Abstract
Background
Malaria poses a significant public health threat globally, particularly in African regions, where asymptomatic malaria is a considerable logistic problem. Individuals with asymptomatic malaria do not seek treatment, and thus they are invisible to health facilities and represent a substantial hidden reservoir of Plasmodium species. This study aimed to determine the prevalence of asymptomatic malaria and its associated factors in Gorgora, western Dembia district, Northwest Ethiopia.
Methods
A community-based cross-sectional study was conducted from May to June 2023 in the Gorgora area, Western Dembia district, Northwest Ethiopia. Data were collected using a semi-structured questionnaire. Giemsa-stained blood smear microscopy was employed for the diagnosis of Plasmodium species. The data were entered into Epi Data version 4.6 and exported to SPSS version 25 for analysis. Bivariate and multivariable binary logistic regression analyses were conducted to identify associated factors.
Results
Among the 357 individuals who participated in this study, 9.2% (33/357) [95% CI 6.40–12.70: p = 0.000] were confirmed to be infected with Plasmodium species. Plasmodium falciparum and Plasmodium vivax accounted for 66.7% and 33.3%, respectively. Not using bed nets [AOR = 7.3, 95% CI 2.08–23.46, p = 0.006)], previous malaria history [AOR = 2.6, 95% CI 1.01–6.45, p = 0.041], outdoor activities at night [AOR = 8.3, 95% CI 3.21–21.30, p = 0.000], and family size [AOR = 3.3, 95% CI 1.18–9.22, p = 0.023] were significantly associated with asymptomatic malaria (p < 0.05).
Conclusions
A considerable proportion of asymptomatic Plasmodium infections was found which likely act as a reservoir of transmission. This has implications for ongoing malaria control programmes that are based on the treatment of symptomatic patients and highlight the need for intervention strategies targeting asymptomatic carriers. Not using bed nets, engaging in outdoor activities at night, and having a family size of more than five increased the odds of developing asymptomatic malaria. The district health office and health extension workers should collaborate to promote the regular use of mosquito bed nets among community residents.
Keywords: Asymptomatic malaria, Associated factors, Gorgora, Prevalence
Background
Malaria poses a substantial public health threat on a global scale [1–4]. In 2022, there were an estimated 249 million malaria cases and 608,000 deaths, a concerning increase of 5 million cases compared with 2021 [5]. The disease is caused by species of Plasmodium, with Plasmodium falciparum and Plasmodium vivax being the most prevalent species in Ethiopia, contributing 60%–70% and 30%–40% of malaria cases, respectively [6]. In Ethiopia, approximately 75% of the landmass is considered malarious, with 68% of the total population living in areas at risk of malaria [7–9]. The main malaria vectors in the country are Anopheles arabiensis followed by Anopheles pharoensis [9]. Malaria prevalence in Ethiopia is seasonally influenced with peaks coinciding with the planting and harvesting seasons, posing an economic challenge. This results in an economic burden, which can adversely affect the fight against poverty [10].
The clinical manifestation of malaria ranges from asymptomatic to severe and complicated infections [11]. The likelihood of contracting asymptomatic malaria can be increased by factors like living conditions and housing structures, as these can promote mosquito vector entry, indoor resting, biting, and ultimately malaria transmission [12]. Repeated exposures to Plasmodium infections can result in the development of partial immunity, offering some protection against further complications [13]. Asymptomatic malaria is the presence of Plasmodium parasites in the blood without fever and other acute malaria symptoms in individuals who have not received recent antimalarial treatment [14, 15]. Furthermore, malaria endemicity and other factors such as age, bed net utilization, genetic background, and the level of partial immunity from previous exposures can all influence the frequency of asymptomatic malaria [16].
Stable endemic regions and areas with unstable transmission have a significant prevalence of asymptomatic malaria carriers [17–19]. Most carriers are unaware of the infection and do not seek treatment, and, therefore, remain invisible to the healthcare system [20, 21]. Parasites from asymptomatic carriers are infectious to Anopheles mosquitoes, and their continuous exposure to mosquito bites fuels the transmission cycle [13, 20, 22]. Asymptomatic Plasmodium species carriers can pose a serious threat to malaria control and elimination efforts [21, 23]. Beyond sustaining transmission, asymptomatic malaria has been linked to adverse health effects, including anaemia among pregnant women and children, as well as chronic malnutrition and cognitive impairment in children [21]. Individuals with asymptomatic malaria may also develop symptomatic malaria at a later stage [24, 25]. In Ethiopia, studies have reported a high prevalence of asymptomatic infections [8, 26, 27].
Intending to eliminate malaria nationwide by 2030, the Federal Ministry of Health has launched a sub-national malaria elimination initiative in 2017 [28]. A successful malaria elimination programme calls for attention to all parasite carriers, including asymptomatic malaria cases [29].
Most epidemiological studies of malaria in Ethiopia have focused on symptomatic malaria. Studies on asymptomatic malaria have been limited, with a primary emphasis on pregnant women and children [18, 30]. Limited data on asymptomatic malaria parasite reservoir potentially under-estimates the malaria burden, undermines efforts for parasite clearance, and compromises opportunities for transmission interruption and subsequent efforts to achieve malaria elimination. Therefore, this study aimed to assess the prevalence and associated factors of asymptomatic malaria among community residents in the study area.
Methods
Study area and period
A community-based cross-sectional study was conducted from May to June 2023 in Gorgora, western Dembia district, Northwest Ethiopia to assess the prevalence of asymptomatic malaria and identify associated factors among community dwellers. Gorgora is situated 65 kms from Gondar town and 808 kms from Addis Ababa, the capital city of Ethiopia [31]. The climate in Gorgora can be described as moist Weina Dega, with rainfall ranging between 900 and 1400 mm, and the altitude falling within the range of 1500 to 2300 m above sea level. The temperature in Gorgora varies from 24 to 15 °C [31]. Its geographical coordinates are 12° 14ʹ N latitude and 37° 18ʹ E longitude. The area has an estimated total population size of 16,270 [31]. The local economy primarily depends on trading, farming, and fishing. Gorgora is characterized by ongoing malaria transmission, with an estimated prevalence rate of 30.3%. The most prevalent Plasmodium species in the area are P. falciparum and P. vivax [7] (Fig. 1).
