Abstract
Chlamydia trachomatis inoculated by any mucosal route colonized multiple murine mucosae and, in most cases, the spleen, liver, and kidneys. Cell-to-cell transmission, systemic dissemination, and autoinoculation of infectious fluids may have contributed to chlamydial spread. Intermucosal trafficking of protective T cells cannot be accurately evaluated by using live chlamydial challenges.
Chlamydia trachomatis is an obligate intracellular bacterium with a tropism for mucosal epithelial cells. The clinical consequences of infection vary according to the site of colonization and may be associated with the development of blindness, pneumonia, infertility, and/or proctitis (28). Once introduced to a susceptible mucosa, Chlamydia spreads canalicularly along adjacent epithelia by a process that is undoubtedly facilitated by the entry and exit of chlamydial elementary bodies from the apical surface of mucosal epithelial cells (8). In most cases, Chlamydia is cleared through the action of neutrophils and mononuclear leukocytes recruited to the site of infection, with a predominant role for type 1 CD4+ T cells and the proinflammatory cytokines that they secrete (4, 9, 11, 16, 22, 30). Unfortunately, Chlamydia-induced inflammation may also contribute to the development of residual tissue pathology and associated infertility (2, 20, 21, 33, 34). For these reasons, limiting the extent and severity of infection remains a primary vaccine goal.
Induction of immunity to epithelial pathogens such as C. trachomatis has relied heavily upon delivery of vaccine antigens to a mucosal surface, particularly the intestinal mucosa, which serves as an inductive site for the common mucosal immune system. This system was defined initially by the ability of enterically introduced antigens to trigger antibody secretion not only locally but also at distant, unrelated mucosae (13–15), a phenomenon attributed to the directed trafficking of reactive B lymphocytes from the gastrointestinal tract to pulmonary and genital tissues (13). In contrast, antigens administered parenterally induced systemic but not mucosal antibody secretion (15), suggesting separation of systemic and mucosal lymphocyte trafficking pathways. Molecular support for this distinction came from the identification of vascular cell adhesion molecule (VCAM) on endothelial cells of the systemic vasculature and mucosal addressin cell adhesion molecule (MAdCAM) on endothelial cells of the intestinal lamina propria. Lymphocytes infiltrating each of these sites expressed distinct profiles of complementary membrane integrins, represented as α4β1 at VCAM+ tissues and α4β7 at MAdCAM+ mucosae (3). These findings strengthened the notion that induction of mucosal immunity required a mucosal immunization strategy, even though direct evidence for intermucosal trafficking by antigen-specific T cells was lacking.
Vaccine trials against C. trachomatis have focused upon immunization with the homotypic major outer membrane protein (MOMP) or with MOMP-expressing bacteria. Oral administration of UV-inactivated Chlamydia (10) or purified or recombinant MOMP induced weak antibody reactivity but failed to alter the course of a subsequent ocular (31) or genital (32) chlamydial infection. Direct inoculation of the Peyer’s patches with recombinant MOMP was equally ineffective in providing protection against Chlamydia-associated salpingitis and infertility (32). However, oral (6, 7, 17) or intranasal (18) administration of live C. trachomatis stimulated widespread immune reactivity at both the T- and B-cell levels and induced effective protection against a subsequent respiratory or genital Chlamydia challenge. It has been suggested that these data support the idea that the gastrointestinal tract is an induction site for Chlamydia-specific T-cell immunity (6), but the apparent requirement for a live rather than killed antigenic stimulus remained unexplained. Given the propensity of C. trachomatis to ascend from the vagina to the ovary in normal mice or to the kidneys in immunodeficient mice (22), the possibility that Chlamydia does not remain confined to the site of inoculation but spreads to adjacent, nontargeted mucosae to induce protective immunity at each site must also be considered. The current experiments were undertaken to evaluate that possibility.
Mice.
C57BL/6J female mice 8 to 12 weeks of age were obtained from Jackson Laboratories, Bar Harbor, Maine. Animals were housed in an American Association for Accreditation of Laboratory Animal Care-accredited facility in filter-top cages under standard environmental conditions and were provided food and water ad libitum.
