Abstract
Background
This study aimed to elucidate the safety and intra-articular elution profiles of vancomycin and gentamicin bone cement in patients undergoing primary total knee arthroplasty (TKA), with a focus on serum safety thresholds and therapeutic efficacy.
Methods
Consecutive patients who underwent unilateral primary TKA were prospectively enrolled. The implants were fixed using gentamicin-impregnated bone cement, and after arthrotomy closure, 1000 mg of vancomycin suspended in 25 mL of normal saline was directly injected into the joint. Peripheral venous blood and drain fluid samples were collected 2, 8, and 24 h postoperatively. The serum and intra-articular concentrations of vancomycin and gentamicin were analyzed using liquid chromatography-tandem mass spectrometry within 24 h.
Results
Clinical data reflecting renal and liver function were recorded preoperatively, and at 24 and 72 h postoperatively. A total of 100 patients were included. At 2, 8, and 24 h postoperatively, the serum vancomycin concentration was 7.0 ± 2.0, 5.7 ± 1.8, and 3.6 ± 1.4 µg/mL, respectively, while the intra-articular concentration was 468.5 (interquartile range [IQR] 286.0 to 774.8), 139.5 (IQR 52.0 to 295.3), and 34.4 (IQR 22.2 to 56.8) µg/mL, respectively; 33.2 (IQR 19.5 to 80.5) mg vancomycin was lost in drainage fluid at 24 h postoperatively. For gentamicin, the overall intra-articular concentration was 70.4 (IQR 35.4 to 109.2), 33.8 (IQR 17.8 to 73.9), and 21.1 (IQR 12.2 to 36.0) µg/mL at 2, 8, and 24 h postoperatively, respectively, with an undetectable serum concentration. No cases of acute renal injury, liver injury, ototoxicity, or anaphylaxis were observed.
Conclusions
Intra-articular injection of 1000 mg vancomycin after arthrotomy closure combined with gentamicin-impregnated bone cement provided a therapeutic intra-articular concentration while avoiding systemic toxicity over the initial 24 h after primary TKA. Therefore, intra-articular vancomycin administration may offer a safer alternative to intravenous antibiotics, reducing systemic toxicity; however, further large-scale studies are necessary.
Trial registration
ClinicalTrials. Gov (registration number: NCT05338021).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-024-05357-9.
Keywords: Vancomycin, Gentamicin, Total knee arthroplasty
Background
Total knee arthroplasty (TKA) is the ultimate treatment for patients with end-stage knee osteoarthritis (OA) [1, 2]. Periprosthetic joint infection (PJI) is a complication that affects 1–2% of patients undergoing TKA and has catastrophic consequences. PJI significantly affects patient satisfaction and can even become limb-threatening, increasing the medical and health care burden [3–5]. Although multiple strategies, including patient screening, perioperative prophylactic antibiotics, and an ultra-clean air ventilation system, have been introduced in primary TKA to prevent PJI [6], the number of PJI cases is expected to increase in the future with the increasing number of TKA procedures performed [7]. Among these strategies, perioperative antibiotic prophylaxis plays a particularly important role [8].
To achieve a high intra-articular concentration of antibiotics and decrease the occurrence of systemic complications, topical administration of antibiotics has drawn increasing interest. One of the most common methods to achieve intra-articular delivery of antibiotics is the use of antibiotic-loaded bone cement (ALBC). Commercial ALBC usually impregnates aminoglycosides into bone cement; however, 41% and 66% of the isolated staphylococci are resistant to gentamicin and tobramycin, respectively [9]. The increasing occurrence of methicillin-resistant Staphylococcus aureus (MRSA) in PJI also narrows the spectrum of available, effective antibiotics. Consequently, adding vancomycin is regarded as a promising complement [10]. Hand-mixing vancomycin powder with commercial ALBC compromises the flexural strength of bone cement and therefore, is not suitable for primary TKA [11]. Although dual ALBC loaded with vancomycin and gentamicin is now commercially available in certain region, it is not available worldwide for primary TKA [12]. Based on findings by Lawrie et al. and Johnson et al., who reported that direct intra-articular injections may achieve superior localized antibiotic concentrations, direct intra-articular injection of vancomycin appears to be a clinically feasible approach [13, 14].
The major concerns of using vancomycin and aminoglycosides are systemic adverse reactions, including nephrotoxicity, ototoxicity, and anaphylaxis [15, 16]. Although Lawrie et al. confirmed the safety of intra-articular vancomycin, given no adverse events occurred during their study [13], data on changes in renal function were not provided. Moreover, previous studies predominantly involved individuals of white and black race; therefore, the results on the antibiotic pharmacokinetic profiles were limited in their extrapolation to Asian population. In this study, we aimed to determine the intra-articular and serum levels of intra-articular vancomycin administered over the first 24 h postoperatively in primary cemented TKA in a large Asian population. We also aimed to evaluate the intra-articular and serum concentrations of gentamicin pre-mixed in bone cement, and the clinical parameters over 72 h postoperatively.
Methods
Ethics statement
This prospective study was approved by the institutional review board prior to patient recruitment (approval number: QDFYKYLL920011921) and registered at ClinicalTrials. Gov (registration number: NCT05338021).