Fig. 1.
Map of the study site at Western Dembia District, Northwest Ethiopia. N: number of households in the Kebele. n: selected household in the kebele
Population and inclusion criteria
The study population for this research included all individuals who were residents of Gorgora, Western Dembia district, Northwest Ethiopia. In each selected kebele, individuals from the selected households were included in the study if they met the following criteria. Firstly, individuals had to be permanent residents of the area, and they had to have resided in the area for a minimum of 6 months or longer. Secondly, individuals were required to show no signs or symptoms of malaria and have an axillary temperature below 37.5 °C. They also should not have had a history of fever within the past 72 h and should not have taken any anti-malarial treatment for at least one month before and during the data collection period. Moreover, individuals were required to provide informed written consent to participate in the study, and for children below 18 years written informed consent should be taken from their parents/guardians. On the other hand, individuals who were severely ill, unable to respond, or unable to provide sufficient blood samples for various reasons were excluded from the study.
Sample size and sampling technique
The required sample size was calculated using the single-population proportion formula considering a 95% confidence interval (CI), a design effect of 2, a margin of error of 5%, and based on a 12% asymptomatic malaria prevalence reported in a prior study [8]. By adding a 10% non-response rate, the final sample size was calculated as follows:
where n is the required sample size. Zα/2 is the value under the standard normal table for the given value of confidence level = 1.96. P is the prevalence of asymptomatic malaria from a previous study conducted at Gondar Zuria district. d is the margin of error. DeEf is the design effect.
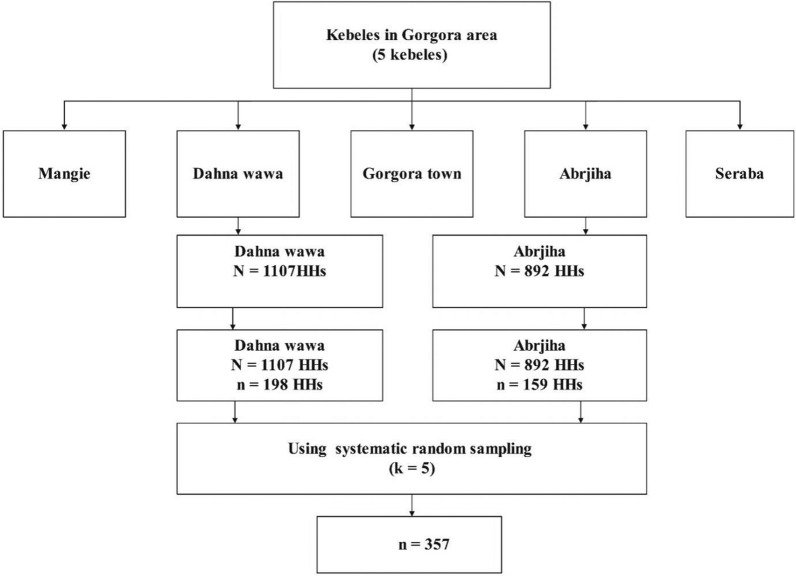
A multi-stage sampling technique was used to select the study participants. In the first stage, two kebeles (Danawawa and Abrjiha) were selected using the lottery method. At the second sampling stage, the number of households (HHs) in each kebele was determined using proportional allocation. Systematic random sampling was then used to select HHs with an interval of five. The sampling interval was calculated by dividing the total number of HHs by the number of HHs to be included in the sample from each kebele. The initial HH was randomly selected by lottery method and the next HH was selected at that interval. One study participant was selected from each HH regardless of family size using a lottery method until 357 individuals were sampled. In case no eligible participant was identified in a selected HH, the next HH was selected keeping the interval constant afterward (Fig. 2).
Fig. 2.
Schematic presentation of sampling procedure
Dependent and independent variables of the study
The dependent variable of the study was the prevalence of asymptomatic malaria. The independent variables included age, sex, family size, occupational status, educational status, presence of holes in the house wall, presence of a kitchen in the main house with no partition, previous history of malaria, family history of malaria, outdoor activities at night, bed net usage, and presence of stagnant water.
Operational definitions of asymptomatic malaria
The presence of Plasmodium parasites in the blood of a person with no history of symptoms and /or signs of malaria within the past 2 days and at the time of the survey, whose body temperature is < 37.5 °C at presentation and no history of fever for the past 72 h [8, 18].
Questionnaire data
The questionnaire, which includes socio-demographics and other variables of the study, was developed in the English language after reviewing previous literature and translated into the local language (Amharic). The questionnaire was pre-tested with 5% of the total sample size among community residents outside of the selected kebeles three weeks before actual data collection time, and necessary modifications were made based on pretest findings. For instance, the variable “sometimes” in bed net utilization and “merchant” in occupation were added. Before starting actual data collection, training was given to data collectors regarding the objective of the study, study participant recruitment techniques among household members, the data collection instrument, data collection techniques, and other ethical issues by the principal investigator of this study. Subsequently, trained questionnaire administrators conducted face-to-face interviews with respondents in the participant house by reading the questionnaires, alongside capillary blood sample collection.
