant microemulsion was then extensively studied through phase diagrams, solubilization parameters and relative phase volume, hydrodynamic diameter, stability, and optimum salt concentration. For the preparation of polymeric surfactant microemulsion, the first desired amount of polymer (Pusher-1000) was added to the surfactant solution of specific concentration and mixed with constant low-speed stirring for 24 h using a magnetic stirrer. The polymeric surfactant solution was then mixed with synthetic oil in a 1:1 volume ratio to formulate the desired polymeric surfactant microemulsion. The system was characterized by droplet size, stability, wettability alteration, adsorption studies, and a subsequent core flooding experiment for EOR.
2.5. Dynamic Light Scattering Studies
The Anton Paar Litesizer 500, used for dynamic light scattering (DLS) at 303 K, provides the hydrodynamic diameter of microemulsion droplets, which is essential for characterizing surfactant- and polymeric surfactant-based formulations. DLS works by analyzing Brownian motion, where smaller particles move faster than larger ones, causing fluctuations in the scattered light intensity. By studying these fluctuations, we can determine the particle size distribution. This technique is crucial for understanding droplet stability and performance in applications like EOR and industrial formulations.44 The average hydrodynamic particle size, which is provided in the equation, is obtained by solving the Stokes–Einstein equation in order to describe this impact
| 2 |
where Df is the diffusion coefficient, KB is the Boltzmann constant (1.38 9 10–23 N m/K), T is the temperature (K), η is the viscosity (Pa·s), and RH is the solute radius. In order to improve the repeatability of the results, each DLS experiment was performed three times in a repetition series.
2.6. Surfactants Surface Adsorption and Thermodynamic Properties Studies
Surfactants are known to form micelles, and it is important to comprehend the process underlying this thermodynamic change.45 The parameters of adsorption include the maximum excess surface concentration (τmax) and minimum area per molecule (Amin). The Gibbs adsorption isotherm equation was used to obtain these parameters. As per Gibb’s law, the adsorption of surfactant at the interface aids in the reduction of surface tension by many folds. The above surface–active parameters at this interface can be calculated through the CMC values obtained from the plot of surface tension v/s surfactant concentration.46
Gibbs free energy of micellization gives an idea of the potential of micellization, taking into consideration the CMC values of surfactants. The Gibbs free energy change of micellization can be calculated by the equation below
| 3 |
where ΔGmic is the molar Gibbs energy of micellization represented in kJ/mol, XCMC is the CMC (CMC/55.4) in molar fraction units, and β is the average degree of counterion binding in the micelle and is determined by the equation as
| 4 |
where α is the degree of ionization, which is taken to be 0 for nonionic surfactants. The Gibbs free energy change due to adsorption can be calculated by the equation
| 5 |
2.7. Ternary Phase Diagram Study
It is crucial to develop ternary phase diagrams in order to identify systems that provide the concentration range where microemulsion production occurs. From an economic perspective, it is crucial to prepare microemulsions with low surfactant concentrations. The phase diagram was determined by using a traditional titration technique. A two-phase combination of the surfactant and oil was titrated against brine in order to determine how much brine was needed to create a single-phase mixture. Every titration’s end point is considered to be the first acceptable permanent cloudiness, signifying the emergence of a second phase in the combination. The temperature used for this experiment was 298.15 K. To determine the critical concentration for phase transition, surfactant solutions were prepared by mixing specific ratios of surfactant and deionized water in a constant volume of 10 mL. Starting with 1 mL of surfactant and 9 mL of deionized water, the concentration was incrementally increased (2:8, 3:7, and 4:6). Each solution was mixed with a magnetic stirrer until turbidity or color change was observed. The first instance of turbidity marked the phase transition point, indicating micelle formation and alteration of the solution properties. The details are reported in our earlier studies.47
2.8. Solubilization Parameter and Relative Phase Volume Calculations
A surfactant system’s capacity to solubilize oil and brine by microemulsion is a key characteristic to consider.48 The amounts of oil and water solubilized by a unit of surfactant in terms of solubilization parameters (V0/Vs and Vw/Vs), where V0, Vw, and Vs represent the volumes of oil, water, and surfactant in the microemulsion phase. The solubilization parameters were calculated under the assumption that the surfactant was completely contained in the microemulsion. To finish the solubilization parameters, the following steps are taken: a combination of solutions of NaCl with weights ranging from 0 to 3% of NaCl and 600 ppm of surfactant was made. To obtain the proper volume of surfactant solution, the two-phase mixture of surfactant solution was agitated for 60 min at 50 rpm in a Rotospin rotary mixer after brine and synthetic oil were added in a 1:1 (v/v) ratio. After that, each tube was removed and let to settle for a day in a specially designed rack that could hold several tubes in order to achieve the equilibrium state.
2.9. Zeta Potential Studies
The potential at the sliding plane within the diffused layers is known as the zeta potential or electrokinetic potential, and is denoted by the symbol ζ. Comprehending zeta potential is crucial for understanding an emulsion’s short- and long-term stability. Higher zeta potential levels, on the other hand, signify strong repulsive forces between charged particles, which will stop aggregation and hence increase the stability. Lower values of zeta potential will cause coagulation, agglomeration, and settling of particles in a very rapid manner. The Litesizer 500 (Anton Paar) was utilized to determine the zeta potential of an oil droplet surface in a prepared microemulsion at 303 K. In order to perform potentiometric measurements in reaction to an electric field, the produced microemulsion was loaded into an omega cuvette and placed in a Litesizer. For the results to be repeatable, every test was run three times.
2.10. Surfactant Adsorption Study
Prior to injecting the surfactant slug into the formation for increased oil recovery, a thorough laboratory (experimental) examination of the surfactant’s adsorption onto the reservoir rock surface must be conducted. This study examines the surfactant’s static adsorption tendency on the rock surface using the dry sand approach. In order to conduct the experiment, a combination of 3 g of sand and 30 mL of surfactant solution was prepared at various concentrations with a solid to liquid ratio of 1 to 1049. The sand used in this study is rich in quartz with minor other clay minerals. The sand, which serves as the adsorption medium, was thoroughly cleaned with acid washing to get rid of any leftovers. The cleaned sand was then dried for 24 h at 368.15 K. Sand particles were sieved to 60–80 mesh for the study. The sand, which serves as the adsorption medium, must first be cleaned by acid washing to get rid of any leftovers. The cleaned sand must then be dried for 24 h at 368.15 K. The sample mixture of different surfactant concentrations of TX-100 viz. (200, 400, 600, 800, and 1000 ppm) is then kept in roto spin for 24 h to make it homogeneous, followed by centrifugation at 4000 rpm for 30 min after adsorption. Lastly, UV–vis spectroscopy is employed to calculate the adsorption of surfactants. The amount of surfactant adsorbed q (mg/g) on the sand surface is then calculated by using eq 6
| 6 |
where Ci and Ce are the values of surfactant concentration (mg/L) in solution before and after balanced with sand particles; Vsol is the total volume of solution (mL); and Msand is the mass of dry sand (gm).
2.11. Rheological Measurements
The modular compact rheometer series, MCR-102, Anton Paar rheometer, was used for rheological studies of formulated microemulsions at two different temperatures (30° and 70 °C). The cub and bob assemblies served as the hardware setup for measuring viscosity. To analyze the microemulsion’s rheological behavior at various desired temperatures, the shear rate was adjusted from 0.01 to 1000 s–1. The rheometer’s measuring cylinder was filled with the microemulsion sample, and after that, the bob was positioned at the zero-gap position and adjusted to the test temperature. The viscosity values were determined by the drag produced by the fluid movement caused by the bob’s rotation on the cylindrical surface. The rheological parameters were then compared for the surfactant to polymeric surfactant microemulsion sample.
2.12. Wettability Studies
Whether they are carbonate or sandstone, reservoir rocks are naturally damp with water. As crude oil builds up in the reservoir, polar components of the oil (asphaltene, resin, etc.) adsorb onto the rocks, changing their wettability and creating circumstances that are either oil-wet or mixed-wet.50 Relative permeability, capillary pressure, and oil recovery are all impacted by the wettability. The main function of surfactants is to reduce IFT, but they also help to convert oil-wet to water-wet wettability.51 Because the contact angle is dynamic, it gradually decreases until reaching equilibrium and the changes become less pronounced. The ability of microemulsion solutions to change the wettability of oil-wet sandstone rock was investigated through contact angle studies. The sessile drop method using a Kruss DSA25 drop shape analyzer measured the contact angle on the sandstone samples. Small slices (30 × 30 × 5 mm) were cut, polished, and aged in crude oil for 45 days to simulate the reservoir conditions. Following aging, the samples were cleaned with n-heptane and dried overnight. For the contact angle test, a microemulsion drop was dispensed through a 0.5 mm needle onto the rock surface at 30 °C, and the dynamic contact angle was recorded. For consistency, each measurement was performed on a fresh, unaltered rock sample. Multiple repetitions ensured reliable, reproducible results. The ability of microemulsion solutions to change the wettability of oil-wet sandstone rock was investigated through contact angle studies. The oil is compelled to remain trapped inside the porous medium and adhere to the pore walls due to the preference for the reservoir rock. This affinity hinders residual oil recovery by encouraging saturation of the oil.1 Therefore, contact angle measurements were required to be made on the oil-saturated rocks that were treated with the microemulsion systems developed in this study in order to alter the reservoir rock affinity.
Wettability alteration is a critical process in EOR that aims to modify the wettability of reservoir rocks, particularly from oil-wet to water-wet conditions, thereby improving oil production efficiency. This alteration is primarily achieved through the application of surfactants, which reduce the IFT between oil and water and facilitate the displacement of trapped oil within the rock pores. Nonionic surfactants have shown significant promise in laboratory studies by effectively altering the wetting state of carbonate and sandstone reservoirs. For instance, research indicates that surfactant flooding can result in a substantial decrease in contact angles, transitioning from oil-wet conditions (contact angle >90°) to water-wet states (contact angle <30°).52,53 This transformation enhances the capillary pressure and relative permeability, enabling more efficient oil recovery through mechanisms such as spontaneous and forced imbibition. Moreover, field applications of smart water flooding, where modified brine with specific ionic compositions is injected, have demonstrated improved recovery rates by leveraging wettability changes induced by ionic interactions with rock surfaces.54 Ultimately, understanding wettability alteration is essential for optimizing EOR strategies and maximizing hydrocarbon extraction from complex reservoir systems.
