Background
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disease, with an estimated global prevalence of 10.3%, affecting approximately 2 million Canadians.1,2 COPD is a leading cause of hospitalization in Canada, responsible for approximately 50,000 inpatient visits annually at an average of 7 days each. 3 Given the average cost of $7800 CAD per Canadian hospital stay and an estimated 45 kg of carbon dioxide emissions per acute care patient per day in the United States, COPD hospitalizations are associated with significant financial and environmental impact.4,5 Most importantly, many people with COPD experience significant morbidity, marked by symptoms of dyspnea, chronic cough, sputum production, and exercise limitation, with more than 60% of patients reporting shortness of breath that has impaired their quality of life. 6 A 2017 study found that 15% of patients with COPD were hospitalized at least 3 times per year, with a cumulative length of stay of more than 30 days. 7
Inhaled therapies are a cornerstone of COPD management, with a variety of drug and device formulations available. Table 1 provides an overview of inhaler devices available in Canada for COPD. Unfortunately, suboptimal inhaler technique is often observed, with a user error rate as high as 50% to 100%. 8 To optimize benefits from inhaled therapies, regimens should be individualized for patient preference and capabilities, in unison with intensive patient counselling and education. 2 In addition, patients and health care providers can consider the climate impacts of inhalers, some of which contain propellant compounds with significant greenhouse gas (GHG) content. 9 Improving COPD control can improve patients’ quality of life, lead to fewer exacerbations and hospitalizations and reduce environmental burden.2,5 Pharmacists are well-positioned to aid in COPD therapy optimization to improve patient outcomes and minimize climate impacts. 10
Table 1.
Overview and comparison of COPD inhaler devices
| Dry powder inhaler | Soft mist inhaler* | Metered-dose inhaler | ||||||
|---|---|---|---|---|---|---|---|---|
| Turbuhaler | Ellipta | Diskus | Genuair | Handihaler | Breezhaler | Respimat | ||
| Available drugs/combinations | SABA Terbutaline (Bricanyl) LAMA Formoterol (Oxeze) LABA + ICS Formoterol + budesonide (Symbicort) |
LAMA Umeclidinium (Incruse) LAMA + LABA Umeclidinium + vilanterol (Anoro) LABA + ICS Vilanterol + fluticasone (Breo) LAMA + LABA + ICS Umeclidinium + vilanterol + fluticasone (Trelegy) |
SABA Salbutamol (Ventolin) LABA Salmeterol (Serevent) LABA + ICS Salmeterol + fluticasone (Advair) |
LAMA Aclidinium (Tudorza) LAMA + LABA Aclidinium + formoterol (Duaklir) |
LAMA Tiotropium (Spiriva) |
LAMA Glycopyrronium (Seebri) LABA Formoterol (Oxeze) LAMA + LABA Glycopyrronium + indacaterol (Ultibro) |
SAMA + SABA Ipratropium + salbutamol (Combivent) LAMA Tiotropium (Spiriva) LAMA + LABA Tiotropium + olodaterol (Inspiolto) |
SAMA Ipratropium (Atrovent) Salbutamol (Ventolin) LAMA + LABA + ICS Glycopyrronium + formoterol + budesonide (Breztri Aerosphere) |
| Inspiratory capacity11,12 | High inspiratory capacity required (30-60 L/min) | Moderate inspiratory capacity required (30 L/min) | Moderate-high inspiratory capacity required (50 L/min) | Low inspiratory capacity required (minimum 10-20 L/min) | ||||
| Require “hard and fast” inhalation | Require “slow and steady” inhalation | |||||||
| Device use/handling | Breath-actuated (timing of actuation and breath not required) | Timing of actuation and breath required | ||||||
| Low dexterity required to load dose | Feedback mechanism (window colour changes and audible click when dose taken) | Multistep process to load, requires the most dexterity | Spring loading requires dexterity and strength (if loaded by pharmacy, low strength and dexterity required) | Actuation requires hand strength | ||||
| Priming not required | Priming required | |||||||
| Cannot be used with a spacer | Can be used with a spacer | |||||||
| Dose counter | Yes—every 20 doses | Yes | Yes | Yes—every 10 doses | Not required—capsules loaded with each dose | Yes | No (Breztri—yes) |
|
| Climate9,13,14 | Lowest carbon footprint (~25 times lower than MDI)*
|
Moderate carbon footprint (~18 times lower than MDI)*
|
Highest carbon footprint—choose brands with lower HFA content if possible (such as Teva-salbutamol or Airomir [salbutamol sulphate]) | |||||
COPD, chronic obstructive pulmonary disease; HFA, hydrofluoroalkane; ICS, inhaled corticosteroid; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; MDI, metered-dose inhaler; SABA, short-acting beta2-agonist; SAMA, short-acting muscarinic antagonist.