Blood sample collection and laboratory diagnosis
Before capillary blood sample collection, the inclusion criteria used to enroll the study participants were screened by a nurse professional with a qualification of BSc degree. After obtaining written informed consent from study participants who fulfilled the inclusion criteria, capillary blood samples were collected aseptically from finger pricks, using sterile blood lancets by two trained laboratory technologists. The first drop of blood was removed, and consecutive drops were taken for blood film preparation. Thin and thick blood smears were prepared on a single slide for each of the study participants by dropping approximately 2–3 μl and 6 μl blood respectively. Each blood smear was air-dried, and the thin smear was fixed by carefully dropping methanol using a Pasteur pipette. The methanol-fixed thin smears were allowed to dry completely in the air by placing the slides on a flat surface. Then, the dried blood smears were transported to the nearby health centre in a slide box to stain with 10% Giemsa for 10 min. Finally, microscopic slides were immediately transported to and examined under oil emulsion (100 X) objective at the University of Gondar medical parasitology laboratory to detect asymptomatic malaria and identify Plasmodium species using thick and thin blood smears respectively. A slide was considered negative if no Plasmodium parasite was detected after examination of at least a hundred fields of the thick smear with a 100X objective [32]. All microscopic slides were examined independently by two experienced laboratory technologists. The first and second laboratory technologists who examine malaria microscopic slides have eight and ten years of malaria microscopy experience at the University of Gondar Comprehensive Specialized Hospital respectively. The discordant results were reexamination by a third trained and certified laboratory technologist blind to the initial examination results. A third laboratory technologist who read discordant results has certification during completing several malaria microscopy trainings such as FHI 360 malaria microscopy training, August 11–15, 2014, University of Gondar Hospital, Gondar, Ethiopia, and training on malaria laboratory diagnosis and quality assessment organized by Ethiopian public health institute (EPHI) in collaborated with ICAP—Colombia University programs in Ethiopia and PMI/USAID Ethiopia held on August 03–07, 2015.
Data processing and analysis
Data were coded, entered into Epidata version 4.6, cleaned up, and analysed using SPSS for Windows version 25 (IBM SPSS Statistics 25). Frequencies and summary statistics, such as mean, standard deviation, and percentages, were generated to describe the study participants in terms of the relevant variables. The Chi-square assumption was checked for all categorical independent variables. Binary logistic regression was used to assess the associations between dependent and independent variables. In the bivariate logistic regression analysis, variables with a p-value < 0.25 were considered potential candidates for the multivariable logistic regression analysis. Variables with a p-value < 0.05 from the multivariable regression analysis were considered statistically significant. The goodness of fit of the model was assessed using the Hosmer–Lemeshow test. Finally, the results of this study were presented in text, tables, and figures accordingly.
Data quality control and management
Quality control measures were implemented for the working equipment and reagents, using standard controls. The quality of the Giemsa solution was ensured by checking stock expiration dates, filtering the Giemsa working solution, and employing known positive and negative control slides.
Results
Socio-demographic characteristics
The study included a total of 357 asymptomatic participants from two kebeles. Of this total, 53.2% (190/357) were females. The mean age of the participants was 26.8 years, with a standard deviation of ± 14.04 years. In terms of educational status, 45.1% (161/357) were reported as illiterate. Additionally, most of the participants, comprising 63.3% (226/357), were engaged in farming (Table 1).
Table 1.
Socio-demographic characteristics of study participants in Gorgora, Northwest Ethiopia 2023
| Variables | Category | Frequency | Percentage |
|---|---|---|---|
| Age | 5–14 | 69 | 19.3 |
| 15–29 | 145 | 40.6 | |
| > 29 | 143 | 40.1 | |
| Sex | Male | 167 | 46.8 |
| Female | 190 | 53.2 | |
| Family size | ≤ 5 | 166 | 46.5 |
| > 5 | 191 | 53.5 | |
| Occupation | Farmer | 226 | 63.3 |
| Government employee | 29 | 8.1 | |
| Merchant | 35 | 9.8 | |
| Student | 67 | 18.8 | |
| Educational status | Illiterate | 161 | 45.1 |
| Elementary | 140 | 39.2 | |
| High school and above | 56 | 15.7 |
Behavioural, environmental, and other factors of study participants
Over one-fifth (23.2%, 83/357) of participants engaged in outdoor activities at night, placing them at increased risk of mosquito bites. Furthermore, a significant proportion (41.7%, 149/357) did not use bed nets, a key preventative measure against malaria transmission. Additionally, 44.0% (157/357) of participants reported a prior malaria infection, highlighting the prevalence of the disease in the study population. Notably, stagnant water, a breeding ground for mosquitoes, was present near the villages of 40.3% (144/357) of participants (Table 2).
Table 2.
Behavioural, Environmental, and other factors of study participants in Gorgora, Northwest Ethiopia 2023
| Variables | Category | Frequency | Percentage |
|---|---|---|---|
| Outdoor activities at night | Yes | 83 | 23.2 |
| No | 274 | 76.8 | |
| Utilization of bed net | Daily using | 92 | 25.8 |
| Sometimes | 116 | 32.5 | |
| Not using | 149 | 41.7 | |
| Presence of stagnant water | Yes | 144 | 40.3 |
| No | 213 | 59.7 | |
| Presence of holes in the house wall | Yes | 150 | 42.0 |
| No | 207 | 58.0 | |
| Presence of a kitchen in the main house with no partition | Yes | 201 | 56.3 |
| No | 156 | 43.7 | |
| Previous history of malaria | Yes | 157 | 44.0 |
| No | 200 | 56.0 | |
| Family history of malaria | Yes | 242 | 67.8 |
| No | 115 | 32.2 | |
| Kebele | Dana Wawa | 198 | 55.5 |
| Abrjiha | 159 | 44.5 |
Prevalence of asymptomatic malaria
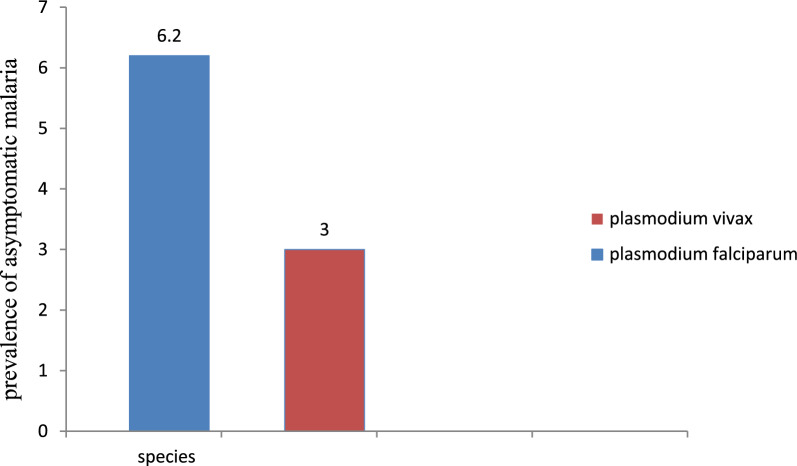
This study found a 9.2% (33/357) prevalence of asymptomatic malaria, with P. falciparum and P. vivax accounting for 6.2% (22/357) and 3% (11/357) of infections, respectively (Fig. 3).