C. trachomatis.
The C. trachomatis strain mouse pneumonitis (MoPn) was grown in HeLa 229 cells, and elementary bodies were purified by discontinuous density gradient centrifugation as previously described (5).
Infection of mice.
Preliminary experiments revealed that the spread of Chlamydia was dependent upon the success of infection over a wide range of challenge doses. Therefore, mice were infected with Chlamydia at doses that provided reproducible infections without causing overt clinical disease. Gastrointestinal infections were performed by depositing 50 μl of 250 mM sucrose–10 mM sodium phosphate–5 mM l-glutamic acid (pH 7.2) (SPG) containing 104 inclusion-forming units (IFU) of C. trachomatis MoPn into the stomach with a Jorgensen feeding needle under methoxyflurane anesthesia. Intranasal and intratracheal infections with 400 IFU of MoPn in 30 μl of SPG were also performed under anesthesia. A midline incision over the trachea with a no. 10 scalpel blade followed by blunt tissue dissection aided exposure of the trachea at the level of the thoracic inlet. Vaginal infection of mice pretreated with 2.5 mg of medroxy-progesterone acetate (Depo-Provera; Upjohn) on day −5 was performed by depositing 5 μl of SPG containing 1.5 × 103 IFU of MoPn into the vaginal vault. The presence of infectious MoPn in relevant tissues was determined by sacrificing mice at 7 or 10 days postinfection and enumerating chlamydial organisms recovered from weighed, minced tissue fragments by IFU formation on HeLa cell monolayers using indirect immunofluorescence as described previously (16). Chlamydial shedding from the genital mucosa was monitored by swabbing the vaginal vault twice weekly with Calgiswabs (Spectrum Medical Laboratories, Los Angeles, Calif.) as previously described (22). In certain experiments, mice were outfitted with 3.5-cm-diameter padded Elizabethan collars cut from exposed X-ray film to prevent self-grooming and autoinoculation of genital and/or intestinal secretions. Continued presence of the collars was monitored daily and animals that lost their collars were excluded from that group.
The ability of a primary chlamydial infection to induce immunity at a distant mucosal site was assessed initially between noncontiguous mucosae of the respiratory and genital tracts. Female mice were infected vaginally with C. trachomatis and monitored for bacterial shedding until immune-mediated clearance was complete by 28 days postinoculation (p.i.) (data not shown). Four weeks later, these genitally immune mice as well as naive control animals were rechallenged with a potentially lethal dose of C. trachomatis by direct intratracheal injection. Several logs of Chlamydia were recovered from the lungs of naive control mice on day 11 p.i., but none were detected in the lungs of immune mice (Table 1). These data were consistent with previous reports and suggested that T cells primed in the genital tract may have trafficked to the pulmonary mucosa in response to a chlamydial challenge. Since the potential usefulness of this model to evaluate mucosal T-cell trafficking patterns depended upon containment of infections to the targeted mucosa, spread from the trachea to adjacent epithelia of the gastrointestinal tract was also assessed. High levels of infectious Chlamydia were detected in the small intestines of normal as well as Chlamydia-immune mice (Table 1), indicating that pulmonary containment had not occurred. Instead it appeared that chlamydial spread to contiguous mucosae may be a complicating factor in in vivo analyses of mucosal trafficking and protection by Chlamydia-specific T cells.
TABLE 1.
Pulmonary protection following vaginal infection with C. trachomatis MoPn
| Primary vaginal infectiona | Pulmonary challengeb | IFU/gram of lung (no. infected/total)c | IFU/gram of intestine (no. infected/total)c |
|---|---|---|---|
| − | + | (2.2 ± 1.3) × 105 (3/3) | (3.5 ± 1.7) × 105 (2/3) |
| + | + | 0 (0/3) | (2.8 ± 1.6) × 104 (2/3) |
Mice were infected with 1,500 IFU of C. trachomatis MoPn intravaginally on day −60 relative to the pulmonary challenge.
Mice were challenged with 400 IFU of MoPn by intratracheal inoculation.
Mean numbers of IFU ± standard errors detected in tissues collected 11 days after pulmonary challenge.