2.13. Core Flooding Test
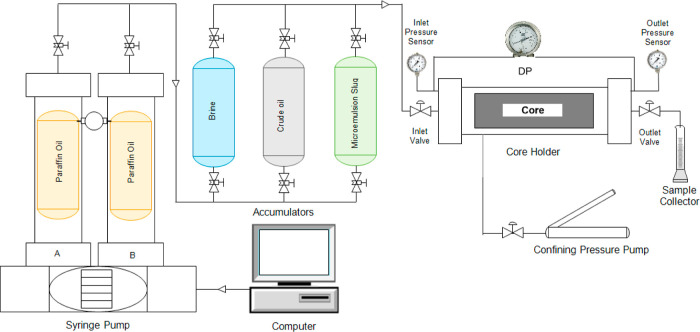
Core flood studies were conducted on a laboratory scale to examine the suitability of different chemical slugs for chemical EOR. The schematic of the core flooding apparatus is shown in Figure 2. These experiments replicate the real-world circumstances of the reservoir. The selected core was first cleaned using the Soxhlet device, and then it was dried. After a week of being saturated with 1 wt % brine, it was weighed to ascertain its porosity. The initial water flood was carried out at 343 K with variable flow rates, and the differential pressure was recorded in order to calculate the permeability of the core. The absolute permeability was calculated from the following equation using the slope approach of the Darcy equation
| 7 |
where q is the volumetric
flow rate (cm3/s), k is the permeability
in Darcy, μ is the fluid viscosity (cP), A is
the cross-sectional area of the sand pack (cm2), and 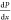 is the pressure gradient (atm/cm). Crude
oil was pumped into the core at a steady flow rate of 0.3 mL/min in
order to displace water and achieve an irreducible water saturation.
Irreducible water saturation and initial oil saturation were calculated
by using the volume balance approach. A traditional water flood was
performed on the core that had the initial oil saturation, and the
effluent was collected in a graduated cylinder. After 95% water reduction
was achieved, the water injection was stopped after four pore volumes
(PVs). After that, a single PV of the formulated microemulsion slug
was inserted and allowed to interact with the remaining oil in the
core. Subsequently, chase water was added at the same flow rate of
0.3 mL/min to maintain the pressure. The final tertiary oil recovery
was calculated by using the entire amount of recovered oil, which
was gathered in a graduated cylinder.
is the pressure gradient (atm/cm). Crude
oil was pumped into the core at a steady flow rate of 0.3 mL/min in
order to displace water and achieve an irreducible water saturation.
Irreducible water saturation and initial oil saturation were calculated
by using the volume balance approach. A traditional water flood was
performed on the core that had the initial oil saturation, and the
effluent was collected in a graduated cylinder. After 95% water reduction
was achieved, the water injection was stopped after four pore volumes
(PVs). After that, a single PV of the formulated microemulsion slug
was inserted and allowed to interact with the remaining oil in the
core. Subsequently, chase water was added at the same flow rate of
0.3 mL/min to maintain the pressure. The final tertiary oil recovery
was calculated by using the entire amount of recovered oil, which
was gathered in a graduated cylinder.
Figure 2.
Schematic of oil displacement experimental setup.
3. Results and Discussion
3.1. Critical Micelle Concentration of the TX-100 Surfactant
Interfacial properties of surfactants play a very important role in the emulsification of oil, which leads to improvement in oil recovery efficiency.55 The CMC of a surfactant is one of the most important features of the surfactant. Surfactants function well and have the best absorption at the CMC point. The variation of surface tension of the surfactant solution with its concentration and the change of CMC of surfactant at different temperatures are depicted in Figure 3. It was found that surface tension reduces as the temperature rises. A decline in the surface tension is observed with an increase in temperature. This is due to an increase in molecular velocity because, at high temperatures, thermal activity combines and diminishes cohesive forces, making surfactant molecules more sticky to the surrounding air.56 Because of the surfactant molecule’s increased adsorption at the air–water interface, surface tension values fall as surfactant solution concentration rises. Following the CMC, the surface tension curve stabilizes. From Figure 3, it is also observed that the surface tension and CMC decrease with an increase in temperature due to heightened molecular kinetic energy, which reduces intermolecular attraction, thereby requiring less surfactant for micelle formation.
Figure 3.
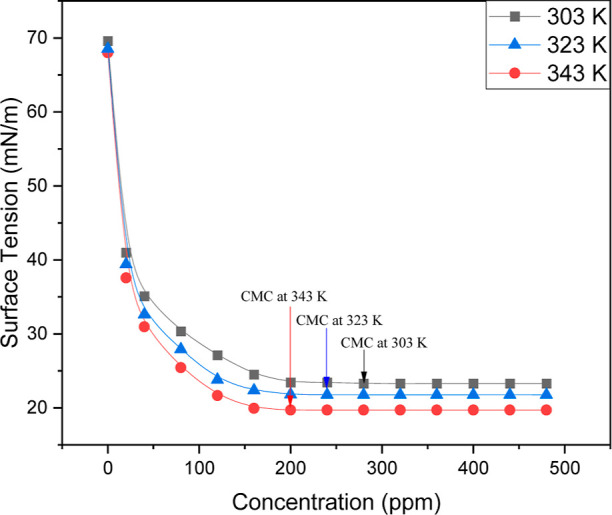
Variation of surface tension as a function of concentration and temperature.
3.2. Thermodynamic Behavior of Adsorption and Micellization
Temperature has a significant impact on the micellization and surface tension characteristics of the surfactants. The processes of micelle generation and equilibrium dynamics are involved in micellization. With the use of surface adsorption characteristics, the Gibbs free energy for both adsorption and micellization can be easily computed. For the selection of the desired surfactant concentration, thermodynamic studies were performed to quantify the adsorption and micellization properties.57 The surface–active parameter values for the surfactants at various temperatures are presented in Table 1. The data indicates that when the temperature rises, it increases as well. This is because higher thermal mobility lowers and deteriorates the existing packing of the adsorbed molecules. The values of surface–active parameters for surfactants at various temperatures. From the data, it has been observed that with an increase in temperature, the surface area per molecule Amin decreases, which suggests that surfactant molecules are packing more efficiently at the air–liquid interface. This could be due to stronger hydrophobic interactions overcoming thermal agitation, allowing for tighter molecular packing despite the rise in the temperature. As maximum surface excess concentration, τmax increases, more surfactant molecules are adsorbing at the interface, which indicates that adsorption becomes more favorable than micellization at elevated temperatures. The decrease in Amin and increase in τmax imply that adsorption is energetically preferred over micelle formation in the bulk solution.58 Negative values for Gibbs free energy of adsorption ΔGads and Gibbs free energy of micellization ΔGmic confirm that both processes are spontaneous, but a higher magnitude of ΔGads suggests that adsorption is more favorable. The surfactant molecules might adopt a configuration that allows for denser packing at higher temperatures, enhancing adsorption despite the thermal mobility. Therefore, the system favors adsorption over micellization as temperature increases.59 A detailed review reveals that thermodynamic properties of TX-100 is highly encouraging for application in EOR.60,61
Table 1. Surface Adsorption Parameters of TX-100 Surfactant at Different Temperatures.
| T (K) | CMC (ppm) | γCMC (mN/m) | πCMC (mN/m) | τmax (μmol/m2) | Amin (nm2/molecule) | ΔGmic (kJ/mol) | ΔGads (kJ/mol) |
|---|---|---|---|---|---|---|---|
| 303 | 280 | 23.3 | 45.8 | 2.565 | 0.65 | –29.54 | –47.40 |
| 323 | 240 | 21.8 | 46.8 | 2.729 | 0.61 | –31.90 | –49.03 |
| 343 | 200 | 19.7 | 48.2 | 2.830 | 0.59 | –34.40 | –51.43 |
3.3. Characteristics of Pseudo Ternary Phase Diagram
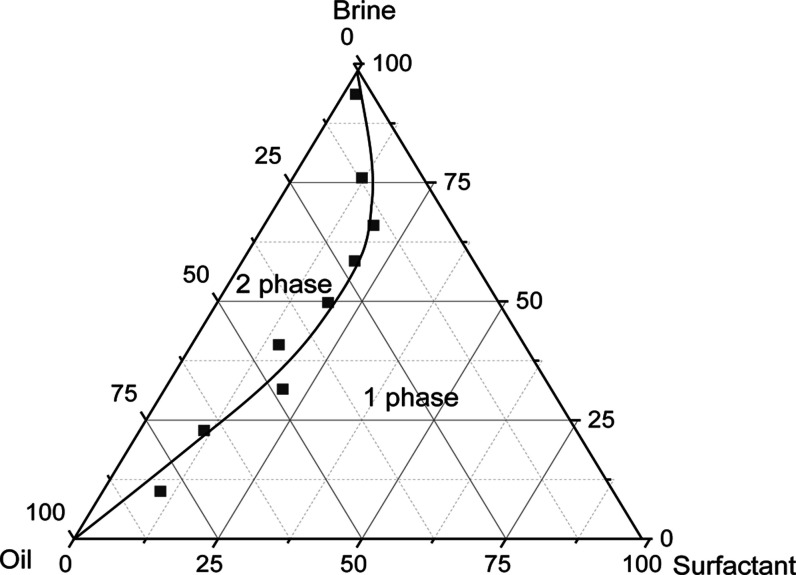
From an economic perspective, the creation of microemulsions with low surfactant concentrations is crucial. The ternary phase diagram for a system containing surfactant (TX-100), brine, and synthetic oil (n decane) is displayed in Figure 4. The microemulsion phase is represented by the single-phase region, while the microemulsion and surplus oil phase are shown by the two-phase region. As can be shown in Figure 4, even at low concentrations of surfactant and oil, a large microemulsion area is formed. It is essential to note that the region is a single phase beyond the binodal curve and is biphasic beneath it. From an economic perspective, it is also crucial to prepare microemulsions with low surfactant concentrations.
Figure 4.
Ternary phase diagram of oil–brine-surfactant (TX-100) system.
3.4. Solubilization Parameters
The volume of oil solubilized by the volume of surfactant in the microemulsion is known as the oil solubilization ratio. It is assumed that all of the surfactants are in the microemulsion phase. After sufficient shaking, the volume of oil solubilized in the microemulsion is equal to the difference between the original oil volume and the surplus oil at the top under equilibrium conditions. Comparably, the volume of water-soluble divided by the volume of surfactant in the microemulsion is the definition of the water solubilization ratio. The difference in volume between the original aqueous phase and excess water (at the bottom) is used to calculate the volume of water solubilized. Drawing oil and water solubilization ratio curves from the individual data points (one data per tube) yields the optimal solubilization ratio, which is reached when the two solubilizations are equal.62
Since solubilization parameters and IFT are correlated at optimal salinity, estimating these attributes is a valuable tool in the design of cost-effective microemulsion flooding compositions. Microemulsions undergo a phase change from lower to middle to upper phases when salinity levels rise. The solubilization parameters for oil in microemulsion, Vo/Vs, is an increasing function of salinity, whereas Vw/Vs is a declining function of salinity, as shown in Figure 5. The meeting point of these functions is termed as “optimal salinity” for phase behavior.