Fig. 3.
Prevalence of asymptomatic malaria in Gorgora, western Dembia, Northwest Ethiopia
In the present study, asymptomatic malaria among males and females were 14/357(3.9%) and 19/357 (5.3%) respectively. Similarly, the prevalence of asymptomatic malaria in the age groups of 15–29 and > 29 years were 4.8% (17/357) and 3.3% (12/357) respectively (Table 3).
Table 3.
Prevalence of asymptomatic malaria by sex and age group in Gorgora, western Dembia, Northwest Ethiopia 2023
| Asymptomatic Plasmodium Infection Status | Total | χ2 | p value | ||||
|---|---|---|---|---|---|---|---|
| Pos | Percentage | Neg | Percentage | ||||
| Sex | |||||||
| Male | 14 | 3.9 | 153 | 42.9 | 167 | 0.599 | 0.599 |
| Female | 19 | 5.3 | 171 | 47.9 | 190 | ||
| Total | 33 | 9.2 | 324 | 90.8 | 357 | ||
| Age | |||||||
| 5–14 | 4 | 1.1 | 65 | 18.2 | 69 | 0.339 | 0.384 |
| 15–29 | 17 | 4.8 | 128 | 35.9 | 145 | ||
| > 29 | 12 | 3.3 | 131 | 36.7 | 143 | ||
| Total | 33 | 9.2 | 324 | 90.8 | 357 | ||
Pos Positive, Neg Negative
Notably, 1.1% (4/357) of the total blood samples collected were positive for the gametocyte stage of P. falciparum. The prevalence of gametocyte carriage among participants within the age group of 15–29 was 0.8% (3/357) (Table 4).
Table 4.
Prevalence of gametocyte carriage by sex and age group in Gorgora, western Dembia, Northwest Ethiopia 2023
| Gametocyte status | Total | χ2 | p value | ||||
|---|---|---|---|---|---|---|---|
| Pos | Percentage | Neg | Percentage | ||||
| Sex | |||||||
| Male | 1 | 0.3 | 166 | 46.5 | 167 | 0.384 | 0.389 |
| Female | 3 | 0.8 | 187 | 52.4 | 190 | ||
| Total | 4 | 1.1 | 353 | 98.9 | 357 | ||
| Age | |||||||
| 5–14 | 0 | 0 | 69 | 19.3 | 69 | 0.335 | 0.639 |
| 15–29 | 3 | 0.8 | 142 | 39.8 | 145 | ||
| > 29 | 1 | 0.3 | 142 | 39.8 | 143 | ||
| Total | 4 | 1.1 | 353 | 98.9 | 357 | ||
Pos Positive, Neg Negative
Factors associated with asymptomatic malaria
From the multivariable regression analysis, bed net utilization (AOR = 7.3, 95% CI 2.08–23.46, p = 0.006), previous history of malaria (AOR = 2.6, 95% CI 1.01–6.45, p = 0.041), outdoor activities at night (AOR = 8.3, 95% CI 3.21–21.30, p = 0.000), and family size (AOR = 3.3, 95% CI 1.18–9.22, p = 0.023) were significantly associated with the prevalence of asymptomatic malaria (p < 0.05). Regarding this finding, respondents who did not use bed nets were 7.3 times (AOR = 7.3, 95% CI 2.08–23.46, p = 0.006) more likely to have asymptomatic malaria than those who use it daily. Study participants who have a previous history of malaria were 2.55 times (AOR = 2.6, 95% CI 1.01–6.45, p = 0.041) more likely to develop asymptomatic malaria than participants who didn’t have it. Engaging in outdoor activities at night was associated with an 8.27-fold increased risk of asymptomatic malaria (AOR = 8.3, 95% CI 3.21–21.30, p = 0.000) compared to those who did not participate in such activities. In addition, participants who had more than five family members were 3.3 times (AOR = 3.3, 95% CI 1.18–9.22, p = 0.023) more likely to have asymptomatic malaria than their counterparts (Table 5).
Table 5.
Bivariate and multivariable logistic regression analysis of associated factors for asymptomatic malaria in Gorgora, Northwest Ethiopia 2023
| Variables | Category | Asymptomatic malaria | OR 95% CI | p-value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | COR 95%CI | AOR 95% CI | |||
| Age | 5–14 | 4 | 65 | 1 | 1 | |
| 15–29 | 17 | 128 | 2.2 (0.70–6.68) | 1.6 (0.41–6.45) | 0.492 | |
| > 29 | 12 | 131 | 1.5 (0.46–4.80) | 0.7 (0.15–2.86) | 0.567 | |
| Bednet utilization | Daily | 3 | 89 | 1 | 1 | |
| Sometimes | 5 | 111 | 1.3 (0.31–5.74) | 0.7 (0.14–3.79) | 0.709 | |
| No | 25 | 124 | 6.0 (1.75–16.97) | 7.3 (2.09–23.47) | 0.006* | |
| Presence of holes in the house wall | Yes | 19 | 131 | 2.0 (0.97–4.13) | 2.4 (0.92–6.15) | 0.072 |
| No | 14 | 193 | 1 | 1 | ||
| History of malaria | Yes | 17 | 140 | 1.4 (0.68–2.86) | 2.6 (1.01–6.46) | 0.041* |
| No | 16 | 184 | 1 | 1 | ||
| Family history of malaria | Yes | 29 | 213 | 3.8 (1.30–11.02) | 2.9 (1.07–8.57) | |
| No | 4 | 111 | 1 | 1 | 0.082 | |
| Outdoor activities at night | Yes | 22 | 61 | 8.6 (3.97–18.73) | 8.3 (3.22–21.31) | 0.000* |
| No | 11 | 263 | 1 | 1 | ||
| Family size | > 5 | 24 | 167 | 2.5 (1.54–5.58) | 3.3 (1.18–9.22) | 0.023* |
| ≤ 5 | 9 | 157 | 1 | 1 | ||
*Statistically significant, COR Crude Odds Ratio, AOR Adjusted Odds Ratio
Discussion
Light microscopy is the gold standard for the diagnosis of malaria because, when properly interpreted, a positive result indicates an active Plasmodium parasite infection and allows evaluation of parasite morphology, and differentiation between Plasmodium species [8, 33]. Despite these advantages, light microscopy may not be sensitive enough to be used in cases of low-density parasite carriage [34]. Polymerase chain reaction (PCR), a very sensitive molecular technique, is still the gold standard for diagnosing submicroscopic malaria parasites [35]. However, it is less useful for malaria mass screening programmes in communities with low resources due to the high prices of the sophisticated equipment required, the time-consuming procedure that delays the release of results, and the requirement for skilled laboratory personnel [36].