 Indicates the most climate-friendly option if clinically appropriate.
Indicates the most climate-friendly option if clinically appropriate.
Overview of COPD pharmacotherapy recommendations
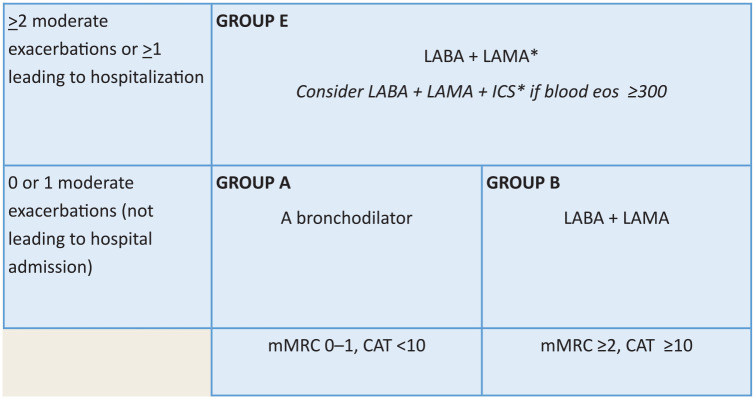
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) and Canadian Thoracic Society (CTS) provide guidelines for COPD management with a tailored approach to initiating and modifying inhaled therapies, depending on disease severity, symptom burden, and exacerbation history.2,11 Tools, including the COPD Assessment Tool (CAT) and Modified Medical Research Council Dyspnea Scale (mMRC), assess airflow limitation and symptom burden.2,12,13 Together, these assessments can be used to further characterize a patient as GOLD group A, B, or E to guide initial pharmacologic therapy (Figure 1). 2 The CTS recently released updated guidelines that closely align with GOLD, with some notable differences. 11 In addition to CAT, mMRC and exacerbation history, CTS guidelines also incorporate forced expiratory volume in 1 second into staging for initial pharmacologic therapy. 11 Given the generalizability to an international audience, as well as the amenability to a concise practice tool, the GOLD recommendations provide the basis for this article.
Figure 1.
Initial pharmacologic treatment (GOLD 2023)2,15
*Single inhaler therapy may be more convenient and effective than multiple inhalers.
Exacerbations refers to the number of exacerbations per year.
CAT, Chronic Obstructive Pulmonary Disease (COPD) Assessment Tool; eos, blood eosinophil count in cells per microliter; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting beta2-agonists; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council dyspnea questionnaire.
Based on figure 4.2 of the 2023 GOLD Guidelines. 2 Used with permission.