Study population
Consecutive patients, aged between 55 years old and 75 years old, with end-stage OA undergoing primary, unilateral cemented TKA using Refobacin® bone cement (ZIMMER BIOMET, USA) containing pre-mixed 0.5 g active gentamicin by two surgeons (YZW and HX) at our institution between May 2022 and August 2022 were screened for eligibility, and 100 patients were subsequently enrolled. Patients with potential risk factors that could influence the elution and toxicity of antibiotics were excluded. The exclusion criteria were: (1) Patients with documented renal disease or a preoperative glomerular filtration rate (GFR) of < 60 mL/min/1.73m2, indicating moderate to severe renal impairment; (2) Patients with known allergy to vancomycin; and (3) Patients who received perioperative intravenous (IV) vancomycin as prophylaxis.
All patients received standard prophylactic infection measures, including one dose of IV cefazolin preoperatively that was continued for the first 24 h postoperatively, iodine-impregnated drape, and intraoperative lavage using 3000 mL of normal saline. An intra-articular drain was then placed and clamped. After arthrotomy closure, 1000 mg of vancomycin powder suspended in 25 mL of normal saline was injected directly into the joint, and the drain was clamped for 2 h.
Sample collection
Serum and drainage samples were collected at 2, 8, and 24 h postoperatively. Drainage samples were collected from the drainage tube; the first 5 mL of fluid was wasted, and the second 5 mL of fluid was collected. The drain was kept open after clamping for 2 h postoperatively. After the final fluid collection, the drain was removed at 24 h postoperatively, and the total volume of the drain was recorded. All samples were stored at 4 °C after collection and analyzed using liquid chromatography-tandem mass spectrometry (LC-MS) within 24 h. The three active congeners of gentamicin—C1, C2, and C1a— were analyzed independently, and the overall gentamicin concentration was calculated. Clinical data reflecting liver and renal function were also recorded preoperatively, and at 24 h and 72 h postoperatively.
LC–MS equipment and conditions
Vancomycin and gentamicin quantification was performed using a high-performance LC-MS method described previously, which could accurately measure the concentration with good reproducibility [17, 18]. The apparatus was composed of a surveyor autosampler, surveyor pump, and triple quadrupole TSQ Quantum Discovery max (AB Sciex, Foster City, CA, USA). A Chrome Core C18 column (50 × 2.1 mm, 3 μm, NanoChrom Technologies, Suzhou, China) was used and maintained at a constant temperature of 40 °C, with a flow rate of 0.4 mL/min. D4-voriconazole was used as the internal reference for vancomycin and etimicin for gentamicin.
Minimum inhibitory concentration (MIC), minimum biofilm eradication concentration (MBEC), and toxic concentration
The MBEC60 and MBEC100 were defined as the concentrations at 60% and 100% eradication of the biofilm. Each antibiotic concentration measurement was compared with the MIC, MBEC60, and MBEC100 of methicillin-susceptible Staphylococcus aureus (MSSA, UMAS-1) and MRSA (USA300LAC) reported by Okae et al. [19], the commonly accepted susceptible breakpoint, and the toxic concentrations of gentamicin and vancomycin [20, 21].
For UMAS-1, the MIC, MBEC60, and MBEC100 of gentamicin were 0.5, 64, and 64 µg/mL, while those of vancomycin were 0.5, 32, and 128 µg/mL, respectively. For USA300LAC, the MIC, MBEC60, and MBEC100 of gentamicin were 0.5, 32, and 128 µg/mL, while those of vancomycin were 0.5, 64, and 128 µg/mL, respectively [19].
The susceptible breakpoint of vancomycin and gentamicin was 2 µg/mL and 4 µg/mL, respectively. The toxic concentration of vancomycin was defined as a serum concentration ≥ 15 µg/mL, according to previous literatures [20, 21].
Statistical analysis
All statistical analyses were performed using SPSS version 23.0 (IBM Inc., Armonk, NY, USA). The Kolmogorov-Smirnov test was used to determine the normality of the distribution of continuous variables. Quantitative variables with normal distribution are presented as mean ± standard deviation (SD), and the one-way analysis of variance (ANOVA) analysis was used to compare data among three or more groups. Variables with an abnormal distribution are presented as median (interquartile range, IQR), and Friedman test was used to compare data among three groups. The level of significance was set at P < 0.05. All concentrations are reported in µg/mL.