Identification of Plasmodium parasites in asymptomatic individuals is crucial for the reduction of transmission, control, and elimination of malaria. However, due to the low health-seeking behaviuor of asymptomatic individuals, asymptomatic malaria is often undetected and remains untreated [17, 37]. At this time, even with appropriate treatment for symptomatic cases, asymptomatic individuals continue to be potential carriers and transmitters of gametocytes, resulting in ongoing malaria transmission within a population [38, 39]. The present results also indicate that in the study area, treating symptomatic cases alone did not stop or reduce malaria transmission as expected. The high prevalence of asymptomatic malaria obtained by this study, 9.2% could be the source of forward transmission. Thus, addressing the problem associated with asymptomatic carriers is required in the study area or elsewhere to achieve the malaria elimination goals.
The current study found a 9.2% (95% CI 6.40–12.70) prevalence of asymptomatic carriers, consistent with findings from other parts of Ethiopia (10.2% and 12%) [8, 10] and Tanzania (8%) [40]. This prevalence is lower than those reported from other areas of Ethiopia (18.4% and 17.5%) [26, 27], Uganda (34.7%) [41], Ghana (27%) [42], and Nigeria (69.9%) [43]. The current result is higher than the findings of 3.75% and 5% from Ethiopia [44, 45], and Myanmar (1.44%) [46].
These variations in the prevalence of asymptomatic malaria could be attributed to several factors. Seasonality of malaria transmission could be one factor. The present study was conducted in a minor transmission season (May to June), while studies in West Armachiho and Metema district were conducted in the major malaria transmission season (September to December) [26, 27], resulting higher numbers of asymptomatic malaria cases [28].
The study population is another factor. This finding was carried out among the general population, whereas the aforementioned studies in West Armachiho and Metema [26, 27] focused on seasonal migrant workers who had repeated malaria exposures due to frequent visits to malaria-endemic lowland areas. This frequent exposure might allow the development of immunity and allow the parasite to persist in their blood for extended periods without exhibiting signs and symptoms, resulting in a higher prevalence of asymptomatic malaria [47].
This single cross-sectional study design differs from the longitudinal surveys conducted in Ghana [42] which were conducted in forest ecological zones with more favourable conditions for mosquito breeding [47]. Additionally, the Nigerian study [43] targeted household members of symptomatic malaria cases, which can lead to a higher detection rate of asymptomatic carriers compared to the general population approach used in the present study [48].
Seasonality and transmission intensity also influence prevalence. While the present study was conducted during the minor malaria transmission season, the study in North Gondar, Ethiopia [44] was conducted during the dry season with low mosquito breeding activity. Conversely, studies in the West Arsi Zone, Ethiopia [45], and Myanmar [46] were conducted in low transmission settings. In high-transmission areas, individuals frequently exposed to malaria might develop partial immunity that renders Plasmodium infection asymptomatic [49], Whereas, in areas of low transmission, populations have limited exposure and thus, are more likely to develop high-density symptomatic parasitaemia.
The present study revealed that the dominant Plasmodium species detected was P. falciparum (66.7%, 22/33). This finding is consistent with previous studies conducted in different areas of the country [10, 50, 51]. In contrast to the present study, studies conducted in East Shewa [52] and Hadiya Zone [53] reported that P. vivax was the dominant Plasmodium species over P. falciparum. This discrepancy may result from variations in the epidemiological distribution of Plasmodium species in various Ethiopian regions, most likely as a result of climate and altitudinal differences [54].
This study revealed that bed net usage, previous history of malaria infection, outdoor activities at night, and family size were significantly associated with the prevalence of asymptomatic malaria (p < 0.05). Respondents who did not use bed nets were 7.3 times more likely to have asymptomatic malaria than those who used bed nets daily. Similar results were reported from studies conducted in Northwest Ethiopia [9, 10, 55] and Uganda [41]. Daily bed net use reduces the risk of malaria infection by preventing human-mosquito contact, which interrupts malaria transmission [18].
Previous history of malaria infection was also a significant factor. Participants with such a history were 2.55 times more likely to have asymptomatic malaria than those who did not. This is consistent with reports from other regions in Ethiopia [8, 26]. The rationale for this finding might be due to the relapsing behavior of P. vivax and the recrudescence behavior of P. falciparum, where the parasite is likely to be present in the blood after medication [49]. For instance, asymptomatic P. falciparum can persist for decades [56]. In addition, encountering multiclonal P. falciparum infections might protect against symptomatic malaria and allow the infected individual to remain an asymptomatic carrier [57].
Study participants who were involved in outdoor activities at night were 8.27 times more likely to develop asymptomatic malaria than those who did not. This finding is consistent with studies conducted in Ethiopia [10, 26, 30]. This could be explained by the fact that individuals who are involved in outdoor activities at night are easily exposed to exophagic-exophilic mosquito bites and get Plasmodium infections [58].