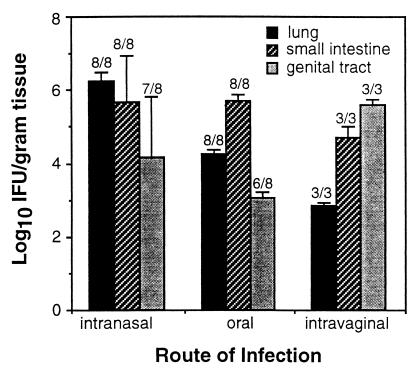
The extent to which Chlamydia deposited at the genital, pulmonary, or gastrointestinal mucosae was capable of colonizing nontargeted mucosal tissues was addressed more thoroughly in mice infected by three commonly used mucosal routes, i.e., intranasal, oral, and vaginal. It was found that Chlamydia deposited intranasally or orally colonized both the pulmonary and gastrointestinal tracts (Fig. 1), as might be predicted by the continuity of epithelia lining these tissues. Surprisingly, however, orally and intranasally infected mice also developed chlamydial infections of the genital tract (Fig. 1), indicating the spread of infection to a noncontiguous mucosa. Transmission of Chlamydia from the genital tract to the pulmonary and gastrointestinal mucosae was also detected by 7 days p.i. (Fig. 1), verifying the capacity of these bacteria to colonize nonadjacent mucosal tissues. Anesthetized mice infected intranasally occasionally developed infections of the conjunctival mucosa as well which was expressed as a mild-to-moderate transient conjunctivitis (data not shown). Spread in this case presumably occurred via ascending infection of the nasolacrimal ducts. These data revealed that primary, experimental Chlamydia infections by any route may lead to colonization of targeted as well as nontargeted mucosae with no apparent requirement for continuity between epithelial surfaces.
FIG. 1.
Mucosal distribution of C. trachomatis 7 days after infection by the intranasal, oral, or intravaginal route. Chlamydia recovered from the lung, small intestine, or genital tract was enumerated on HeLa cell monolayers as described in Materials and Methods and is presented as the mean numbers of IFU ± standard errors of the means. Numbers above each bar represent the fraction of mice displaying chlamydial colonization at each tissue site.
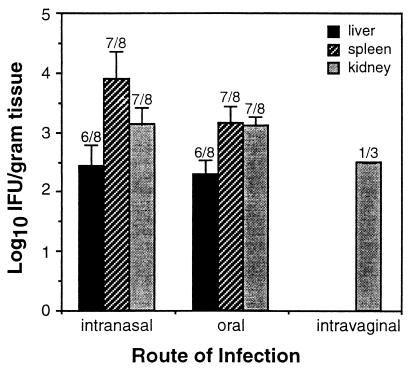
The mechanism of chlamydial spread between contiguous epithelia of the nasal, respiratory, and gastrointestinal tracts probably involved direct cell-to-cell transmission of infectious elementary bodies, or at least it would be difficult to rule out this possibility. The mechanism of spread to noncontiguous mucosae was less clear, however. It was considered that Chlamydia penetrating the epithelial barrier might disseminate systemically via the vascular or lymphatic systems to lodge wherever susceptible target cells were encountered. Under these conditions, colonization of distant mucosae requires that chlamydial elementary bodies gain access to the epithelial layer and not just the submucosal stroma, which may be difficult to achieve without directed migration. Nevertheless, dissemination was monitored by enumerating bacterial burdens in the internal organs of mice infected by the oral, intranasal, or vaginal route. The majority of orally or intranasally infected mice developed significant chlamydial burdens in the liver, spleen, and/or kidneys (Fig. 2) but not in the heart or peripheral blood (data not shown). Systemic dissemination was less apparent following vaginal chlamydial infection in that organisms were detected primarily in the kidneys (Fig. 2), possibly due to retrograde transport of bacteria along the epithelial lining of the ureters. Therefore, chlamydial spread from the lung or gastrointestinal tract to the genital tract may occur via cell-to-cell transmission and/or systemic dissemination of infectious elementary bodies, while alternative pathways may be more relevant to spread from the genital mucosa.
FIG. 2.