Bronchodilators are the mainstay of inhaled therapy, including short-acting beta2-agonists (SABA), long-acting beta2-agonists (LABA), short-acting muscarinic antagonists (SAMA), and long-acting muscarinic antagonists (LAMA), in addition to inhaled corticosteroids (ICS). Combinations of long-acting bronchodilators and ICS are recommended as maintenance therapy based on the GOLD group A, B, or E and levels of blood eosinophils. 2 CTS and GOLD guidelines recommend short-acting therapy to be used as-needed for acute symptom control.2,11 Unlike GOLD, CTS no longer recommends short-acting monotherapy for mild disease with low symptom burden and instead promotes long-acting therapy for all patients. 11 In addition, the CTS guidelines recommend the use of LABA, LAMA, and ICS for all patients with severe disease, whereas GOLD recommends the addition of ICS only in select patients in this category.2,11 While inhaled pharmacotherapy is the focus of this article, this should be considered in the context of other important measures, such as smoking cessation, education on self-management, vaccinations, and pulmonary rehabilitation.2,11
Climate impacts
The health care sector contributes significantly to GHG emissions, comprising 4.6% of the national total in Canada. 14 Pharmaceuticals comprise one-quarter of this, making it the largest single category. 14 Metered-dose inhalers (MDIs), in particular, contain significant amounts of GHG, due to their hydrofluoroalkane (HFA) propellants. 15 The propellant compounds are released into the atmosphere when the inhaler is actuated and continue to leak when not in use, including with improper disposal. 16 United Kingdom data suggest that MDIs alone account for 4% of the UK National Health Service’s overall carbon footprint. 17 It is estimated that the carbon footprint from 1 MDI is equivalent to driving a car approximately 290 km. 15 While all inhalers carry a carbon footprint due to upstream manufacturing processes, MDIs are associated with a 10 to 37 times higher carbon footprint compared with dry powder inhalers (DPIs) and soft mist inhalers (SMIs). 9 In addition, inhaler devices are not recyclable in many places across Canada, and the large amounts dispensed have a significant impact on pharmaceutical and plastic waste. 18 Unfortunately, the GHG content in inhaled COPD treatments could be considered an accelerant of warmer global temperatures and the subsequent deterioration in air quality, negatively affecting those living with COPD.19,20
Optimizing COPD therapy and selecting inhalers with minimal GHG emissions can both improve patient outcomes and mitigate climate change.2,21 This includes selecting optimal pharmacotherapy based on the patient’s COPD severity, selecting an appropriate device through shared decision-making, selecting a DPI or SMI over an MDI when appropriate, selecting an MDI with lower HFA content, and minimizing the number of inhalers prescribed and dispensed.18,21,22 Together, these actions have the potential to reduce COPD exacerbations, hospitalizations, and environmental impacts.
The pharmacist’s role in inhaler assessment
While treatment guidelines provide stepwise recommendations for selection of drug therapy, device selection is largely up to the prescriber based on their knowledge and experience. To optimize patient outcomes, inhaled therapy needs to be individualized to the patient and continually reassessed.2,23 Pharmacists are well-positioned to help patients and interprofessional colleagues navigate inhaled therapies due their accessibility, expertise in drug therapy, and experience managing cost and insurance considerations. 10 Pharmacist interventions can occur during initiation of therapy, regular medication assessments, assessment of prescription fill history, and review of exacerbation history. This practice tool aims to assist pharmacists in determining optimal devices for people with COPD, with consideration to environmental impacts.
How to tailor therapy
In this section, we propose a stepwise approach to assist providers in selection of optimal inhaled pharmacotherapy.
1. Confirm indication for therapy
a. Identify COPD indication for prescribed inhalers with the patient, electronic health record, or appropriate health care provider.
• Although spirometry is not often done in COPD, both GOLD and CTS guidelines, as well as Choosing Wisely Canada, endorse objective spirometry diagnosis prior to initiating long-term maintenance therapy.2,11,24,25
2. Select appropriate drug therapy for disease severity
a. Assess disease severity based on patient symptom burden and exacerbation history.
• Pharmacists can assess disease severity through eliciting subjective symptoms from patients (using the mMRC or CAT scoring tools) or through information from another health care provider.