Results
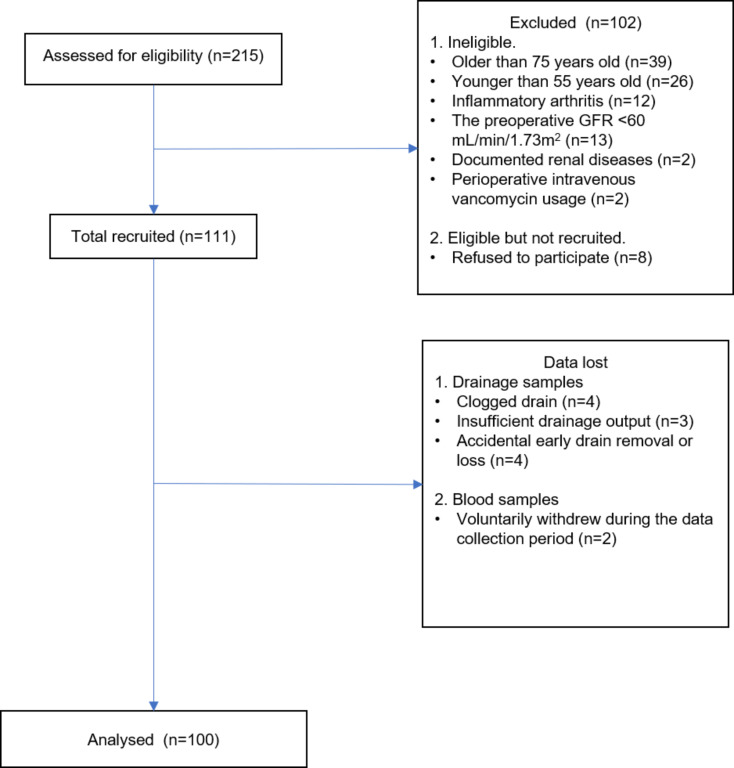
A total of 100 patients (30 male and 70 female) were enrolled (Fig. 1), and their clinical data were collected. The mean age and body mass index (BMI) of the patients was 69.67 ± 6.51 years and 27.92 ± 2.83 kg/m2, respectively. Detailed information on the included patients and their baseline data are listed in Table 1.
Fig. 1.
Subject disposition
Table 1.
Baseline data of the included patients (n = 100)
| Characteristic | Mean ± SD* | Range (min - max) |
|---|---|---|
| Age (years) | 69.7 ± 6.5 | 55.0–75.0 |
| BMI (kg/m²) | 27.9 ± 2.8 | 20.2–39.4 |
| Gender (M: F) | 30:70 | - |
| Albumin (g/L) | 41.1 ± 3.6 | 28.2–50.8 |
| ALT (U/L) | 15.0 (11.0 to 19.0) | 7.0–64.0 |
| AST (U/L) | 19.0 (16.0 to 22.0) | 7.0–52.0 |
| AKP (U/L) | 76.0 (65.0 to 97.0) | 47.0–145.0 |
| GGT (U/L) | 17.0 (13.0 to 21.0) | 8.0–72.0 |
| Bilirubin (umol/L) | 16.5 (13.7 to 20.2) | 7.3–41.4 |
| BUN (mmol/L) | 6.4 ± 1.4 | 4.0–11.0 |
| Cr (umol/L) | 79.0 (73.0 to 89.0) | 60.0–103.0 |
| GFR (ml/min) | 73.2 (68.9 to 78.9) | 60.1 – 144.6 |
*Data with abnormal distribution is listed as median (interquartile range)
ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; AKP, Alkaline phosphatase; GGT, Glutamyl transpeptidase; BUN, Blood urea nitrogen; Cr, Creatinine; GFR, Glomerular filtration rate
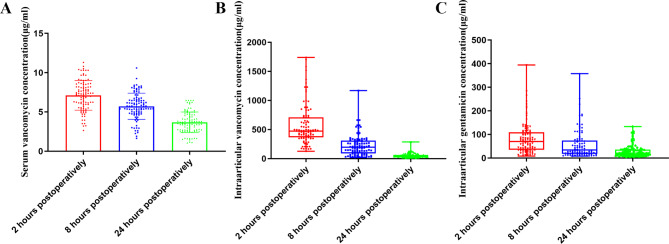
The serum concentration of vancomycin continuously declined at 2, 8, and 24 h postoperatively to 7.0 ± 2.0, 5.7 ± 1.8, and 3.6 ± 1.4 µg/mL, respectively (Table 2; Fig. 2A). Our results demonstrated that the serum vancomycin concentration was below the toxic level in all patients (Table 3). The intra-articular vancomycin concentration also continuously decreased at 2, 8, and 24 h postoperatively to 468.5 (IQR 286.0 to 774.8), 139.5 (IQR 52.0 to 295.3), and 34.4 (IQR 22.2 to 56.8) µg/mL, respectively (Table 2; Fig. 2B). Although the intra-articular concentration of vancomycin successfully reached MIC of MSSA and MRSA and susceptible breakpoint in all patients at 24 h postoperatively, the MBEC100 of MSSA and MRSA was surpassed in only three patients (Table 3). A total of 33.2 (IQR 19.5 to 80.5) mg of vancomycin was lost in the drainage fluid (Table 2). Patients with different BMI did not show significantly different serum or intra-articular distribution of vancomycin (Table S1).
Table 2.
Serum and intra-articular concentration of vancomycin (n = 100) *
| 2 h | 8 h | 24 h | |
|---|---|---|---|
| Serum concentration (µg/ml) | 7.0 ± 2.0 | 5.7 ± 1.8 | 3.6 ± 1.4 |
| Intra-articular concentration (µg/ml) |
468.5 (286.0 to 774.8) |
139.5 (52.0 to 295.3) |
34.4 (22.2 to 56.8) |
| Concentration of drainage fluid (µg/ml) | - | - |
127.0 (77.1 to 238.0) |
| Drainage volume(ml) | - | - |
320.0 (210.0 to 420.0) |
| Vancomycin loss (mg) | - | - |
33.2 (19.5 to 80.5) |
* Data with normal distribution is listed as Mean ± Standard deviation, and data with abnormal distribution is listed as Median (Interquartile range)
Fig. 2.