Figure 5.
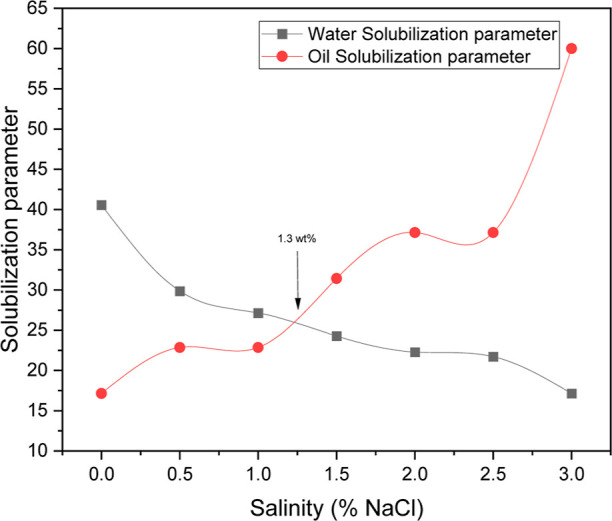
Solubilization parameter vs salinity as % of NaCl.
It is evident from the salinity scan in Figure 5 that the optimum salinity for the microemulsion system would be around 1.3 wt %, which will further be used in all polymeric surfactant microemulsion systems in this paper. It is also to be noted that at the point of optimal salinity, the values of Vo/Vs and Vw/Vs are equal. The IFT value at optimum salinity has been calculated using the Chun-Huh equation.63 The Chun-Huh equation provides a thermodynamic approach for estimating the IFT between oil and water in microemulsion systems stabilized by surfactants. This equation connects the IFT reduction with factors such as surfactant concentration relative to its CMC, surfactant efficiency, and structural properties of microemulsions, which may exist as Winsor type I, II, or III phases (bicontinuous, o/w, or w/o). Surfactants reduce the interfacial energy by adsorbing at the oil–water interface, lowering the IFT and thus facilitating more efficient mixing and stability. The equation emphasizes the role of the HLB of surfactants, which determines their effectiveness in minimizing IFT. Parameters such as effective surfactant volume at the interface and characteristic length scales also play a role. By reduction of the IFT, the Chun-Huh model significantly aids EOR processes by improving fluid mobility and oil displacement efficiency. The calculated value of IFT was found to be around 4.43 × 10–4 mN/m between the oil/microemulsion and water/microemulsion interface at optimum salinity. IFT between water-microemulsion and oil-microemulsion values can be calculated by the following eq 8
| 8 |
Table 2 provides data on the relationship between salinity levels and solubilization parameters of water and oil of the microemulsion, which is related to the stability and effectiveness of emulsion in oil recovery processes. IFT is crucial for optimizing oil solubilization (Vo/Vs) and water solubilization (Vw/Vs) ratios, determined using the Chun-Huh equation between the oil/microemulsion and water/microemulsion interfaces. As salinity increases from 0.0 to 3.0 wt %, significant changes in both the water solubilization parameter (Vw/Vs) and the oil solubilization parameter (Vo/Vs) are observed, which in turn affect the IFT values.
Table 2. IFT Value Determination by Chun-Huh Equation.
| salinity (wt %) | water solubilization parameter (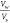 ) ) |
oil solubilization
parameter 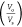
|
IFTwater-microemulsion (mN/m) | IFToil-microemulsion (mN/m) |
|---|---|---|---|---|
| 0.0 | 40.57 | 17.14 | 1.82 × 10–4 | 1.02 × 10–3 |
| 0.5 | 29.86 | 22.86 | 3.37 × 10–4 | 5.74 × 10–4 |
| 1.0 | 27.14 | 22.86 | 4.07 × 10–4 | 5.74 × 10–4 |
| 1.5 | 24.29 | 31.43 | 5.09 × 10–4 | 3.04 × 10–4 |
| 2.0 | 22.29 | 37.14 | 6.04 × 10–4 | 2.17 × 10–4 |
| 2.5 | 21.71 | 37.14 | 6.36 × 10–4 | 2.17 × 10–4 |
| 3.0 | 17.14 | 60.00 | 1.02 × 10–3 | 8.33 × 10–5 |
Table 2 shows ultralow IFT values at both the water-microemulsion and oil-microemulsion interfaces. This indicates high miscibility of the trapped crude oil with the microemulsion, leading to significantly improved displacement efficiency.64 While the water-microemulsion IFT increases with salinity, the IFT at the oil-microemulsion interfaces decreases significantly with an increase in salinity. The reduction in IFT between the oleic phase and the microemulsion, achieved through the addition of NaCl, is attributed to the increased conductivity of the microemulsion. This enhancement facilitates greater oil migration to the oil-microemulsion interface by increasing the number of available vacant adsorption sites with low interfacial energy.65 At optimum salinity, both of the solubilization parameters are the same with the IFT value of 4.43 × 10–4 mN/m. This relationship underscores the importance of salinity in optimizing microemulsion formulations for effective oil recovery.
3.5. Stability Analysis of Surfactant Microemulsion System
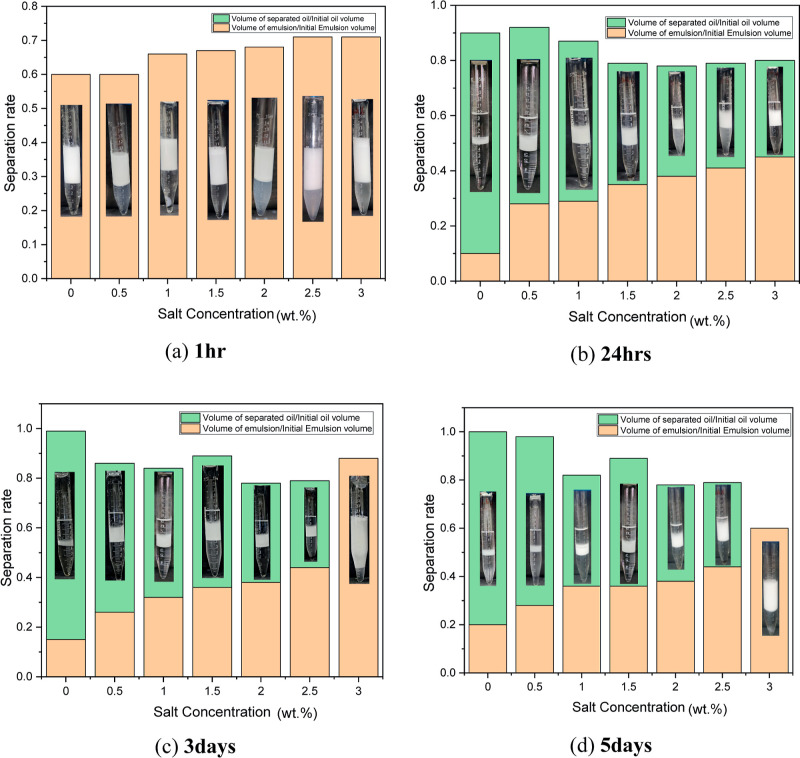
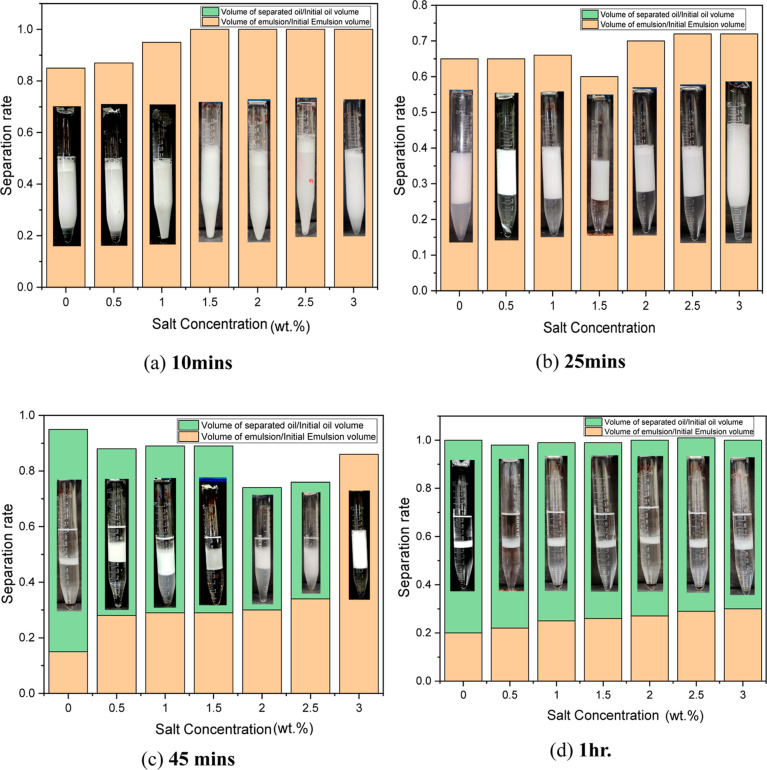
A low energy method was used in this to prepare the microemulsion by dissolving a suitable amount of salt (0.0, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 wt %) in the prepared solution, and the resulting solution was agitated for 30 min in Rotospin to generate chemical solutions of different salinities. While preparing a sample with an aqueous phase and an oil phase, it is preferable to maintain a volume ratio of 1:1. The formulated solutions were kept under observation for a time period of 5 days in a sealed glass vile to see the stability of the different samples at temperature 303 K. Figure 6 depicts that the solutions were kept for a very long time, and initially, it was observed that only 2 phases were in existence for the first few hours, and after a time period of 24 h, 3 phases (middle phase microemulsion) came into existence. The variation of optimal salinity with temperature (both high and low) has been shown here at different time periods. Hence, it is evident from Figure 6 that at the initial time, Winsor (II) was noticeable, and slowly, it changed to Winsor (III), which continued for quite a few days. Type III emulsion with a middle phase is considered best for EOR application as it offers an ultralow IFT.66 The optimal salinity is found to be at 1.3 wt % at 303 K, which was determined using the optimal salinity curve. Similarly, stability analysis was carried out at higher temperatures (343 K) as well. The results (Figure 7) indicate that at higher temperatures, the stability time decreases significantly because of thermal agitation disrupting surfactant effectiveness and altering the thermodynamic balance, leading to phase separation.67 It was initially observed that WINSOR II was exhibited at high temperatures, and after 45 min, WINSOR III came into existence. At later times, it was observed that at high temperatures, the middle phase microemulsion degraded and almost vanished after 1 h. It is also to be noted that all of the plots are between separation rate and salinity, in which the separation rate denotes the volume of separated oil/initial oil volume and the volume of emulsion to initial emulsion volume. Also, as salinity increases, in all of the time frames, it is noted that the volume of oil decreases as salinity increases and the volume of microemulsion (WINSOR II or WINSOR III) increases. This is a typical characteristic of nonionic surfactant microemulsion.2 The variation of the relative separation rates has been shown in Figures 6 and 7 at both 303 and 343 K. It is well noted that in nonionic systems, the microemulsion phase grows while the oil phase progressively declines with increasing salt.68 It is found that the oil phase almost entirely transforms into a biphasic system with a microemulsion at the top and water at the bottom at a notably high NaCl concentration.