Furthermore, this study revealed that the odds of developing asymptomatic malaria among participants with more than five family members were 3.3 times higher than those with five or fewer family members. Studies conducted in Ethiopia also showed similar findings [59–61]. Studies carried out in Ethiopia also showed similar findings [59–61]. The reason behind this could be that, as family size increases, there might be individuals who don’t have a chance to use bed nets as universal coverage of mosquito nets is low in the country in general. Moreover, when the number of residents in the HH increases, the olfactory cues to attract the Anopheles mosquito become stronger and increase the chance of being bitten by the vector [62].
Limitations of the study
The study had a few limitations that need to be acknowledged. As a cross-sectional study, it was unable to establish a direct temporal association between asymptomatic malaria and its potentially associated factors. Due to limited resources, the study was unable to utilize molecular tools for detecting asymptomatic malaria, which could have provided additional support to the microscopic investigation. Not using RDTs was another limitation of this study due to a shortage of resources. Therefore, the use of microscopy alone may underestimate the prevalence of asymptomatic malaria. Despite these limitations, the study provides valuable insights into the prevalence and potential factors associated with asymptomatic malaria, albeit without establishing a definitive causal relationship.
Conclusions
This study reveals a considerable prevalence of asymptomatic malaria. Factors such as the lack of bed net utilization, previous history of malaria, engagement in outdoor activities at night, and larger family size were found to increase the odds of developing asymptomatic malaria. Therefore, the district health office and health extension workers should collaborate to increase the distribution of mosquito bed nets, considering family size, and promote their daily utilization to reduce the prevalence of asymptomatic malaria. Based on these findings, the authors recommend that the responsible authorities focus on eliminating asymptomatic malaria in the study area. Furthermore, in addition to passive case detection, active case detection at the community level could play a crucial role in reducing these silent transmission reservoirs. In addition, further research on the role of asymptomatic carriers in malaria transmission is recommended.
Acknowledgements
The material contribution of the University of Gondar made this research possible, and we are deeply grateful for their support. We extend our sincere gratitude to the data collectors who played a crucial role in gathering the information that forms the foundation of this work. Their dedication and meticulousness were instrumental in ensuring the quality and integrity of our data. Most importantly, we would like to express our heartfelt appreciation to the study participants. Their willingness to share their time and insights is what truly made this research possible. We are humbled by their trust and generosity, and we recognize that their participation is the cornerstone of this project's success.
Abbreviations
- CI
Confidence interval
- HH
Household
Author contributions
TC, AD, WL, AA and YT contributed to the study’s design. TC, AD, WL, AA, TD, BT, GGM, AB and YT supervised the data collection process, and all authors equally contributed to the data analysis and interpretation of the findings. TC, AD, WL, AA and YT contributed to writing the draft the manuscript. AD, WL, AA, TD, BT, GGM, AB, and YT revised the final manuscript. TC, AD, WL, AA, TD, BT, GGM, AB and YT contributed significantly from its inception up to the manuscript preparation. All authors read and approved the final manuscript.
Funding
There was no fund for this research.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Prior to commencing the study, ethical clearance was obtained from the ethical review committee of the School of Biomedical and Laboratory Sciences, University of Gondar, with reference number SBMLS/516. Additionally, permission was obtained from the Western Dembia district health office and Kebele administrators, where the data were collected. Written informed consent was obtained from eligible study participants who willingly agreed to be part of the study after being informed about the purpose, procedures, benefits, and risks involved. For eligible children, verbal assent was sought before obtaining written informed consent from their parents/legal guardians. The study participants were informed that participation in the study was voluntary, and the confidentiality of their information was ensured by using codes instead of names and identification in the data collection form. Finally, malaria-positive asymptomatic study participants were referred to nearby health centers for appropriate treatment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shi D, Wei L, Liang H, Yan D, Zhang J, Wang Z. Trends of the global, regional and national incidence, mortality, and disability-adjusted life years of malaria, 1990–2019: an analysis of the global burden of disease study 2019. Risk Manag Healthc Policy. 2023;16:1187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fikadu M, Ashenafi E. Malaria: an overview. Infect Drug Resist. 2023;16:3339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murdoch J, Baum J. A sting in the tail-are antibodies against the C-terminus of Plasmodium falciparum circumsporozoite protein protective? EMBO Mol Med. 2023;15: e17556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zekar L, Sharman T. Plasmodium falciparum malaria. In: StatPearls. Treasure Island (FL) Publ. 2024. [PubMed]
- 5.WHO. World malaria report 2023. Geneva: World Health Organization; 2023. [Google Scholar]