Concentration of vancomycin and gentamicin. (A) Postoperative serum concentration of vancomycin, presented as mean ± standard deviation; (B) Postoperative intra-articular concentration of vancomycin, presented as median (Range); (C) Postoperative intra-articular concentration of vancomycin, presented as median (Range)
Table 3.
The percentage of patients at each time point who met the target concentration of vancomycin
| MSSA (UMAS-1) | MRSA (USA300LAC) | Vancomycin | ||||||
|---|---|---|---|---|---|---|---|---|
| MIC (0.5 µg/ml) | MBEC60 (32 µg/ml) | MBEC100 (128 µg/ml) | MIC (0.5 µg/ml) | MBEC60 (64 µg/ml) | MBEC100 (128 µg/ml) | Susceptible breakpoint (2 µg/ml) | Toxic concentration (15 µg/ml) | |
| Serum concentration | ||||||||
| 2 h | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 |
| 8 h | 100 | 0 | 0 | 100 | 0 | 0 | 98 | 0 |
| 24 h | 100 | 0 | 0 | 100 | 0 | 0 | 89 | 0 |
| Intraarticular concentration | ||||||||
| 2 h | 100 | 100 | 99 | 100 | 100 | 99 | 100 | - |
| 8 h | 100 | 92 | 69 | 100 | 82 | 69 | 100 | - |
| 24 h | 100 | 68 | 3 | 100 | 25 | 3 | 100 | - |
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; MIC, minimum inhibitory concentration; MBEC, minimum biofilm eradication concentration
The serum concentration of gentamicin was undetectable at each postoperative time point (Table 4). The intra-articular concentrations of each congener at 2, 8, and 24 h postoperatively are listed in Table 4. The overall intra-articular gentamycin concentration at 2, 8, and 24 h postoperatively was 70.4 (IQR 35.4 to 109.2), 33.8 (IQR 17.8 to 73.9), and 21.1 (IQR 12.2 to 36.0) µg/mL, respectively (Table 3). At 24 h postoperatively, MBEC100 of MSSA was reached in eleven patients and MBEC100 of MRSA was reached in one patient, although the intra-articular concentration of gentamicin in 99 patients remained above the susceptible breakpoint (Table 5).
Table 4.
Serum and intra-articular concentration of gentamycin
| 2 h | 8 h | 24 h | |
|---|---|---|---|
| Serum concentration (µg/ml) | Undetectable | Undetectable | Undetectable |
| Intra-articular concentration (µg/ml) | |||
| C1 | 20.4 (10.2 to 30.4) | 11.5 (7.0 to 22.6) | 8.4 (2.2 to 10.7) |
| C1a | 13.2 (4.3 to 20.5) | 8.1 (2.5 to 16.1) | 3.9 (1.7 to 9.2) |
| C2 | 32.7 (9.1 to 50.7) | 15.6 (5.3 to 43.0) | 11.2 (3.5 to 17.5) |
| Overall concentration (µg/ml) | 70.4 (35.4 to 109.2) | 33.8 (17.8 to 73.9) | 21.1 (12.2 to 36.0) |
Table 5.
The percentage of patients at each time point who met the intraarticular target concentration of gentamicin
| MSSA (UMAS-1) | MRSA (USA300LAC) | Gentamicin | ||||
|---|---|---|---|---|---|---|
| MIC (0.5 µg/ml) | MBEC*(64 µg/ml) | MIC (0.5 µg/ml) | MBEC60 (32 µg/ml) | MBEC100 (128 µg/ml) | Susceptible breakpoint (4 µg/ml) | |
| 2 h | 100 | 53 | 100 | 79 | 17 | 100 |
| 8 h | 100 | 30 | 100 | 51 | 11 | 100 |
| 24 h | 100 | 11 | 100 | 36 | 1 | 99 |
* MBEC60 equals to MBEC100 for UMAS-1
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; MIC, minimum inhibitory concentration; MBEC, minimum biofilm eradication concentration
The preoperative and postoperative levels of blood urea nitrogen (BUN), creatinine (Cr), GFR, albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AKP), and glutamyl transpeptidase (GGT) are listed in Table 6. At 24 h postoperatively, the level of ALB significantly decreased to 34.9 ± 2.1 g/L (p < 0.000), and the levels of AKP, GGT, bilirubin also significantly decreased to 65.0 (IQR 56.0 to 79.0, p < 0.000) U/L, 14.0 (IQR 11.0 to 17.0, p < 0.000) U/L, and 13.8 (IQR 11.1 to 17.4, p < 0.000) umol/L, respectively. Although the BUN level significantly elevated to 7.4 ± 1.7 mmol/L (p < 0.000) at 72 h postoperatively, the Cr level was significantly decreased (77.0 [IQR 71.0 to 85.5], p = 0.004). However, the GFR were not significantly changed postoperatively (p = 0.143). No patient developed postoperative acute renal injury, defined as a postoperative Cr level increase of 26.4 umol/L or a 50% increase from the baseline level. Ototoxicity and anaphylaxis were not observed.