b. Based on the above assessment, use Figure 1 to classify based on GOLD group A, B, or E criteria 2 :
• Group A: One or fewer exacerbations not leading to hospital admission and low symptom burden (mMRC 0-1 and CAT < 10). Recommended initial therapy is a long-acting bronchodilator (e.g., LAMA or LABA). Long-acting agents are preferred over short-acting agents by GOLD, except in patients with very occasional dyspnea. 2
• Group B: One or fewer exacerbations not leading to hospital admission and greater symptom burden (mMRC 2 or greater and CAT 10 or greater). Recommended initial therapy is a LABA and LAMA. 2
• Group E: Two or more moderate exacerbations or 1 or more exacerbation leading to hospitalization. Recommended initial therapy is a LABA and LAMA, with or without ICS. Factors such as a blood eosinophil count of 300 cells/µL or greater, more frequent or severe exacerbations, and concomitant asthma would favour the use of an ICS. Eosinophils of less than 100 cells/µL, multiple previous episodes of pneumonia, or history of mycobacterial infection are a less favourable set of factors. These would indicate lower expected clinical benefits and greater potential harms of ICS usage. 2
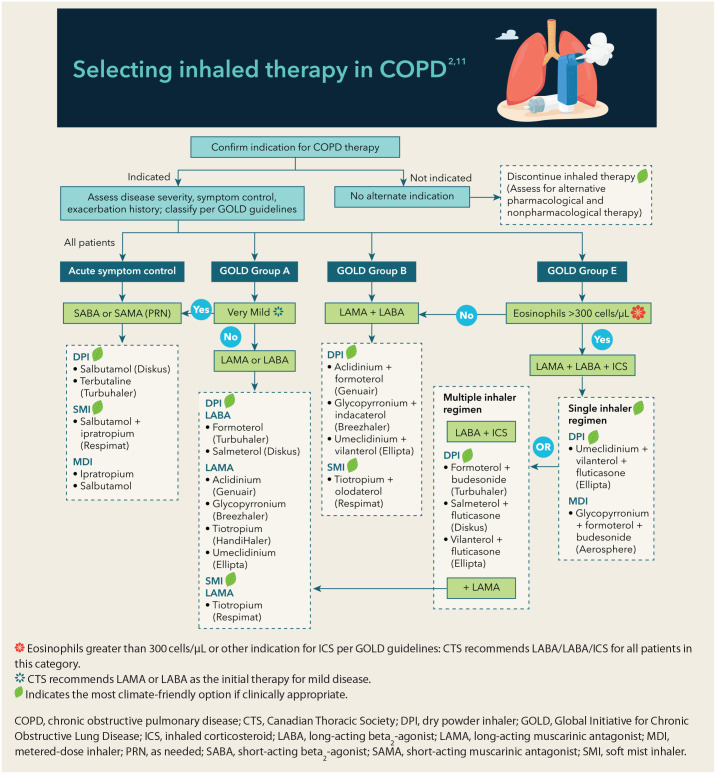
3. Select an appropriate device
Determining appropriate devices for patients requires dedicated time and ideally some additional supplies, including placebo inhaler devices, a peak inspiratory flow (PIF) meter, and patient information handouts.
a. Engage with patient:
• Prepare for discussion by gathering relevant information and supplies as identified above.
• Discuss expectations for inhaler device assessment. Elicit their baseline understanding of COPD and the role of inhaled therapies.
• An essential first step in the assessment is to estimate inspiratory capacity. This process will help include or exclude certain inhaler devices. Capacity can be assessed in the following ways:
▪ Consider the use of a PIF meter, such as the In-Check DIAL. 26 This can provide an objective measure of inspiratory capacity and determine which devices may be suitable. Individuals with limited inspiratory capacity may not generate enough force to effectively use some DPIs.
▪ If a PIF meter is not available, a subjective assessment can be made. Observe the patient take a deep, controlled breath with a placebo device to estimate if a forceful and sustained inspiration is possible. For patients with advanced symptoms, this can be a challenge.
b. Reassess device options:
• Using the above information in combination with Figure 2, determine available drug combination devices that may be suitable.
c. Review device options with patient:
• Discuss options, including the pros/cons of each regarding use and handling (see Table 1). Having placebo devices available for all suitable device types is an asset.
• If possible, observe inhaler technique with placebo devices to assess ability to use, including dexterity, cognitive ability, and inspiratory capacity.
• At this point, if appropriate, promote DPIs or SMIs over MDIs to minimize environmental impacts. Acknowledge that patients may require or prefer an MDI due to inspiratory capacity, drug costs, or preference. If continuing with MDI devices, select a low-volume HFA brand, such as Teva-salbutamol or Airomir (salbutamol sulphate), and educate about proper inhaler technique and disposal of the device to promote adherence and mitigate environmental impacts. 22
d. Select therapy:
• Based on patient preferences, demonstrated technique, and cost, select appropriate therapy.