Figure 6.
Stability analysis of surfactant microemulsion system along with separation rate at 303 K.
Figure 7.
Stability analysis of surfactant microemulsion system along with separation rate at 343 K.
3.6. Stability of Polymeric Surfactant Microemulsion System at Optimal Salinity
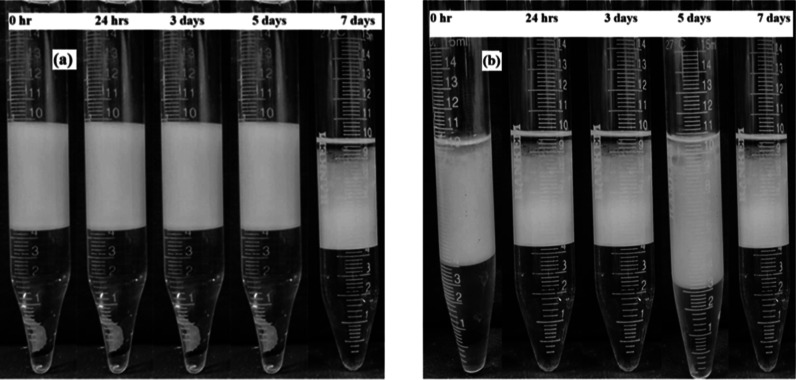
Salinity affects microemulsion stability in EOR by changing phase behavior, micellar structure, cosurfactant interactions, and electrostatic stabilization mechanisms essential for stability.69 The rheological behavior of microemulsions is influenced by polymer presence, which affects their flow characteristics during injection in EOR processes.70 The viscosity of microemulsions can be optimized by adjusting polymer concentrations, improving their ability to displace trapped oil in porous media. This is particularly important as it ensures that the injected slugs maintain favorable mobility ratios with crude oil. In this study, the samples were kept in 15 mL sealed glass tubes at room temperature, and their temporal physical appearance was observed to confirm the long-term stability (up to 7 days) of polymer-augmented surfactant microemulsions at both low (303 K) and high temperatures (343 K). The low energy (hand-shaken) method was used in preparing the sample using TX-100 surfactant (600 ppm) and PHPA polymer(1000 ppm) along with synthetic n-decane oil, with 1.3 wt % salt concentration as predetermined from previous stability studies and hence visualizing their long-term stability. The formulated polymeric microemulsion system was kept under observation for a time period of 7 days starting from 0 h. From Figure 8, it is clear that polymeric surfactant microemulsion at 303 K showed the change from Winsor type II to Winsor type III at a time period of 7 days. It is also evident that at a higher temperature, the water phase separated at 0 h; however, the microemulsion phase (Winsor type III) was found to be stable until a time period of 1 week. While laboratory experiments provide valuable insights, real reservoir conditions are dynamic, with varying temperature, salinity, and shear forces. Microemulsion instability can lead to phase separation, pore plugging, and a reduced oil recovery. The injected microemulsion slug will encounter various factors that can affect its stability as it travels through the rock formation. Reservoir temperatures can vary significantly compared to static test conditions. As shown in the experiment, higher temperatures (343 K) caused immediate water phase separation. During injection, the microemulsion experiences shear forces due to flow through the rock. While the hand-shaking method used in the experiment is a low-energy approach, real reservoir flow involves higher shear forces. Understanding how the microemulsion behaves under these forces is crucial. Hence, it is also very important to keep in mind the dynamic conditions of the reservoir, as well.
Figure 8.
Stability analysis of polymeric surfactant microemulsion system at (a) 303 K and (b) 343 K.
3.7. Dynamic Light Scattering and Zeta Potential Results
When it comes to the EOR, the size of the distributed particles in microemulsions is crucial. The durability of the microemulsion, the capacity of the emulsion to migrate through the pore throats in rocks, and the colloidal interaction between the dispersed particles all increase with decreasing size. A HLB value is typically assigned to all nonionic surfactants. This number describes the surfactant’s hydrophile/lipophile behavior and determines the stability of the emulsion.71 The particle size distribution of micelles in aqueous solution is assessed using the DLS method. The formation of microemulsion is confirmed by droplet size measurements by the DLS technique. At 600 ppm concentration in aqueous solution, DLS tests were carried out for the TX-100 surfactant microemulsion. A maximum peak that corresponds to the average particle diameter of 115 nm has been detected when comparing the intensity (wt %) vs average particle diameter. Furthermore, the effect of adding polymer to the microemulsion stabilized by surfactant was investigated. This study showed that the inclusion of PHPA polymer increased the microemulsion’s diameter because these polymers have the ability to coil up and take up space inside the droplets of microemulsion, hence expanding the droplets’ total size. Polymers’ ability to adsorb at the interface of a microemulsion’s water and oil phases is another explanation for this. The thicker interfacial coating produced by this adsorption causes the droplet diameter to rise.33 For surfactant + polymer-stabilized microemulsions containing 600 ppm surfactant and 1000 ppm polymer, an average particle diameter of 196 nm has been observed, indicating an increase in droplet size as compared to surfactant-stabilized microemulsion. This research is crucial for measuring the size of the oil droplets scattered in the microemulsion phase. The DLS data of different concentrations is displayed in Table 3.
Table 3. Average Diameter and Zeta Potential of Formulated Microemulsions.
| S.no | type of system | average particle diameter (nm) | zeta potential (mV) |
|---|---|---|---|
| 1 | TX-100 surfactant (600 ppm) microemulsion | 115 | –31.1 |
| 2 | PHPA (1000 ppm) + surfactant (600 ppm) microemulsion | 196 | –33.2 |
The zeta potential measures the electrical charge on dispersed particles in an emulsion, influencing the stability through electrostatic repulsion. A higher zeta potential, whether positive or negative, enhances the repulsive forces between similarly charged droplets, preventing aggregation and coalescence. This increased repulsion helps maintain separation between droplets, which is crucial for emulsion stability.72,73 Conversely, a low zeta potential allows attractive van der Waals forces to dominate, leading to instability. Factors such as ionic strength and pH can affect the zeta potential, with a critical threshold determining stability; exceeding this threshold is vital for ensuring well-dispersed systems in applications such as EOR. The effect of adding polymers on the zeta potential in a surfactant-based microemulsion can be complex and depends on several factors. The zeta potential values of several microemulsion systems are depicted in Table 3, too. For the TX-100 surfactant-stabilized microemulsion, a potential of −31.1 mV was measured, whereas after adding the PHPA polymer, a potential of −33.2 was measured. If the added polymer carries a charge (ionic), as in the case of PHPA, it can adsorb onto the microemulsion droplet surface, depending on its charge and affinity. This adsorption can create a stronger electrical repulsion between droplets, leading to a higher zeta potential.44 From both results, we have observed that adding polymer showed improved stability, as higher electrostatic repulsive forces and a higher stability will result from a larger zeta potential, which is consistent with our studies. Higher zeta potential makes the initiation of undesired aggregates less likely to happen.
3.8. Surfactant Adsorption Study
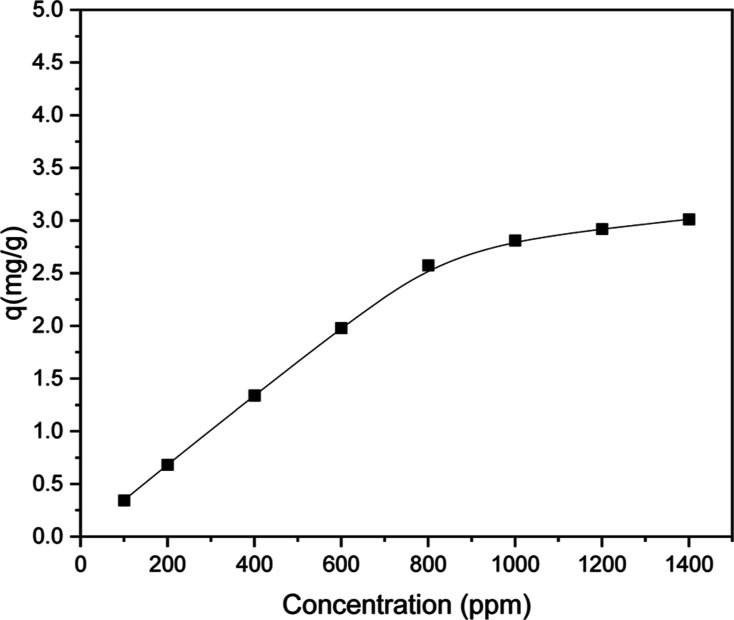
To determine the ideal surfactant concentration for chemical EOR techniques, surfactant adsorption plays a crucial role. Given their high cost, the monetary consequences of surfactant loss and adsorption must be taken into account. Thus, one of the biggest obstacles in chemical EOR projects is minimizing surfactant loss by adsorption on rock surfaces. The effectiveness of surfactant-based improved oil recovery methods may be adversely affected by surfactant adsorption, which results in a decrease in surfactant concentration in the aqueous phase.33 Therefore, reducing surfactant loss by adsorption on rock surfaces is one of the biggest problems with chemical EOR initiatives. The performance of surfactant-based EOR may be adversely affected by surfactant adsorption, which results in a decrease in surfactant concentration.74Figure 9 illustrates the adsorption behavior of the TX-100 surfactant at 25 °C. The data show that the surfactant exhibits low adsorption on the sand surface. This behavior is attributed to the nonionic nature of the surfactant, which lacks the charged groups necessary to form strong electrostatic interactions with the sand’s surface. As a result, the surfactant does not adhere well to the sand particles, leading to minimal adsorption. After 24 h, the measured adsorption value for the 200 ppm (CMC) TX-100 surfactant solution was 0.54 mg/g, which is within the acceptable range for surfactant flooding. However, the dynamic adsorption of surfactants offers clear benefits for EOR from surfactant flooding. It ensures continuous reduction of IFT and consistent wettability alteration throughout the recovery process, enhancing oil displacement more effectively than static adsorption, which often leads to excessive surfactant retention and inefficiencies. Dynamic adsorption also optimizes surfactant use, reducing costs and improving the overall recovery factor by maintaining desired surfactant levels at the rock surface under reservoir flow conditions.75
Figure 9.