Internal dissemination of C. trachomatis 7 days after infection by the intranasal, oral, or intravaginal route. Chlamydia recovered from the spleen, liver, or kidneys of infected mice was enumerated as described in Fig. 1 and is presented as the mean numbers of IFU ± standard errors of the means. Numbers above each bar represent the fraction of mice displaying chlamydial colonization at each tissue site. Differences between groups were not statistically significant due to the small size of the intravaginal group.
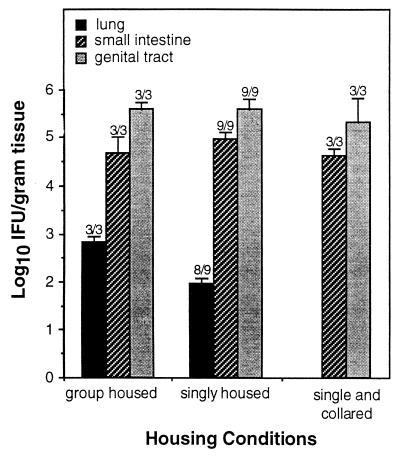
Under conventional group housing conditions, mutual grooming is a natural behavior in mice. Chlamydia present in intestinal or genital secretions could provide a ready source of unintentional oropharyngeal infection that is not usually considered. The possibility of transmission by this route was tested by comparing rates of chlamydial spread from the genital tract to the respiratory or gastrointestinal tracts of mice housed singly and in groups. Singly housed mice were further separated into those wearing restrictive Elizabethan collars to prevent orovaginal autoinoculation and those without. The spread of infection from the genital mucosa to unrelated mucosal tissues in all three groups of mice is presented in Fig. 3. Animals without restrictive collars, whether housed singly or in groups, developed infections of both the pulmonary and intestinal mucosae within 10 days of a primary genital infection. In contrast, singly housed animals wearing restrictive collars to prevent self-grooming showed no evidence of pulmonary infection, suggesting that autoinoculation may play a role in the spread of Chlamydia from the genital tract to the lungs. The presence of collars did not prevent spread of infection to the intestinal tract, however, suggesting that an alternative mechanism was in play. External exchange of contaminated fluids between the genital and rectal ori should be considered in this regard.
FIG. 3.
Mucosal distribution of C. trachomatis in vaginally infected mice housed under various conditions. Tissue burdens of Chlamydia from tissues recovered 10 days postinfection were enumerated as described in Fig. 1 and are presented as the mean numbers of IFU ± standard errors of the means. Numbers above each bar represent the fraction of mice displaying chlamydial colonization at each site. Chlamydia were not recovered from lung tissues of singly housed mice wearing restrictive Elizabethan collars. Differences between groups were not statistically significant due to the small size of the single, collared group.
The propensity of Chlamydia to infect contiguous as well as noncontiguous mucosae by any of several pathways suggests that experimental containment of infections to a single mucosal site may be an unrealistic goal. Indeed, Chlamydia-infected children from areas where trachoma is endemic also display involvement of multiple mucosal tissues (12, 29), suggesting a similar potential for bacterial spread during human infection. Further investigations will be required to determine the relevance of these observations to the course of human disease. For example, the fact that enterically infected mice maintain approximately 4 logs of Chlamydia per gram of large intestine for over 8 months p.i. with no clinical or histological evidence of disease (23a) raises the possibility that the intestine may act as a reservoir for chronic reinfection of other mucosal tissues. The degree of mucosal cross-protection afforded by T cells primed at a distant mucosal site is also poorly understood. In the present experiments, C57BL/6 mice infected intratracheally with 400 IFU of C. trachomatis MoPn consistently developed clinical signs of pneumonia that included dyspnea, tachypnea, lethargy, and wasting. In contrast, mice infected orally or vaginally who developed pulmonary infections as a result of chlamydial spread showed no clinical signs of pulmonary disease, even given additional time for bacterial replication to occur (data not shown).