• If possible, consider using the same device type for the maintenance and as-needed inhaler. This can help build consistency with technique and avoid potential confusion from using 2 separate inhalation techniques.
• Consider drug costs for the patient, including insurance coverage and ability to pay out of pocket.
• If the prescribed regimen requires further optimization, take the appropriate steps to obtain or provide a prescription, depending on your practice setting and available resources.
e. Education and documentation:
• Provide education regarding medication benefits, potential adverse effects, treatment expectations, and disposal (return to pharmacy).
• Particular attention should be given to instructing on optimal inhaler technique, as this is a common point of deficiency, even when an ideal regimen is prescribed. 8 Use of written or online resources to supplement in-person instruction is a major asset (e.g., lung.ca). 27
• Document the assessment in the patient’s health record.
f. Follow-up:
• Continually reassess the appropriateness of therapy and devices, including at refills, medication assessments, and upon discharge from the hospital.
• Upon dispensation, especially at refills or upon discharge from the hospital, determine the patient’s supply at home and dispense only those that are required.
Figure 2.
Selecting inhaled therapy in COPD
Conclusion
COPD is a highly prevalent condition in Canada with significant patient impacts, including symptom burden and exacerbations. Optimal selection of inhaled therapy is prudent to improve patient outcomes as well as mitigate climate impacts. Pharmacists are well-positioned to support appropriate inhaler prescribing, including DPIs or SMIs in place of MDIs where appropriate, and patient education, including proper technique and disposal to benefit both patients and the environment.
Footnotes
Author Contributions: J. Goldak performed a literature search, summarized data, wrote the first draft of the manuscript, revised each draft, and approved the final draft; S. Tri initiated the project, reviewed and revised each draft, and reviewed and approved final draft; C. Roy reviewed and revised each draft and reviewed and approved final draft. L. Underwood reviewed and revised each draft and reviewed and approved final draft.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Joyce Goldak  https://orcid.org/0009-0006-0975-2008
https://orcid.org/0009-0006-0975-2008
Caitlin Roy  https://orcid.org/0000-0001-8018-0336
https://orcid.org/0000-0001-8018-0336
Logan Underwood  https://orcid.org/0000-0001-5316-0081
https://orcid.org/0000-0001-5316-0081
Contributor Information
Joyce Goldak, Saskatchewan Health Authority – Regina, Saskatchewan.
Samantha Tri, Saskatchewan Health Authority – Regina, Saskatchewan.
Caitlin Roy, Saskatchewan Health Authority – Regina, Saskatchewan.
Logan Underwood, Saskatchewan Health Authority – Regina, Saskatchewan.
References
- 1. Canada.ca. Canadian Chronic Disease Surveillance System (CCDSS). Updated March 30, 2024. Available: https://health-infobase.canada.ca/ccdss/data-tool/ (accessed Aug. 27, 2023).
- 2. Global Initiative for Chronic Obstructive Lung Disease – GOLD. 2023 GOLD report. 2024. Available: https://goldcopd.org/2023-gold-report-2/ (accessed Aug. 13, 2023). [DOI] [PMC free article] [PubMed]
- 3. Canadian Institute for Health Information. Inpatient hospitalization, surgery and newborn statistics, 2021–2022. Ottawa (ON): CIHI; 2023. [Google Scholar]
- 4. Canadian Institute for Health Information. Cost of a standard hospital stay. Available: https://yourhealthsystem.cihi.ca/hsp/inbrief?lang=en#!/indicators/015/cost-of-a-standard-hospital-stay/;mapC1;mapLevel2; (accessed Aug. 28, 2023).