- 6.Deress T, Girma M. Plasmodium falciparum and Plasmodium vivax prevalence in Ethiopia: a systematic review and meta-analysis. Malar Res Treat. 2019;2019:7065064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adugna F, Wale M, Nibret E. Prevalence of malaria and its risk factors in Lake Tana and surrounding areas, northwest Ethiopia. Malar J. 2022;21:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minwuyelet A, Eshetu T, Milikit D, Aschale Y. Prevalence and risk factors of asymptomatic Plasmodium infection in Gondar Zuria District, Northwest Ethiopia. Infect Drug Resist. 2020;13:3969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abebaw A, Aschale Y, Kebede T, Hailu A. The prevalence of symptomatic and asymptomatic malaria and its associated factors in Debre Elias district communities, Northwest Ethiopia. Malar J. 2022;21:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debash H, Tesfaw G, Ebrahim H, Shibabaw A, Melese Y, Tilahun M, et al. Symptomatic and asymptomatic malaria prevalence and its determinant factors in pastoral communities of Waghemira Zone, Northeast Ethiopia: a community-based cross-sectional study. Health Sci Rep. 2023;6: e1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laishram DD, Sutton PL, Nanda N, Sharma VL, Sobti RC, Carlton JM, et al. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J. 2012;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dlamini N, Hsiang MS, Ntshalintshali N, Pindolia D, Allen R, Nhlabathi N, et al. Low-quality housing is associated with increased risk of malaria infection: a national population-based study from the low transmission setting of Swaziland. Open Forum Infect Dis. 2017;4:dofx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barry A, Bradley J, Stone W, Guelbeogo MW, Lanke K, Ouedraogo A, et al. Higher gametocyte production and mosquito infectivity in chronic compared to incident Plasmodium falciparum infections. Nat Commun. 2021;12:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Rev Anti Infect Ther. 2013;11:623–39. [DOI] [PubMed] [Google Scholar]
- 15.Mosawi SH, Dalimi A, Najibullah S, Ghaffarifar F, Sadraei J. Evaluation of asymptomatic malaria status in eastern of Afghanistan using high resolution melting analysis. Iran J Parasitol. 2020;15:177. [PMC free article] [PubMed] [Google Scholar]
- 16.Singh R, Godson II, Singh S, Singh RB, Isyaku NT, Ebere UV. High prevalence of asymptomatic malaria in apparently healthy schoolchildren in Aliero, Kebbi state, Nigeria. J Vector Borne Dis. 2014;51:128. [PubMed] [Google Scholar]
- 17.Hailemeskel E, Tebeje SK, Behaksra SW, Shumie G, Shitaye G, Keffale M, et al. The epidemiology and detectability of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in low, moderate and high transmission settings in Ethiopia. Malar J. 2021;20:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balcha F, Menna T, Lombamo F. Prevalence of asymptomatic malaria and associated factors among pregnant women at Boset District in East Shoa Zone, Oromia Region, Ethiopia: a cross-sectional study. Malar J. 2023;22:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiyama T, Pongvongsa T, Phrommala S, Taniguchi T, Inamine Y, Takeuchi R, et al. Asymptomatic malaria, growth status, and anaemia among children in Lao People’s Democratic Republic: a cross-sectional study. Malar J. 2016;15:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galatas B, Bassat Q, Mayor A. Malaria parasites in the asymptomatic: looking for the hay in the haystack. Trends Parasitol. 2016;32:296–308. [DOI] [PubMed] [Google Scholar]
- 21.Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, et al. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLoS Med. 2016;13: e1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andolina C, Rek JC, Briggs J, Okoth J, Musiime A, Ramjith J, et al. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis. 2021;21:1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starzengruber P, Fuehrer H-P, Ley B, Thriemer K, Swoboda P, Habler VE, et al. High prevalence of asymptomatic malaria in south-eastern Bangladesh. Malar J. 2014;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Njama-Meya D, Kamya MR, Dorsey G. Asymptomatic parasitaemia as a risk factor for symptomatic malaria in a cohort of Ugandan children. Trop Med Int Health. 2004;9:862–8. [DOI] [PubMed] [Google Scholar]
- 25.Fogang B, Biabi MF, Megnekou R, Maloba FM, Essangui E, Donkeu C, et al. High prevalence of asymptomatic malarial anemia and association with early conversion from asymptomatic to symptomatic infection in a Plasmodium falciparum hyperendemic setting in Cameroon. Am J Trop Med Hyg. 2022;106:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aschale Y, Mengist A, Bitew A, Kassie B, Talie A. Prevalence of malaria and associated risk factors among asymptomatic migrant laborers in West Armachiho District, Northwest Ethiopia. Res Rep Trop Med. 2018;9:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilaye T, Tessema B, Alemu K. High asymptomatic malaria among seasonal migrant workers departing to home from malaria endemic areas in northwest Ethiopia. Malar J. 2022;21:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Federal Democratic Republic of Ethiopia Ministry of Health. National malaria elimination strategic plan: 2021–2025. Addis Ababa: Federal Democratic Republic of Ethiopia Ministry of Health; 2020. [Google Scholar]
- 29.Sattabongkot J, Suansomjit C, Nguitragool W, Sirichaisinthop J, Warit S, Tiensuwan M, et al. Prevalence of asymptomatic Plasmodium infections with sub-microscopic parasite densities in the northwestern border of Thailand: a potential threat to malaria elimination. Malar J. 2018;17:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biruksew A, Demeke A, Birhanu Z, Golassa L, Getnet M, Yewhalaw D. Schoolchildren with asymptomatic malaria are potential hotspot for malaria reservoir in Ethiopia: implications for malaria control and elimination efforts. Malar J. 2023;22:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asefa GM. Community based ecotourism potentials for sustainable development in Gorgora, Ethiopia. J Hosp Manag Tourism. 2020;11:40–51. [Google Scholar]
- 32.Geleta G, Ketema T. Severe malaria associated with Plasmodiumfalciparum and P.vivax among children in Pawe Hospital Northwest Ethiopia. Malar Res Treat. 2016;2016:40962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim AO, Bello IS, Ajetunmobi AO, Ayodapo A, Afolabi BA, Adeniyi MA. Prevalence of asymptomatic malaria infection by microscopy and its determinants among residents of Ido-Ekiti, Southwestern Nigeria. PLoS ONE. 2023;18: e0280981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aydin-Schmidt B, Xu W, González IJ, Polley SD, Bell D, Shakely D, et al. Loop mediated isothermal amplification (LAMP) accurately detects malaria DNA from filter paper blood samples of low density parasitaemias. PLoS ONE. 2014;9: e103905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Febrer-Sendra B, Crego-Vicente B, Nindia A, Martínez-Campreciós J, Aixut S, Mediavilla A, et al. First field and laboratory evaluation of LAMP assay for malaria diagnosis in Cubal, Angola. Parasit Vectors. 2023;16:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvarajah D, Naing C, Htet NH, Mak JW. Loop-mediated isothermal amplification (LAMP) test for diagnosis of uncomplicated malaria in endemic areas: a meta-analysis of diagnostic test accuracy. Malar J. 2020;19:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golassa L, Enweji N, Erko B, Aseffa A, Swedberg G. Detection of a substantial number of sub-microscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malar J. 2013;12:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girma S, Cheaveau J, Mohon AN, Marasinghe D, Legese R, Balasingam N, et al. Prevalence and epidemiological characteristics of asymptomatic malaria based on ultrasensitive diagnostics: a cross-sectional study. Clin Infect Dis. 2019;69:1003–71. [DOI] [PubMed] [Google Scholar]