Adsorption loss of TX-100 surfactant at different concentrations.
3.9. Rheology of Microemulsions
Emulsification is a key mechanism in EOR, improving the displacement of trapped oil and increasing extraction efficiency.76 Injecting microemulsions as slugs can significantly improve the displacement efficiency in EOR due to their favorable rheological properties. On the other hand, the presence of surfactant as an emulsion stabilizer reduces the IFT and hence improves the displacement efficiency. The mobility ratio between the injected slug and the crude oil being displaced must be maintained to control the displacement of the chemical slug without fingering. Both at the microscopic and macroscopic levels, polymeric surfactant microemulsion flooding contributes to an increase in oil recovery from the reservoir. The inclusion of polymer slug typically facilitates the displacement of oil in the reservoir by increasing the macroscopic sweep efficiency, while the injection of surfactant flooding helps to mobilize the oil at the microscopic level by minimizing the IFT and wettability alteration.77 Rheological properties of polymer, surfactant, and polymer-stabilized microemulsion slug were investigated in detail at set temperatures of 303 and 343 K. The flow behavior of the surfactant microemulsion system and the polymeric surfactant microemulsion system at 303 and 343 K degrees centigrade are shown in Figure 10.
Figure 10.
Viscosity curve for (a) PHPA polymer (1000 ppm), (b) surfactant (600 ppm)-stabilized microemulsion, and (c) polymer (1000 ppm)-augmented surfactant (600 ppm)-stabilized microemulsion at 303 and 343 K.
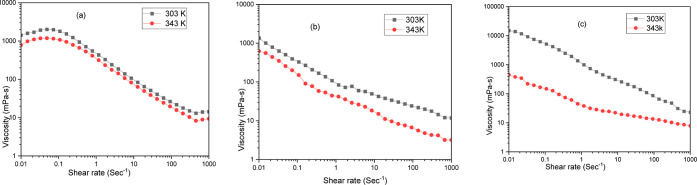
In this study, shear rates were varied from 0.01 to 1000 s–1 for all the systems. The viscous nature of fluid during its displacement in oil reservoirs is understood. Temperature is kept at 303 and 343 K to take both the low- and high-temperature studies into consideration. It has been observed that a rise in temperature leads to a decrease in viscosity in all of the rheological studies. This is because of an increase in Brownian motion.78 In Figure 10a, the graph between viscosity and shear rate for the PHPA polymer appears to show a shear-thinning relationship, which is a common characteristic of many fluids. In a shear-thinning relationship, the viscosity of the fluid declines as the shear rate increases.43 This means that the fluid becomes thinner and easier to flow as it is subjected to more stress. At the highest shear rate shown on the graph (around 1000 s–1), the viscosity is down to around 10 cP at a higher temperature. It is also evident from Figure 10c that at 343 K, the polymeric surfactant microemulsion has a better viscosity of above 10 cP, even at very high shear rates. Polymeric surfactants act like tiny dissolved thickeners within the microemulsion. These polymers create physical entanglement between chains, resisting flow and maintaining viscosity even at higher shear rates. In underground reservoirs, a more viscous microemulsion displaces oil more efficiently by resisting separation from the injected water.79 This is especially helpful in displacing the fluids uniformly in reservoir conditions and avoiding any potential viscous fingering as compared to simple surfactant microemulsion in Figure 10b, where at a high temperature of 343 K, the microemulsion system tends to lose viscosity control at higher shear rates. The primary conclusion drawn from the experiment is that the microemulsion bears a shear-thinning nature with a pseudoplastic nature, which implies that it is a non-Newtonian fluid. However, for the surfactant microemulsion system, the drop in viscosity at higher shear rates is much higher than the gradual decline in viscosity for polymeric surfactant microemulsion systems.78 This viscosity retention at higher shear rates helps reduce viscous fingering and creates a favorable viscosity balance. This reason can be attributed to polymer network formation, which creates a more structured system.80 The high molecular weights of polymers and their interactions with surfactants form larger aggregates. Additionally, hydrophilic polymers absorb water and swell, further increasing the viscosity. These factors combine to make polymer–surfactant microemulsions more viscous than simple-surfactant microemulsions. Applying shear can partially disentangle the polymer chains, but it is a slower process compared to breaking the surfactant network. This results in a more gradual decrease in viscosity with increasing shear rate.81
3.10. Wettability Alteration (Contact Angle)
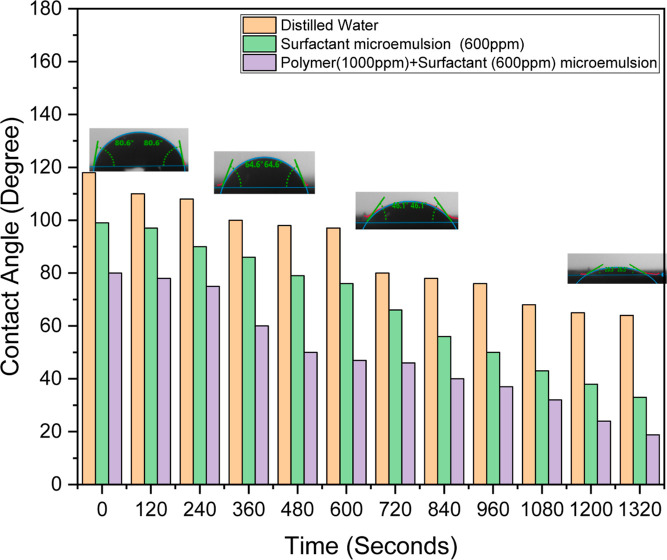
Altering an oil-wet reservoir to a water-wet state enhances oil recovery by improving the fluid flow and mobilization of trapped oil. Water-wet conditions increase capillary pressure on oil, facilitating its displacement toward production wells while enhancing sweep efficiency during water or chemical flooding by promoting uniform fluid distribution. Wettability alteration reduces residual oil saturation and minimizes oil adhesion to rock surfaces, thereby improving the relative permeability of the oil. This transformation also makes chemical EOR methods, such as surfactant flooding, more effective by better reducing IFT.82 Overall, water-wet conditions enable more efficient oil mobilization and displacement, which boost recovery rates. Whether they are carbonate or sandstone, reservoir rocks are naturally damp with water. As crude oil builds up in the reservoir, polar components of the oil (asphaltene, resin, etc.) adsorb onto the rocks, changing their wettability and creating oil- or mixed-wet conditions.44 Relative permeability, capillary pressure, and oil recovery are all impacted by wettability. The main action of surfactants is to reduce IFT, but they also help to convert oil-wet to water-wet wettability.68 Because the contact angle is dynamic, it gradually decreases until reaching equilibrium, and the changes become less pronounced. Contact angle experiments were carried out to determine whether microemulsion solutions may change the wettability of oil-wet sandstone rock. The oil is compelled to remain trapped inside the porous medium and adhere to the pore walls due to the preference for reservoir rock. This affinity hinders residual oil recovery by encouraging saturation of the oil.1 Therefore, contact angle measurements were required to be made on the oil-saturated rocks that were treated with the microemulsion systems developed in this study in order to alter the reservoir rock affinity.
To ascertain the role of the microemulsion, including surfactant and polymer ingredients, in the wettability inversion of the sandstone core sample, contact angle examination was carried out in triplicate. Surfactant microemulsion (600 ppm) and polymeric (1000 ppm) + surfactant (600 ppm) microemulsion systems were used to assess the dynamic contact angles. Regarding the modification of wettability by contact angle changes, both microemulsion systems exhibited extremely encouraging outcomes. The sandstone core was originally oil-soaked. The oil-wet surface eventually became water-wet due to the application of microemulsion systems, which are preferred for improved oil recovery mechanisms.
Under normal conditions, the reservoir is water-wet, so water tends to stick to the rock surface while oil lies between the water phases. This is a normal condition, where oil can flow by itself to be produced to the surface. In the case of tertiary recovery conditions, where oil is trapped in rock pores and cannot move on its own, in general, the reservoir is oil-wet.1 In an oil-wet system, oil occupies a narrow pore and is present as a film on the pore wall, while water is present as water droplets in the middle of the pore. In this condition, the so-called oil droplets stick to the pore walls. A surfactant is needed to release the oil attached to the pore wall, which functions to reduce the IFT between the oil grains on the pore wall. Figure 11 shows the contact angle of the three different systems, viz., distilled water, surfactant microemulsion, and polymeric surfactant microemulsion, with time. Because of the sample’s viscous solution, the polymeric surfactant microemulsion has a greater initial contact angle. Due to the viscous nature of the polymer-assisted surfactant microemulsion, a stable thin coating forms on the core surface, initially providing a larger contact angle, which then reduces over time. The reduction in contact angle for the polymeric surfactant microemulsion system can be attributed to the fact that the hydrophilic (water-loving) portions of the polymeric surfactants preferentially interact with the rock surface, forming a water-wet film. The polymers’ hydrophobic (oil-loving) tails project outward, creating a steric hindrance that repels oil molecules from readsorbing onto the rock surface. Numin et al.78 investigated the wettability alteration of oil-wet sandstone cores using polymeric microemulsions. Their findings showed a significant shift toward water-wetness due to the adsorption of the polymeric surfactants and steric repulsion effect.
Figure 11.
Contact angle variation of DW, surfactant microemulsion, and polymeric-surfactant microemulsion at different time intervals.
3.11. Core Flooding
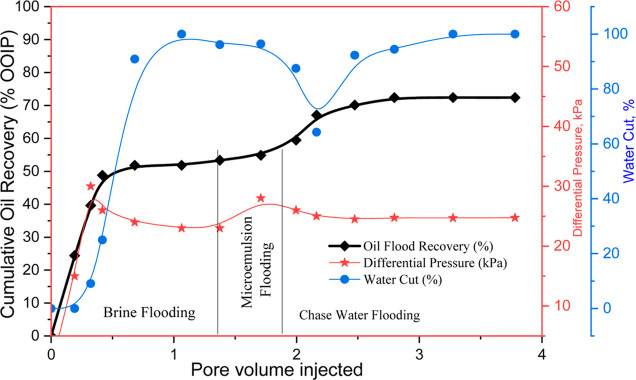
The EOR method, like microemulsion flooding, has been demonstrated to be efficacious in curtailing the IFT to achieve oil residual saturation in field and lab studies between oil and water phases.48 Based on the foregoing tests, the ideal concentrations of brine, surfactant, and polymer were chosen. In EOR operations, core flooding experiments are carried out to test the viability of the formulated chemical slugs for potential applications. These experiments help estimate the secondary and tertiary recoveries achievable for their application in the industry. In this study, the characterized formulations, namely, TX-100 surfactant (600 ppm) and PHPA polymer (1000 ppm)-assisted microemulsions, are injected into the sandstone cores, and their performances are examined. Table 4 shows the petrophysical properties of the porous media and flooding data for the selected chemical slugs. The cumulative oil recovery and differential pressure drop vs PV injection for two different systems are shown in Figure 12.