- 5. Prasad PA, Joshi D, Lighter J, et al. Environmental footprint of regular and intensive inpatient care in a large US hospital. Int J Life Cycle Assess 2022;27:38-49. [Google Scholar]
- 6. May SM, Li JTC. Burden of chronic obstructive pulmonary disease: healthcare costs and beyond. Allergy Asthma Proc 2015;36:4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canadian Institute for Health Information. COPD in Alberta: examining the characteristics and health care use of high users. Ottawa (ON): CIHI; 2017. [Google Scholar]
- 8. Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med 2017;27:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilkinson AJK, Braggins R, Steinbach I, Smith J. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open 2019;9:e028763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hesso I, Gebara SN, Kayyali R. Impact of community pharmacists in COPD management: inhalation technique and medication adherence. Respir Med 2016;118:22-30. [DOI] [PubMed] [Google Scholar]
- 11. Bourbeau J, Bhutani M, Hernandez P, et al. 2023 Canadian Thoracic Society guideline on pharmacotherapy in patients with stable COPD. Can J Respir Crit Care Sleep Med 2023;7:173–191. [DOI] [PubMed] [Google Scholar]
- 12. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648-54. [DOI] [PubMed] [Google Scholar]
- 13. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eckelman MJ, Sherman JD, MacNeill AJ. Life cycle environmental emissions and health damages from the Canadian healthcare system: an economic-environmental-epidemiological analysis. PLoS Med 2018;15:e1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fidler L, Green S, Wintemute K. Pressurized metered-dose inhalers and their impact on climate change. CMAJ 2022;194:E460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fulford B, Mezzi K, Aumônier S, Finkbeiner M. Carbon footprints and life cycle assessments of inhalers: a review of published evidence. Sustainability 2022;14:7106. [Google Scholar]
- 17. NHS England. Improving health outcomes for respiratory patients while reducing carbon emissions. Available: https://www.england.nhs.uk/greenernhs/whats-already-happening/improving-health-outcomes-for-respiratory-patients-while-reducing-carbon-emissions/ (accessed Aug. 27, 2023).
- 18. Wintemute K, Bursque G, Chang B, et al. Climate conscious inhaler prescribing in primary care. Creating a Sustainable Canadian Health System in a Climate Crisis (CASCADES). 2022. Available: https://cascadescanada.ca/resources/sustainable-inhaler-prescribing-in-primary-care-playbook/ (accessed Oct. 16, 2023).
- 19. Climate Atlas of Canada. Climate change, air quality, and public health. Available: https://climateatlas.ca/climate-change-air-quality-and-public-health (accessed Aug. 27, 2023).
- 20. Canada.ca. Climate change and public health factsheets. 2013. Available: https://www.canada.ca/en/public-health/services/health-promotion/environmental-public-health-climate-change/climate-change-public-health-factsheets-air.html (accessed Aug. 27, 2023).
- 21. Stoynova V, Culley C. Climate conscious inhaler practicesin inpatient care.Creating a Sustainable Canadian Health System in a Climate Crisis (CASCADES). 2023. Available: https://cascadescanada.ca/resources/climate-conscious-inhaler-practices-in-inpatient-care-playbook/ (accessed Sep. 13, 2023).
- 22. Creating a Sustainable Canadian Health System in a Climate Crisis (CASCADES). Sustainable inhaler alternatives: cost and coverage for asthma and COPD inhalers in Canada. 2023. Available: https://view.publitas.com/5231e51e-4654-42c2-accd-b722e21f3093/visual-inhaler-comparison-chart-1/page/1 (accessed Oct. 29, 2023).
- 23. Bourbeau J, Bhutani M, Hernandez P, et al. Canadian Thoracic Society clinical practice guideline on pharmacotherapy in patients with COPD – 2019 update of evidence. Can J Respir Crit Care Sleep Med 2019;3:210-32. [Google Scholar]
- 24. Choosing Wisely Canada. Respiratory medicine: seven things physicians and patients should question. 2022. Available: https://choosingwiselycanada.org/recommendation/respiratory-medicine/ (accessed Sep. 25, 2023).
- 25. Gershon AS, Hwee J, Chapman KR, et al. Factors associated with undiagnosed and overdiagnosed COPD. Eur Respir J 2016;48:561-4. [DOI] [PubMed] [Google Scholar]
- 26. Sanders MJ. Guiding inspiratory flow: development of the in-check DIAL G16, a tool for improving inhaler technique. Pulm Med 2017;2017:1495867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Canadian Lung Association. How to use your inhaler. Available: https://www.lung.ca/lung-health/how-use-your-inhaler (accessed Oct. 3, 2023).