Table 6.
Renal and liver function at 24 h and 72 h postoperatively$
| Baseline | 24 h postoperatively | 72 h postoperatively | P value* | |
|---|---|---|---|---|
| Albumin (g/L) | 41.1 ± 3.6 | 34.9 ± 2.1 | 34.3 ± 2.6 | 0.000 |
| ALT (U/L) | 15.0 (11.0 to 19.0) | 15.0 (11.0 to 19.0) | 14.0 (12.0 to 18.0) | 0.000 |
| AST (U/L) | 19.0 (16.0 to 22.0) | 19.0 (16.0 to 22.0) | 20.0 (16.0 to 24.0) | 0.133 |
| AKP (U/L) | 76.0 (65.0 to 97.0) | 65.0 (56.0 to 79.0) | 65.0 (56.0 to 74.0) | 0.000 |
| GGT (U/L) | 17.0 (13.0 to 21.0) | 14.0 (11.0 to 17.0) | 15.0 (12.0 to 18.0) | 0.000 |
| Bilirubin (umol/L) | 16.5 (13.7 to 20.2) | 13.8 (11.1 to 17.4) | 9.9 (7.9 to 11.9) | 0.000 |
| BUN (mmol/L) | 6.4 ± 1.4 | 6.4 ± 1.4 | 7.4 ± 1.7 | 0.000 |
| Cr (umol/L) | 79.0 (73.0 to 89.0) | 79.0 (73.0 to 88.0) | 77.0 (71.0 to 85.5) | 0.004 |
| GFR (ml/min) | 73.2 (68.9 to 78.9) | 73.9 (68.1 to 78.9) | 73.5 (66.8 to 79.3) | 0.143 |
ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; AKP, Alkaline phosphatase; GGT, Glutamyl transpeptidase; BUN, Blood urea nitrogen; Cr, Creatinine; GFR, Glomerular filtration rate
$ Data with normal distribution is listed as Mean ± Standard deviation, and data with abnormal distribution is listed as Median (Interquartile range)
* One-way ANOVA analysis or Friedman test
Discussion
In this study, we measured the intra-articular and serum levels of vancomycin injected into the articular capsule during primary TKA as well as the local and systemic concentrations of gentamicin preblended in commercial ALBC. Our results demonstrated that a 1000 mg dose of vancomycin suspension provided a bactericidal intra-articular concentration for at least 24 h postoperatively, while the serum concentration remained sub-toxic. Gentamicin pre-mixed with ALBC also provided intra-articular therapeutic concentrations and sub-toxic serum concentrations within the first 24 h. Further, our results demonstrated the safety of the combination of gentamicin-impregnated bone cement and intra-articular vancomycin during primary TKA.
ALBC is the most common strategy for delivering intra-articular antibiotics in primary TKA, and the use of ALBC is associated with a lower risk of revision [22]. Gentamicin and tobramycin are the most common antibiotics impregnated in bone cement. Studies have demonstrated long-term elution and high local concentrations of gentamicin and tobramycin in ALBC-made spacers in two-stage revision TKA [23, 24]. An earlier study also found that the intra-articular concentration of tobramycin can be maintained for at least 48 h after primary TKA using tobramycin-impregnated bone cement [25]. However, the elution profile of gentamicin-impregnated bone cement has never been reported in primary TKA in a large Chinese population. Our results demonstrated that preblended gentamicin in commercial ALBC could provide an intra-articular concentration higher than the MIC of common MSSA and MRSA for at least 24 h postoperatively. Moreover, the intra-articular concentration of gentamicin exceeded 4 µg/mL, which is defined as the susceptible breakpoint of gentamicin in 99 patients at 24 h postoperatively, indicating that the gentamicin-impregnated bone cement provided effective local prevention for gentamicin-susceptible bacteria. However, the intra-articular gentamicin concentration at 24 h postoperatively was lower than the MBEC60 and MBEC100 in most patients, indicating the incapacity of ALBC in eradicating the biofilm formed by the bacteria. Similar results were acquired in another newly published literature [26]. Although Lizcano et al. found that the intra-articular concentration of tobramycin or gentamicin failed to exceed the MIC for most of the pathogens at 24 h postoperatively, the intra-articular concentration in 95% of the included patients exceeded the MIC of UMAS-1 and USA300LAC.