Table 4. Petrophysical Properties of the Core and the Additional Oil Recovery Percentage.
| core sample | porosity (%) | perm. (mD) | slug design | secondary recovery (%) | tertiary recovery (%) | Swi (%) |
|---|---|---|---|---|---|---|
| sandstone | 18.068 | 97.94 | PSME/TX-100(600 ppm) + PHPA (1000 PPM) | 51.83 | 20.58 | 21.5 |
Figure 12.
Core flooding performance of PSME slugs representing recovery, differential pressure, and water cut as a function of pore volume.
An initial step in core flooding experiments involved establishing the initial oil saturation by oil flood within the cores, i.e., Soi was 78.5%. Subsequently, the core underwent a three-day aging process to attain an oil-wet state before initiating waterflooding. In the experimental run, the pressure drops initially increased around 17–19 psi until the point of water breakthrough. Following this breakthrough, the differential pressure decreased and eventually stabilized toward the end of the water flooding phase. This initial pressure increase is a typical response, as it is necessary to overcome capillary forces and displace the oil from the pore spaces. Following the breakthrough, multiple water channels formed within the core’s porous structure, resulting in water fingering.
Consequently, there was an increase in the water cut and a decrease in the differential pressure as water infiltrated through the highly permeable capillaries. Upon injecting roughly 1.75 PVs of water in this experiment, secondary recovery was executed until more than 94% water cut was reached, resulting in the plateauing of the recovery curve. Consequently, the secondary recovery phase concluded, and the injection strategy transitioned to tertiary recovery. This transition involved the introduction of a chemical slug comprising TX-100 along with PHPA polymer microemulsion, with a volume of approximately 1 PV, followed by a chase water flood spanning around 1.25 PV. The oil recovery achieved during the secondary oil recovery phase amounted to 51.83% of the initial OOIP for the experiment. The remaining oil between the pore throats is due to the action of inertial, gravitational, and capillary forces. In EOR operations, the injection of chemical slugs into the porous medium tries to destabilize these forces by their chemical action and accounts for more recovery.
The injection of polymer-stabilized surfactant-based microemulsion resulted in an additional oil recovery of 20.58% beyond water flooding, with a residual oil saturation (Sor) of 17.9%. The core flooding experiments have unveiled a compelling narrative of EOR. Microemulsions consisting of oil, water, and surfactant molecules act as microscopic carriers that can penetrate rock formations and mobilize trapped oil droplets. The combination of surfactants (lower IFT) and polymers (viscosity control) within the microemulsion aids in this process. This orchestrated combination of reduced IFT, heightened wettability alteration facilitated by the incorporation of polymeric surfactant microemulsions, and the increased mobility control by the interaction process all contribute synergistically to the success of the recovery process.81 The polymeric component of PSMEs can act as a thickening agent for the injected fluid. This increased viscosity can create a more favorable mobility ratio between the injected fluid and the oil, improving sweep efficiency and reducing viscous fingering.83 This effect demonstrated that PSME flooding with the appropriate viscosity improvement resulted in better areal sweep and higher oil recovery. This aided in the polymer solution’s effective piston-like movement of the in situ emulsion that was generated in the irregular pore network.
4. Conclusions
This study demonstrates the efficacy of microemulsions stabilized by the synergistic action of polymer and surfactant, with a focus on their application in EOR. Utilizing the nonionic surfactant TX-100, we systematically analyzed these microemulsions under varying salinity conditions and compared them to traditional surfactant-stabilized systems.
Our findings indicate that while the water-microemulsion IFT increases with salinity, the oil-microemulsion IFT decreases significantly. At optimal salinity, both solubilization parameters equalize, achieving an IFT of 4.43 × 10–4 mN/m. The microemulsions exhibited exceptional stability across different temperatures, transitioning between Winsor type II and III phases, which is crucial for EOR. The addition of polymer notably increased the viscosity of the surfactant-stabilized emulsion from 50 mPa·s at a shear rate of 10 s–1 to 300 mPa·s and significantly improved the stability, as confirmed by zeta potential measurements of −31.1 and −33.2 mV for the surfactant and polymer-augmented surfactant systems, respectively. These enhancements indicate a higher performance and improvement in sweep efficiency. Furthermore, the microemulsions effectively altered sandstone wettability from oil-wet to water-wet, facilitating improved oil recovery. Core flooding experiments demonstrated that injecting one PV of polymer-augmented surfactant-stabilized microemulsion yielded an additional 20.58% oil recovery over conventional water flooding. In summary, the polymer-augmented surfactant-stabilized microemulsions exhibit superior properties for EOR applications, including enhanced stability, increased viscosity, effective wettability alteration, and significant improvements in oil recovery.
Overall, the study underscores the effectiveness of polymer-augmented surfactant-based microemulsions in the EOR by demonstrating their stability, optimal salinity conditions, and significant improvements in oil recovery rates. These findings not only advance our understanding of microemulsion systems in EOR but also open avenues for further research to optimize formulations for even higher efficiency in field applications.
Acknowledgments
We extend our sincere appreciation to SPARC, Ministry of Human Resource Development (SPARC/2019-2020/P1672/SL), for financial support to the Department of Petroleum Engineering, Indian Institute of Technology (ISM), Dhanbad, India.
The authors declare no competing financial interest.
References
- Alvarado V.; Manrique E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3 (9), 1529–1575. 10.3390/en3091529. [DOI] [Google Scholar]
- Bera A.; Ojha K.; Mandal A.; Kumar T. Interfacial Tension and Phase Behavior of Surfactant-Brine-Oil System. Colloids Surf., A 2011, 383 (1–3), 114–119. 10.1016/j.colsurfa.2011.03.035. [DOI] [Google Scholar]
- Guo H.; Song K.; Hilfer R.. A Critical Review of Capillary Number and Its Application in Enhanced Oil Recovery; 2020. 10.2118/200419-MS. [DOI]
- Deng X.; Tariq Z.; Murtaza M.; Patil S.; Mahmoud M.; Kamal M. shahzad. Relative Contribution of Wettability Alteration and Interfacial Tension Reduction in EOR: A Critical Review. J. Mol. Liq. 2021, 325, 115175. 10.1016/j.molliq.2020.115175. [DOI] [Google Scholar]
- Mandal A.; Ojha K.. Enhanced Oil Recovery: Mechanisms, Technologies and Feasibility Analyses; CRC Press: Boca Raton, 2023; . 10.1201/9781003098850. [DOI] [Google Scholar]
- Zhang Z.; Azad M. S.; Trivedi J. J. IFT or Wettability Alteration: What Is More Important for Oil Recovery in Oil-Wet Formation?. Fuel 2021, 291, 119986. 10.1016/j.fuel.2020.119986. [DOI] [Google Scholar]
- Massarweh O.; Abushaikha A. S. The Use of Surfactants in Enhanced Oil Recovery: A Review of Recent Advances. Energy Rep. 2020, 6, 3150–3178. 10.1016/j.egyr.2020.11.009. [DOI] [Google Scholar]
- Negin C.; Ali S.; Xie Q. Most Common Surfactants Employed in Chemical Enhanced Oil Recovery. Petroleum 2017, 3 (2), 197–211. 10.1016/j.petlm.2016.11.007. [DOI] [Google Scholar]
- Porter M. R.Handbook of Surfactants; Springer, 2013. [Google Scholar]
- Li J.; Zhu R.; Liu Y.; Yin L.; Wu W. Synthesis and Performance of Novel Sulfonate Gemini Surfactant with Trialkyl Chains. J. Dispersion Sci. Technol. 2016, 37 (3), 374–379. 10.1080/01932691.2015.1028069. [DOI] [Google Scholar]
- Yu F.; Jiang H.; Fan Z.; Xu F.; Su H.; Li J. Formation and Flow Behaviors of in Situ Emulsions in Heavy Oil Reservoirs. Energy Fuels 2019, 33 (7), 5961–5970. 10.1021/acs.energyfuels.9b00154. [DOI] [Google Scholar]
- Salehpour M.; Sakhaei Z.; Salehinezhad R.; Mahani H.; Riazi M. Contribution of Water-in-Oil Emulsion Formation and Pressure Fluctuations to Low Salinity Waterflooding of Asphaltic Oils: A Pore-Scale Perspective. J. Pet. Sci. Eng. 2021, 203, 108597. 10.1016/j.petrol.2021.108597. [DOI] [Google Scholar]