- 39.Umunnakwe FA, Idowu ET, Ajibaye O, Etoketim B, Akindele S, Shokunbi AO, et al. High cases of submicroscopic Plasmodium falciparum infections in a suburban population of Lagosn, Nigeria. Malar J. 2019;18:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumari D, Mwingira F, Selemani M, Mugasa J, Mugittu K, Gwakisa P. Malaria prevalence in asymptomatic and symptomatic children in Kiwangwa, Bagamoyo district, Tanzania. Malar J. 2017;16:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agaba BB, Rugera SP, Mpirirwe R, Atekat M, Okubal S, Masereka K, et al. Asymptomatic malaria infection, associated factors and accuracy of diagnostic tests in a historically high transmission setting in Northern Uganda. Malar J. 2022;21:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mensah BA, Myers-Hansen JL, Obeng AE, Opoku M, Abuaku BK, Ghansah A. Prevalence and risk factors associated with asymptomatic malaria among school children: repeated cross-sectional surveys of school children in two ecological zones in Ghana. BMC Public Health. 2021;21:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onyiah AP, Ajayi IO, Dada-Adegbola HO, Adedokun BO, Balogun MS, Nguku PM, et al. Long-lasting insecticidal net use and asymptomatic malaria parasitaemia among household members of laboratory-confirmed malaria patients attending selected health facilities in Abuja, Nigeria, 2016: a cross-sectional survey. PLoS ONE. 2018;13: e0203686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Getie S, Genetu A, Fola AA, Worku L, Getnet G, Birhanie M, et al. Preventive measures and diagnosis of asymptomatic malaria in Northwest Ethiopia. Ethiop J Health Biomed Sci. 2017;8:31–9. [Google Scholar]
- 45.Golassa L, Baliraine FN, Enweji N, Erko B, Swedberg G, Aseffa A. Microscopic and molecular evidence of the presence of asymptomatic Plasmodium falciparum and Plasmodium vivax infections in an area with low, seasonal and unstable malaria transmission in Ethiopia. BMC Infect Dis. 2015;15:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaw MT, Thant M, Hlaing TM, Aung NZ, Thu M, Phumchuea K, et al. Asymptomatic and sub-microscopic malaria infection in Kayah State, eastern Myanmar. Malar J. 2017;16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alemu K, Worku A, Berhane Y, Kumie A. Men traveling away from home are more likely to bring malaria into high altitude villages, northwest Ethiopia. PLoS ONE. 2014;9: e95341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stresman GH, Kamanga A, Moono P, Hamapumbu H, Mharakurwa S, Kobayashi T, et al. A method of active case detection to target reservoirs of asymptomatic malaria and gametocyte carriers in a rural area in Southern Province, Zambia. Malar J. 2010;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gemechu T, Dedecha W, Gelchu M, Husen O, Jarso H. Asymptomatic malaria during pregnancy: prevalence, influence on anemia and associated factors in West Guji Zone, Ethiopia: a community based sdtudy. Infect Drug Resist. 2023;16:6747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Workie GY, Tesema GA, Lakew AM, Akalu TY. Malaria outbreak investigation in Argoba District, South Wello Zone, Northeast Ethiopia, 2016: a case control study. Research Square. 2019:(pre-print).
- 51.Tesfay K, Assefa B, Addisu A. Malaria outbreak investigation in Tanquae Abergelle district, Tigray region of Ethiopia: a case–control study. BMC Res Notes. 2019;12:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tadesse F, Fogarty AW, Deressa W. Prevalence and associated risk factors of malaria among adults in East Shewa Zone of Oromia Regional State, Ethiopia: a cross-sectional study. BMC Public Health. 2018;18:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delil RK, Dileba TK, Habtu YA, Gone TF, Leta TJ. Magnitude of malaria and factors among febrile cases in low transmission areas of Hadiya zone, Ethiopia: a facility based cross sectional study. PLoS ONE. 2016;11: e0154277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yadav CP, Hussain SS, Mullick R, Rahi M, Sharma A. Climate zones are a key component of the heterogeneous presentation of malaria and should be added as a malariometric for the planning of malaria elimination. PLOS Glob Public Health. 2023;3: e0001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Worku L, Damtie D, Endris M, Getie S, Aemero M. Asymptomatic malaria and associated risk factors among school children in Sanja town, Northwest Ethiopia. Int Sch Res Notices. 2014;2014: 303269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashley EA, White NJ. The duration of Plasmodium falciparum infections. Malar J. 2014;13:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sondén K, Doumbo S, Hammar U, Vafa Homann M, Ongoiba A, Traoré B, et al. Asymptomatic multiclonal Plasmodium falciparum infections carried through the dry season predict protection against subsequent clinical malaria. J Infect Dis. 2015;212:608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bilal ME. Mapping of Anopheles mosquitoes (Diptera, Culicidae) in West Kassala, Wad Al Hileio and Khashm El Girba localities, Kassala State, Sudan (2014−2015). Thesis, University of Gezira; 2018.
- 59.Getachew H, Demissew A, Abossie A, Habtamu K, Wang X, Zhong D, et al. Asymptomatic and submicroscopic malaria infections in sugar cane and rice development areas of Ethiopia. Malar J. 2023;22:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melese Y, Alemu M, Yimer M, Tegegne B, Tadele T. Asymptomatic malaria in households and neighbors of laboratory confirmed cases in Raya Kobo District, Northeast Ethiopia. Ethiop J Health Sci. 2022;32:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zemene E, Koepfli C, Tiruneh A, Yeshiwondim AK, Seyoum D, Lee M-C, et al. Detection of foci of residual malaria transmission through reactive case detection in Ethiopia. Malar J. 2018;17:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Argaw MD, Woldegiorgis AG, Workineh HA, Akelom BA, Abebe ME, Abate DT, et al. Access to malaria prevention and control interventions among seasonal migrant workers: a multi-region formative assessment in Ethiopia. PLoS ONE. 2021;16: e0246251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.