Although gentamicin-impregnated bone cement displayed promising results in the prevention of PJI, up to 40% of the isolated staphylococci are resistant to gentamicin, and vancomycin became a reasonable complement [9, 10]. However, IV prophylactic vancomycin administration in arthroplasty failed to show superiority in PJI prevention, with a mean intra-articular concentration of 6.3 µg/mL [27, 28]. Another concern of IV vancomycin is drug toxicity when the serum concentration exceeds the toxic level. Consequently, the topical administration of vancomycin has attracted increasing attention. A meta-analysis demonstrated that intra-wound vancomycin usage significantly lowered the PJI rate in primary and revision TKA [29]. The results of this study elucidated the reason why combining intra-articular vancomycin with routine IV antibiotic prophylaxis lowered the PJI rate in primary TKA, which was reported in our previous study [30]. Although the MBEC100 of MRSA and MSSA was reached in only three patients at 24 h postoperatively, the intra-articular concentrations within the first 24 h postoperatively surpassed the susceptible breakpoint of vancomycin, indicating its effectiveness in preventing MRSA infection given its time-dependent bactericidal pattern. Two studies also reported the elution profile of intra-articular vancomycin in primary TKA at different postoperative time points, which could be a good complement for our study [13, 14]. Notably, the mean concentration in the previous studies was higher than MBEC100 at 24 h postoperatively, which was 162.2 µg/mL in and 163 µg/mL, respectively. The difference in local concentration between the current study and these previous studies might be attributed to differences in race, age, renal function, and BMI of the study population, which are common confounders that influence drug distribution and clearance rates. However, in this study, after stratifying the patients according to BMI, our results failed to demonstrate a significant different drug distribution pattern in serum or in joint cavity. The renal function also influences the drug distribution. However, the GFR of 90 patients included was between 60 and 89 mL/min/1.73 m2, indicating a G2 chronic kidney disease. Therefore, the vancomycin distribution is not likely to be significantly influenced by renal function in this study. Another factor that can influence the vancomycin distribution is the amount of drug lost in in the drainage fluid, which has never been reported before. Our results demonstrated that approximately 30 mg of vancomycin (3%) was lost in the drainage fluid before drain removal. Given the current request for enhanced recovery after TKA, routine use of drains is not recommended [31]. Therefore, our results indicate that if drains were not used, the intra-articular and serum vancomycin concentrations might be slightly different. In recent years, studies have recommended a single preoperative dose of IV cephalosporin prophylaxis [32]; however, high quality evidence of its safety and effectiveness from randomized controlled trials is still lacking. Therefore, we followed the routine 24-hour-usage of cephalosporin for antibiotic prophylaxis for TKA. Nonetheless, our data suggests that 1000 mg of intra-articular vancomycin could be a reasonable complement to traditional IV antibiotic prophylaxis. Literatures regarding the elution profiles of intra-articular vancomycin injection and the usage of ALBC in primary total joint arthroplasty are summarized in Table S2.
The incidence of nephrotoxicity was reported to increase when combining vancomycin with aminoglycosides; therefore, the safety of combining intra-articular vancomycin and gentamicin-impregnated bone cement must be addressed [16]. In the present study, the serum concentration of vancomycin remained below the toxic concentration, defined as 15 µg/mL, and the serum concentration of gentamicin was undetectable. Our results are in accordance with previous literatures, showing sub-toxic serum concentration of intra-articular vancomycin within the first 24 h postoperatively [13, 14]. The insignificant change of GFR at 24 and 72 h postoperatively further substantiated the safety of the combination of intra-articular vancomycin and gentamicin-impregnated bone cement. The significantly elevated BUN level at 72 h postoperatively indicated a negative nitrogen balance caused by surgical trauma, rather than renal toxicity caused by antibiotics. Elevated levels of liver enzymes are markers for liver injury. However, in this study, the levels of liver enzymes were significantly decreased, also suggesting a negative nitrogen balance status. Ototoxicity was also not observed. Our results provide evidence of the safety of combining 1000 mg of intra-articular vancomycin with gentamicin-impregnated bone cement during primary TKA. Although the serum vancomycin concentration exceeded the toxic concentration in two patients, Lawrie et al. also validated the renal safety of 1000 mg of intra-articular vancomycin, considering none of their patients experienced acute renal injury.
This study has several limitations. First, the antibiotic concentration was measured at only three postoperative time points. Although the serum concentration of vancomycin continuously declined, we could not identify whether the serum concentration between 2 and 8 h postoperatively peaked above the toxic thresholds. Second, excluding patients with renal disease may limit the generalizability of the findings, as these patients represent a significant proportion of those undergoing TKA who may have altered antibiotic pharmacokinetics. Further investigations are needed to evaluate the safety in patients with renal diseases. Third, we measured only three active components of gentamicin; two other active components, C2a and C2b, were not analyzed. Therefore, the concentration of gentamicin reported in our study might be lower. Finally, we administered the dose of intra-articular vancomycin recommended by Lawrie et al. to all patients. However, whether the dose should be adjusted in patients with different BMI or comorbidities was not addressed in this study. Therefore, further research is needed to investigate the value of a personalized strategy and the minimum effective dose of intra-articular delivery of vancomycin.
Conclusions
Our results demonstrated that intra-articular injection of 1000 mg vancomycin during primary TKA using gentamicin-impregnated bone cement provided intra-articular concentrations beyond the susceptible breakpoints of both vancomycin and gentamicin for at least 24 h, without resulting in toxic serum levels. These findings suggest that intra-articular vancomycin administration may offer a safer alternative to IV antibiotics, reducing systemic toxicity; nonetheless, further large-scale studies are necessary.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- TKA
Total Knee Arthroplasty
- IV
Intravenous
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- AKP
Alkaline Phosphatase
- GGT
Glutamyl Transpeptidase
- BUN
Blood Urea Nitrogen
- Cr
Creatinine
- GFR
Glomerular Filtration Rate
Author contributions
XWL performed all the analyses and write the paper; JHL collected the data; XY helped in data collection; HX revised the manuscript; SX designed the study and revised the manuscript.