- Zhao X.; Feng Y.; Liao G.; Liu W. Visualizing In-Situ Emulsification in Porous Media during Surfactant Flooding: A Microfluidic Study. J. Colloid Interface Sci. 2020, 578, 629–640. 10.1016/j.jcis.2020.06.019. [DOI] [PubMed] [Google Scholar]
- Alzahid Y. A.; Mostaghimi P.; Alqahtani N. J.; Sun C.; Lu X.; Armstrong R. T. Oil Mobilization and Solubilization in Porous Media by in Situ Emulsification. J. Colloid Interface Sci. 2019, 554, 554–564. 10.1016/j.jcis.2019.07.009. [DOI] [PubMed] [Google Scholar]
- Karambeigi M. S.; Abbassi R.; Roayaei E.; Emadi M. A. Emulsion Flooding for Enhanced Oil Recovery: Interactive Optimization of Phase Behavior, Microvisual and Core-Flood Experiments. J. Ind. Eng. Chem. 2015, 29, 382–391. 10.1016/j.jiec.2015.04.019. [DOI] [Google Scholar]
- Jeirani Z.; Jan B. M.; Ali B. S.; See C. H.; Saphanuchart W. Pre-Prepared Microemulsion Flooding in Enhanced Oil Recovery: A Review. Pet. Sci. Technol. 2014, 32 (2), 180–193. 10.1080/10916466.2011.586968. [DOI] [Google Scholar]
- Pal N.; Kumar N.; Mandal A. Stabilization of Dispersed Oil Droplets in Nanoemulsions by Synergistic Effects of the Gemini Surfactant, PHPA Polymer, and Silica Nanoparticle. Langmuir 2019, 35 (7), 2655–2667. 10.1021/acs.langmuir.8b03364. [DOI] [PubMed] [Google Scholar]
- Zhu T.; Kang W.; Yang H.; Li Z.; Zhou B.; He Y.; Wang J.; Aidarova S.; Sarsenbekuly B. Advances of Microemulsion and Its Applications for Improved Oil Recovery. Adv. Colloid Interface Sci. 2022, 299, 102527. 10.1016/j.cis.2021.102527. [DOI] [PubMed] [Google Scholar]
- Kahlweit M.; Strey R.; Busse G. Microemulsions: A Qualitative Thermodynamic Approach. J. Phys. Chem. 1990, 94 (10), 3881–3894. 10.1021/j100373a006. [DOI] [Google Scholar]
- Ruckenstein E. Thermodynamic Approaches to Microemulsions. J. Colloid Interface Sci. 1998, 204 (1), 143–150. 10.1006/jcis.1998.5555. [DOI] [PubMed] [Google Scholar]
- Bera A.; Mandal A.; Guha B. B. Synergistic Effect of Surfactant and Salt Mixture on Interfacial Tension Reduction between Crude Oil and Water in Enhanced Oil Recovery. J. Chem. Eng. Data 2014, 59 (1), 89–96. 10.1021/je400850c. [DOI] [Google Scholar]
- Rezaie A.; Ghasemi H.; Eslami F. An In-Depth Investigation of the Impact of Salt Nature on the Formulation of Microemulsion Systems. Sci. Rep. 2023, 13 (1), 14362. 10.1038/s41598-023-40761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M.; Lindman B.; Holmberg K. Effect of Polymer Addition on the Phase Behavior of Oil–Water–Surfactant Systems of Winsor III Type. Phys. Chem. Chem. Phys. 2024, 26 (5), 3699–3710. 10.1039/D3CP04730J. [DOI] [PubMed] [Google Scholar]
- Chen X.; Adkins S. S.; Nguyen Q. P.; Sanders A. W.; Johnston K. P. Interfacial Tension and the Behavior of Microemulsions and Macroemulsions of Water and Carbon Dioxide with a Branched Hydrocarbon Nonionic Surfactant. J. Supercrit. Fluids 2010, 55 (2), 712–723. 10.1016/j.supflu.2010.08.019. [DOI] [Google Scholar]
- De Gennes P. G.; Taupin C. Microemulsions and the Flexibility of Oil/Water Interfaces. J. Phys. Chem. 1982, 86 (13), 2294–2304. 10.1021/j100210a011. [DOI] [Google Scholar]
- Schabes B. K.; Altman R. M.; Richmond G. L. Come Together: Molecular Details into the Synergistic Effects of Polymer–Surfactant Adsorption at the Oil/Water Interface. J. Phys. Chem. B 2018, 122 (36), 8582–8590. 10.1021/acs.jpcb.8b05432. [DOI] [PubMed] [Google Scholar]
- De Gennes P. G. Interactions between Polymers and Surfactants. J. Phys. Chem. 1990, 94 (22), 8407–8413. 10.1021/j100385a010. [DOI] [Google Scholar]
- Goodwin J. W.Colloids and Interfaces with Surfactants and Polymers-An Introduction; John Wiley & Sons, Ltd: Chichester, UK, 2004; . 10.1002/0470093919. [DOI] [Google Scholar]
- Nie C.; Han G.; Ni J.; Guan S.; Du H.; Zhang Y.; Wang H. Stability Dynamic Characteristic of Oil-in-Water Emulsion from Alkali–Surfactant–Polymer Flooding. ACS Omega 2021, 6 (29), 19058–19066. 10.1021/acsomega.1c02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker I. M.; Petkov J. T.; Jones C.; Penfold J.; Thomas R. K.; Rogers S. E.; Terry A. E.; Heenan R. K.; Grillo I. Adsorption of Polymer–Surfactant Mixtures at the Oil–Water Interface. Langmuir 2012, 28 (42), 14974–14982. 10.1021/la303563j. [DOI] [PubMed] [Google Scholar]
- Pal N.; Alzahid Y.; AlSofi A. M.; Ali M.; Hoteit H. Review on Microemulsions for Conformance Improvement Technology: Fundamentals, Design Considerations, and Perspectives. Energy Fuels 2023, 37 (2), 858–875. 10.1021/acs.energyfuels.2c03148. [DOI] [Google Scholar]
- Gradzielski M.; Duvail M.; De Molina P. M.; Simon M.; Talmon Y.; Zemb T. Using Microemulsions: Formulation Based on Knowledge of Their Mesostructure. Chem. Rev. 2021, 121 (10), 5671–5740. 10.1021/acs.chemrev.0c00812. [DOI] [PubMed] [Google Scholar]
- Bera A.; Mandal A. Microemulsions: A Novel Approach to Enhanced Oil Recovery: A Review. J. Pet. Explor. Prod. Technol. 2015, 5 (3), 255–268. 10.1007/s13202-014-0139-5. [DOI] [Google Scholar]
- Yang W.; Lu J.; Wei B.; Yu H.; Liang T. Micromodel Studies of Surfactant Flooding for Enhanced Oil Recovery: A Review. ACS Omega 2021, 6 (9), 6064–6069. 10.1021/acsomega.0c05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsal E.; Broens M.; Armstrong R. T. Pore Scale Dynamics of Microemulsion Formation. Langmuir 2016, 32 (28), 7096–7108. 10.1021/acs.langmuir.6b00821. [DOI] [PubMed] [Google Scholar]
- Neuma De Castro Dantas T.; Viana F. F.; Thaise Costa De Souza T.; Dantas Neto A. A.; Aum P. T. P. Study of Single-Phase Polymer-Alkaline-Microemulsion Flooding for Enhancing Oil Recovery in Sandstone Reservoirs. Fuel 2021, 302, 121176. 10.1016/j.fuel.2021.121176. [DOI] [Google Scholar]
- Santanna V. C.; Curbelo F. D. S.; Castro Dantas T. N.; Dantas Neto A. A.; Albuquerque H. S.; Garnica A. I. C. Microemulsion Flooding for Enhanced Oil Recovery. J. Pet. Sci. Eng. 2009, 66 (3), 117–120. 10.1016/j.petrol.2009.01.009. [DOI] [Google Scholar]
- Tao Q.; Li A.; Liu X.; Gao H.; Zhang Z.; Ma R.; An Y.; Shi L. Improved Thermal Stability of Lipase in W/O Microemulsion by Temperature-Sensitive Polymers. Colloids Surf., B 2013, 111, 587–593. 10.1016/j.colsurfb.2013.06.055. [DOI] [PubMed] [Google Scholar]
- Biruss B.; Valenta C. The Advantage of Polymer Addition to a Non-Ionic Oil in Water Microemulsion for the Dermal Delivery of Progesterone. Int. J. Pharm. 2008, 349 (1), 269–273. 10.1016/j.ijpharm.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Lu M.; Lindman B.; Holmberg K. Effect of Polymer Addition on the Phase Behavior of Oil–Water–Surfactant Systems of Winsor III Type. Phys. Chem. Chem. Phys. 2024, 26 (5), 3699–3710. 10.1039/D3CP04730J. [DOI] [PubMed] [Google Scholar]
- Hamouma M.; Delbos A.; Dalmazzone C.; Colin A. Polymer Surfactant Interactions in Oil Enhanced Recovery Processes. Energy Fuels 2021, 35 (11), 9312–9321. 10.1021/acs.energyfuels.1c00562. [DOI] [Google Scholar]
- Lu M.; Lindman B.; Holmberg K. Effect of Polymer Addition on the Phase Behavior of Oil–Water–Surfactant Systems of Winsor III Type. Phys Chem Chem Phys 2024, 26, 3699–3710. 10.1039/D3CP04730J. [DOI] [PubMed] [Google Scholar]
- Karambeigi M. S.; Abbassi R.; Roayaei E.; Emadi M. A. Emulsion Flooding for Enhanced Oil Recovery: Interactive Optimization of Phase Behavior, Microvisual and Core-Flood Experiments. J. Ind. Eng. Chem. 2015, 29, 382–391. 10.1016/j.jiec.2015.04.019. [DOI] [Google Scholar]
- Kumar N.; Mandal A. Wettability Alteration of Sandstone Rock by Surfactant Stabilized Nanoemulsion for Enhanced Oil Recovery—A Mechanistic Study. Colloids Surf., A 2020, 601 (May), 125043. 10.1016/j.colsurfa.2020.125043. [DOI] [Google Scholar]
- Negi K.; Chauhan S. Multifaceted Insights into the Molecular Interactions of Tetraalkylammonium-Based Quaternary Ammonium Salts with Cetyltrimethylammonium Bromide: Unraveling Structural, Thermodynamic, and Antibacterial Effects. J. Dispersion Sci. Technol. 2024, 0 (0), 1–14. 10.1080/01932691.2024.2315482. [DOI] [Google Scholar]
- Jangid L.; Dey S.; Joshi D.; Saxena N.; Ojha K.; Mandal A. Exploring Interfacial Properties and Thermodynamic Parameters of Synthesized Biodegradable Surfactants from Brassica Juncea and Their Emulsification Characteristics. J. Mol. Liq. 2024, 408, 125326. 10.1016/j.molliq.2024.125326. [DOI] [Google Scholar]
- Bera A.; Ojha K.; Kumar T.; Mandal A. Phase Behavior and Physicochemical Properties of (Sodium Dodecyl Sulfate + Brine + Propan-1-Ol + Heptane) Microemulsions. J. Chem. Eng. Data 2012, 57 (3), 1000–1006. 10.1021/je2013796. [DOI] [Google Scholar]
- Healy R. N.; Reed R. L.; Carpenter C. W.. Laboratory Study of Microemulsion Flooding, 1974; pp 191–208.
- Saxena N.; Kumar A.; Mandal A. Adsorption Analysis of Natural Anionic Surfactant for Enhanced Oil Recovery: The Role of Mineralogy, Salinity, Alkalinity and Nanoparticles. J. Pet. Sci. Eng. 2019, 173, 1264–1283. 10.1016/j.petrol.2018.11.002. [DOI] [Google Scholar]
- Alhammadi A. M.; Gao Y.; Akai T.; Blunt M. J.; Bijeljic B. Pore-Scale X-Ray Imaging with Measurement of Relative Permeability, Capillary Pressure and Oil Recovery in a Mixed-Wet Micro-Porous Carbonate Reservoir Rock. Fuel 2020, 268 (February), 117018. 10.1016/j.fuel.2020.117018. [DOI] [Google Scholar]
- Santanna V. C.; Silva A. C. M.; Lopes H. M.; Sampaio Neto F. A. Microemulsion Flow in Porous Medium for Enhanced Oil Recovery. J. Pet. Sci. Eng. 2013, 105, 116–120. 10.1016/j.petrol.2013.03.015. [DOI] [Google Scholar]
- Nouqabi R. Q. M.; Zargar G.; Takassi M. A.; Moradi S. Wettability Alteration in Enhanced Oil Recovery Process Using New Amphoteric and Cationic Surfactants. Biosci. Biotechnol. Res. Comm 2017, 10 (4), 704–709. 10.21786/bbrc/10.4/14. [DOI] [Google Scholar]
- Ratanpara A.; Kim M. Wettability Alteration Mechanisms in Enhanced Oil Recovery with Surfactants and Nanofluids: A Review with Microfluidic Applications. Energies 2023, 16 (24), 8003. 10.3390/en16248003. [DOI] [Google Scholar]
- Ahmadi S.; Hosseini M.; Tangestani E.; Mousavi S. E.; Niazi M. Wettability Alteration and Oil Recovery by Spontaneous Imbibition of Smart Water and Surfactants into Carbonates. Pet. Sci. 2020, 17 (3), 712–721. 10.1007/s12182-019-00412-1. [DOI] [Google Scholar]
- Hoff E.; Nyström B.; Lindman B. Polymer-Surfactant Interactions in Dilute Mixtures of a Nonionic Cellulose Derivative and an Anionic Surfactant. Langmuir 2001, 17 (1), 28–34. 10.1021/la001175p. [DOI] [Google Scholar]
- Pal N.; Saxena N.; Mandal A. Synthesis, Characterization, and Physicochemical Properties of a Series of Quaternary Gemini Surfactants with Different Spacer Lengths. Colloid Polym. Sci. 2017, 295 (12), 2261–2277. 10.1007/s00396-017-4199-1. [DOI] [Google Scholar]
- Sulthana S. B.; Bhat S. G. T.; Rakshit A. K. Thermodynamics of Micellization of a Non-Ionic Surfactant Myrj 45: Effect of Additives. Colloids Surf., A 1996, 111 (1–2), 57–65. 10.1016/0927-7757(95)03491-9. [DOI] [Google Scholar]
- Asadov Z. H.; Ahmadova G. A.; Rahimov R. A.; Abilova A. Z.; Nazarov I. G.; Zubkov F. I. Surface Activity, Adsorption, and Micellization Parameters of Ammonium Surfactants Containing a Hydroxyethyl and Hydroxyisopropyl Head Group. J. Chem. Eng. Data 2017, 62 (10), 3297–3305. 10.1021/acs.jced.7b00347. [DOI] [Google Scholar]
- Mańko D.; Zdziennicka A.; Jańczuk B. Thermodynamic Properties of Rhamnolipid Micellization and Adsorption. Colloids Surf., B 2014, 119, 22–29. 10.1016/j.colsurfb.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Bhatt D.; Maheria K.; Parikh J. Mixed System of Ionic Liquid and Non-Ionic Surfactants in Aqueous Media: Surface and Thermodynamic Properties. J. Chem. Thermodyn. 2014, 74, 184–192. 10.1016/j.jct.2014.01.032. [DOI] [Google Scholar]
- Mushahid Zafar M.; Yusaf A.; Usman M.; Rauf A.; Rasool N.; Nawaz S.; Rasool L. Effect of TX-100 on Solubilizing Power of CTAB and CPC for H Acid: An Experimental and Computational Analysis. J. Mol. Liq. 2024, 402, 124779. 10.1016/j.molliq.2024.124779. [DOI] [Google Scholar]
- Nadir N.; Shahruddin S.; Othman J. Surfactant Evaluation for Enhanced Oil Recovery: Phase Behavior and Interfacial Tension. Open Chem. 2022, 20 (1), 1110–1120. 10.1515/chem-2021-0115. [DOI] [Google Scholar]
- Huh C. Interfacial Tensions and Solubilizing Ability of a Microemulsion Phase That Coexists with Oil and Brine. J. Colloid Interface Sci. 1979, 71 (2), 408–426. 10.1016/0021-9797(79)90249-2. [DOI] [Google Scholar]
- Mahboob A.; Kalam S.; Kamal M. S.; Hussain S. M. S.; Solling T. EOR Perspective of Microemulsions: A Review. J. Pet. Sci. Eng. 2022, 208, 109312. 10.1016/j.petrol.2021.109312. [DOI] [Google Scholar]
- Santanna V. C.; Curbelo F. D. S.; Castro Dantas T. N.; Dantas Neto A. A.; Albuquerque H. S.; Garnica A. I. C. Microemulsion Flooding for Enhanced Oil Recovery. J. Pet. Sci. Eng. 2009, 66 (3), 117–120. 10.1016/j.petrol.2009.01.009. [DOI] [Google Scholar]
- Budhathoki M.; Hsu T. P.; Lohateeraparp P.; Roberts B. L.; Shiau B. J.; Harwell J. H. Design of an Optimal Middle Phase Microemulsion for Ultra High Saline Brine Using Hydrophilic Lipophilic Deviation (HLD) Method. Colloids Surf., A 2016, 488, 36–45. 10.1016/j.colsurfa.2015.09.066. [DOI] [Google Scholar]
- Flanagan J.; Kortegaard K.; Neil Pinder D.; Rades T.; Singh H. Solubilisation of Soybean Oil in Microemulsions Using Various Surfactants. Food Hydrocolloids 2006, 20, 253–260. 10.1016/j.foodhyd.2005.02.017. [DOI] [Google Scholar]
- Neuma de Castro Dantas T.; Viana F. F.; Thaise Costa de Souza T.; Dantas Neto A. A.; Aum P. T. P. Study of Single-Phase Polymer-Alkaline-Microemulsion Flooding for Enhancing Oil Recovery in Sandstone Reservoirs. Fuel 2021, 302 (March), 121176. 10.1016/j.fuel.2021.121176. [DOI] [Google Scholar]
- Riswati S. S.; Bae W.; Park C.; Permadi A. K.; Efriza I.; Min B. Experimental Analysis to Design Optimum Phase Type and Salinity Gradient of Alkaline Surfactant Polymer Flooding at Low Saline Reservoir. J. Pet. Sci. Eng. 2019, 173, 1005–1019. 10.1016/j.petrol.2018.09.087. [DOI] [Google Scholar]
- Annable T.; Buscall R.; Ettelaie R.; Shepherd P.; Whittlestone D. Influence of Surfactants on the Rheology of Associating Polymers in Solution. Langmuir 1994, 10 (4), 1060–1070. 10.1021/la00016a018. [DOI] [Google Scholar]
- Trapani G.; Altomare C.; Franco M.; Latrofa A.; Liso G. Determination of Hydrophile-Lipophile Balance of Some Polyethoxylated Non-Ionic Surfactants by Reversed-Phase Thin Layer Chromatography. Int. J. Pharm. 1995, 116 (1), 95–99. 10.1016/0378-5173(94)00279-E. [DOI] [Google Scholar]
- Wiącek A.; Chibowski E. Zeta Potential, Effective Diameter and Multimodal Size Distribution in Oil/Water Emulsion. Colloids Surf., A 1999, 159 (2), 253–261. 10.1016/S0927-7757(99)00281-2. [DOI] [Google Scholar]
- Stachurski J.; MichaŁek M. The Effect of the ζ Potential on the Stability of a Non-Polar Oil-in-Water Emulsion. J. Colloid Interface Sci. 1996, 184 (2), 433–436. 10.1006/jcis.1996.0637. [DOI] [PubMed] [Google Scholar]
- Budhathoki M.; Barnee S. H. R.; Shiau B.-J.; Harwell J. H. Improved Oil Recovery by Reducing Surfactant Adsorption with Polyelectrolyte in High Saline Brine. Colloids Surf., A 2016, 498, 66–73. 10.1016/j.colsurfa.2016.03.012. [DOI] [Google Scholar]
- Sakthivel S. Imidazolium Based Ionic Liquids for Enhanced Oil Recovery on the Carbonate Reservoir. J. Mol. Liq. 2022, 366, 120284. 10.1016/j.molliq.2022.120284. [DOI] [Google Scholar]
- Walker D. L.; Britton C.; Kim D. H.; Dufour S.; Weerasooriya U.; Pope G. A.. The Impact of Microemulsion Viscosity on Oil Recovery. In All Days; SPE: Tulsa, Oklahoma, USA, 2012; p SPE-154275-MS. 10.2118/154275-MS. [DOI]
- Saw R. K.; Rane P. M.; Joshi D.; Prakash S.; Jangid L.; Mandal A. Enhanced Oil Recovery Using a Novel Non-Ionic Surfactant Synthesized from Olive Oil: Performance and Synergistic Effects. J. Mol. Liq. 2023, 392 (P1), 123452. 10.1016/j.molliq.2023.123452. [DOI] [Google Scholar]
- Numin M. S.; Jumbri K.; Ramli A.; Borhan N. Microemulsion Rheological Analysis of Alkaline, Surfactant, and Polymer in Oil-Water Interface. Processes 2020, 8 (7), 1–16. 10.3390/pr8070762. [DOI] [Google Scholar]
- Zhou Y.; Luo Z.; Xu M.; Zhao T.; Ma X.; Zhou S.; Wen B.; Yang D. Preparation and Properties of Temperature-Sensitive P(NIPAM-AM) Nano-Microspheres in Enhanced Oil Recovery. Energy Fuels 2023, 37 (1), 204–213. 10.1021/acs.energyfuels.2c02972. [DOI] [Google Scholar]
- Gale W. W.Microemulsions Which Compatibly Incorporate Viscosifiers and Their Use in Enhanced Oil Recovery (Patent) | OSTI.GOV U.S. Patent 4,271,907 AI, 1981. .
- Lu M.; Lindman B.; Holmberg K. Effect of Polymer Addition on the Phase Behavior of Oil-Water-Surfactant Systems of Winsor III Type. Phys. Chem. Chem. Phys. 2024, 26 (5), 3699–3710. 10.1039/D3CP04730J. [DOI] [PubMed] [Google Scholar]
- Kathel P.; Mohanty K. K. Wettability Alteration in a Tight Oil Reservoir. Energy Fuels 2013, 27 (11), 6460–6468. 10.1021/ef4012752. [DOI] [Google Scholar]
- Sheng J. J.; Leonhardt B.; Azri N. Status of Polymer-Flooding Technology. J. Can. Pet. Technol. 2015, 54 (2), 116–126. 10.2118/174541-PA. [DOI] [Google Scholar]