Funding
This research is funded by the Youth Program of National Natural Science Foundation of China (No. 82002349), the Youth Program of Natural Science Foundation of Shandong Province (ZR2020QH079), and the Postdoctoral Research Science Foundation of China (2022M721751). The funders played an important role in the design of the study, data collection and analysis, interpretation of data, and in writing the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
The study was approved Institutional Review Board (IRB) of the affiliated hospital of Qingdao University (Local IRB number: QDFYKYLL920011921), and was performed in accordance with the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publication
Patients signed informed consent regarding publishing their data and photographs.
Competing interests
The authors declare no competing interests.
Footnotes
Xuwen Li and Junhao Lai contributed equally to this study.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sanjeev Jain M, Mch, Dnb ACP, Ms, K, Kalaivanan M. Minimum 5-year follow-up results and functional outcome of rotating-platform high-flexion total knee arthroplasty: A prospective study of 701 knees. Arthroplasty Today. 2016;2(3):127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu HM, Wang YF, Wu JM, Li BY, Lian YY. A comparative study of clinical effect of total knee arthroplasty in the treatment of primary osteoarthritis and osteoarthritis of Kashin-Beck disease. Int Orthop. 2020(3). [DOI] [PubMed]
- 3.Meyer JA, Zhu M, Cavadino A, Coleman B, Young SW. Infection and periprosthetic fracture are the leading causes of failure after aseptic revision total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141(Suppl 1). [DOI] [PubMed]
- 4.Lum ZC, Natsuhara KM, Shelton TJ, Mauro G, Pereira GC, Meehan JP. Mortality During Total Knee Periprosthetic Joint Infection. J Arthroplast. 2018;33:S0883540318307253. [DOI] [PubMed] [Google Scholar]
- 5.Migliorini F, Weber CD, Bell A, Betsch M, Maffulli N, Poth V, et al. Bacterial pathogens and in-hospital mortality in revision surgery for periprosthetic joint infection of the hip and knee: analysis of 346 patients. Eur J Med Res. 2023;28(1):177. 10.1186/s40001-023-01138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco JF, Diaz A, Melchor FR, da Casa C, Pescador D. Risk factors for periprosthetic joint infection after total knee arthroplasty. Arch Orthop Trauma Surg. 2020;140(2):239–45. 10.1007/s00402-019-03304-6. [DOI] [PubMed] [Google Scholar]
- 7.Premkumar A, Kolin DA, Farley KX, Wilson JM, Sculco PK. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J Arthroplast. 2020. [DOI] [PubMed]
- 8.Dougall TW, Duthie R, Maffulli N, Hutchison JD. Antibiotic prophylaxis: theory and reality in orthopaedics. J R Coll Surg Edinb. 1996;41(5):321–2. [PubMed] [Google Scholar]
- 9.Anguita-Alonso P, Hanssen AD, Osmon DR, Trampuz A, Steckelberg JM, Patel R. High rate of aminoglycoside resistance among staphylococci causing prosthetic joint infection. Clin Orthop Relat Res. 2005;439:43–7. 10.1097/01.blo.0000182394.39601.9d. [DOI] [PubMed] [Google Scholar]
- 10.Lawrie CM, Jo S, Barrack T, Roper S, Wright RW, Nunley RM, Barrack RL. Local delivery of tobramycin and vancomycin in primary total knee arthroplasty achieves minimum inhibitory concentrations for common bacteria causing acute prosthetic joint infection. Bone Joint J. 2020;102–B(6SuppleA):163–9. 10.1302/0301-620X.102B6.BJJ-2019-1639.R1. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Bishop AR, Squire MW, Rose WE, Ploeg HL. Mechanical, elution, and antibacterial properties of simplex bone cement loaded with vancomycin. J Mech Behav Biomed Mater. 2020;103:103588. 10.1016/j.jmbbm.2019.103588. [DOI] [PubMed] [Google Scholar]
- 12.Kwong JW, Abramowicz M, Kuhn KD, Foelsch C, Hansen EN. High and Low Dosage of Vancomycin in Polymethylmethacrylate Cements: Efficacy and Mechanical Properties. Antibiot (Basel). 2024;13(9). 10.3390/antibiotics13090818. [DOI] [PMC free article] [PubMed]
- 13.Lawrie CM, Kazarian GS, Barrack T, Nunley RM, Barrack RL. Intra-articular administration of vancomycin and tobramycin during primary cementless total knee arthroplasty: determination of intra-articular and serum elution profiles. Bone Joint J. 2021;103–B(11):1702–8. 10.1302/0301-620X.103B11.BJJ-2020-2453.R1. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JD, Nessler JM, Horazdovsky RD, Vang S, Thomas AJ, Marston SB. Serum and Wound Vancomycin Levels After Intrawound Administration in Primary Total Joint Arthroplasty. J Arthroplasty. 2017;32(3):924–8. 10.1016/j.arth.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Turnidge J. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am. 2003;17(3):503 – 28, v. 10.1016/s0891-5520(03)00057-6 [DOI] [PubMed]
- 16.Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–9. 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 17.Oyaert M, Peersman N, Kieffer D, Deiteren K, Smits A, Allegaert K, et al. Novel LC-MS/MS method for plasma vancomycin: comparison with immunoassays and clinical impact. Clin Chim Acta. 2015;441:63–70. 10.1016/j.cca.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Lucha S, Taibon J, Pongratz S, Geletneky C, Huber E, Wintterle-Roehm C, et al. An LC-MS/MS based candidate reference method for the quantification of total gentamicin in human serum and plasma using NMR characterized calibrator material. Clin Chim Acta. 2017;464:211–7. 10.1016/j.cca.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 19.Okae Y, Nishitani K, Sakamoto A, Kawai T, Tomizawa T, Saito M, et al. Estimation of Minimum Biofilm Eradication Concentration (MBEC) on In Vivo Biofilm on Orthopedic Implants in a Rodent Femoral Infection Model. Front Cell Infect Microbiol. 2022;12:896978. 10.3389/fcimb.2022.896978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeffres MN. The Whole Price of Vancomycin: Toxicities, Troughs, and Time. Drugs. 2017;77(11):1143–54. 10.1007/s40265-017-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kularatne R, Kufa T, Gumede L, Maseko V. Comparison of gentamicin MICs by agar dilution and Etest for clinical isolates of Neisseria gonorrhoeae. J Antimicrob Chemother. 2020;75(9):2599–604. 10.1093/jac/dkaa202. [DOI] [PubMed] [Google Scholar]
- 22.Jameson SS, Asaad A, Diament M, Kasim A, Bigirumurame T, Baker P, et al. Antibiotic-loaded bone cement is associated with a lower risk of revision following primary cemented total knee arthroplasty: an analysis of 731,214 cases using National Joint Registry data. Bone Joint J. 2019;101–B(11):1331–47. 10.1302/0301-620X.101B11.BJJ-2019-0196.R1. [DOI] [PubMed] [Google Scholar]
- 23.Anagnostakos K, Wilmes P, Schmitt E, Kelm J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009;80(2):193–7. 10.3109/17453670902884700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty. 1998;13(3):331–8. 10.1016/s0883-5403(98)90179-6. [DOI] [PubMed] [Google Scholar]
- 25.Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the Intra-articular Concentration of Tobramycin Using Low-dose Tobramycin Bone Cement in TKA: An In Vivo Analysis? Clin Orthop Relat Res. 2016;474(11):2441–7. 10.1007/s11999-016-5006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lizcano JD, Fernandez-Rodriguez D, Goh GS, DeMik DE, Hughes AJ, Parvizi J, et al. In Vivo Intra-Articular Antibiotic Concentrations at 24 Hours After TKA Fall Below the Minimum Inhibitory Concentration for Most Bacteria: A Randomized Study of Commercially Available Bone Cement. J Bone Joint Surg Am. 2024;106(18):1664–72. 10.2106/JBJS.23.01412. [DOI] [PubMed] [Google Scholar]
- 27.Roy ME, Peppers MP, Whiteside LA, Lazear RM. Vancomycin concentration in synovial fluid: direct injection into the knee vs. intravenous infusion. J Arthroplasty. 2014;29(3):564–8. 10.1016/j.arth.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Peel TN, Astbury S, Cheng AC, Paterson DL, Buising KL, Spelman T, et al. Trial of Vancomycin and Cefazolin as Surgical Prophylaxis in Arthroplasty. N Engl J Med. 2023;389(16):1488–98. 10.1056/NEJMoa2301401. [DOI] [PubMed] [Google Scholar]
- 29.Movassaghi K, Wang JC, Gettleman BS, Mayfield CK, Oakes DA, Lieberman JR, Heckmann ND. Systematic Review and Meta-Analysis of Intrawound Vancomycin in Total Hip and Total Knee Arthroplasty: A Continued Call for a Prospective Randomized Trial. J Arthroplasty. 2022;37(7):1405–e151. 10.1016/j.arth.2022.03.047. [DOI] [PubMed] [Google Scholar]
- 30.Hu M, Zhang Y, Yang X, Wang Y, Xu H, Xiang S. Intra-articular vancomycin decreased the risk of acute postoperative periprosthetic joint infection without increasing complication in primary total joint arthroplasty-a prospective study. Int J Infect Dis. 2023;136:64–9. 10.1016/j.ijid.2023.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Wainwright TW, Gill M, McDonald DA, Middleton RG, Reed M, Sahota O, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. Acta Orthop. 2020;91(1):3–19. 10.1080/17453674.2019.1683790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan TL, Shohat N, Rondon AJ, Foltz C, Goswami K, Ryan SP, et al. Perioperative Antibiotic Prophylaxis in Total Joint Arthroplasty: A Single Dose Is as Effective as Multiple Doses. J Bone Joint Surg Am. 2019;101(5):429–37. 10.2106/JBJS.18.00336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.