The ability of Chlamydia to spread to multiple mucosae complicates attempts to define the inductive sites for mucosally protective CD4+ T cells. However, based upon the relative efficacies of Chlamydia-specific vaccines administered by distinct mucosal routes, nonenteric mucosae may be most important in this regard. Thus, enteral exposure to UV-inactivated or subunit vaccines (10, 32) failed to provide significant protection against the pathologic consequences of a subsequent genital challenge, even though type 1 T-cell immunity was successfully induced (10). In contrast, the use of a parenteral immunization scheme achieved a measurable level of host protection (19, 32). The relative success of a nonenteric immunization strategy may be related to the stimulation of a population of T cells capable of migrating between systemic and mucosal sites due to the shared expression of lymphocyte-homing molecules. In this regard, we have shown that T cells homing to the Chlamydia-infected genital mucosa express predominantly the α4β1 integrin that exhibits binding specificity for the VCAM addressin that is expressed systemically as well as in the genital mucosa. In contrast, intestinal T cells express predominantly the α4β7 integrin, which allows binding to the locally expressed MAdCAM+ addressin (23). Use of a VCAM-dominated homing pathway at the genital mucosa suggests that genital T cells may be capable of migrating to other VCAM-dominated tissues, such as the liver and lung (1, 23a–27), but not to the VCAM−, MAdCAM+ mucosa of the gastrointestinal tract. In return, α4β1+ T cells primed in response to systemic immunization may be capable of trafficking to VCAM+ mucosae of the genital tract and lung but not to the VCAM− intestine. Sharing of lymphocyte-homing markers between systemic tissues and nonenteric mucosae suggests that the common mucosal immune system defined for B lymphocytes may be more complex at the T-cell level. Further investigations are required to address the precise pathways of mucosal T-cell trafficking and the potential for inducing protection by systemic versus mucosal vaccination with nonreplicating C. trachomatis immunogens.
REFERENCES
- 1.Barquin N, Chou P, Ramos C, Montano M, Pardo A, Selman M. Increased expression of intercellular adhesion molecule 1, CD11/CD18 cell surface adhesion glycoproteins and alpha 4 beta 1 integrin in a rat model of chronic interstitial lung fibrosis. Pathobiology. 1996;64:187–192. doi: 10.1159/000164034. [DOI] [PubMed] [Google Scholar]
- 2.Beatty W L, Byrne G I, Morrison R P. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 1994;2:257–259. doi: 10.1016/0966-842x(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 3.Butcher E C, Picker L J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 4.Cain T K, Rank R G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui Z-D, LaScolea L J, Jr, Fisher J, Ogra P L. Immunoprophylaxis of Chlamydia trachomatis lymphogranuloma venereum pneumonitis in mice by oral immunization. Infect Immun. 1989;57:739–744. doi: 10.1128/iai.57.3.739-744.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui Z-D, Tristram D, LaScolea L J, Kwiatkowski T, Jr, Kopti S, Ogra P L. Induction of antibody response to Chlamydia trachomatis in the genital tract by oral immunization. Infect Immun. 1991;59:1465–1469. doi: 10.1128/iai.59.4.1465-1469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis C H, Wyrick P B. Differences in the association of Chlamydia trachomatis serovar E and serovar L2 with epithelial cells in vitro may reflect biological differences in vivo. Infect Immun. 1997;65:2914–2924. doi: 10.1128/iai.65.7.2914-2924.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, TH1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 10.Kelly K A, Robinson E A, Rank R G. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landers D V, Erlich K, Sung M, Schachter J. Role of L3T4-bearing T-cell populations in experimental murine chlamydial salpingitis. Infect Immun. 1991;59:3774–3777. doi: 10.1128/iai.59.10.3774-3777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malaty R, Zaki S, Said M E, Vastine D W, Dawson D W, Schachter J. Extraocular infections in children in areas with endemic trachoma. J Infect Dis. 1981;143:853. doi: 10.1093/infdis/143.6.853. [DOI] [PubMed] [Google Scholar]
- 13.McDermott M R, Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–1898. [PubMed] [Google Scholar]
- 14.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 15.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 16.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogra P L, Okamoto Y, Freihorst J, LaScolea L J, Jr, Merrick J M. Immunization of the gastrointestinal tract with bacterial and viral antigens: implications in mucosal immunity. Immunol Investig. 1989;18:559–570. doi: 10.3109/08820138909112263. [DOI] [PubMed] [Google Scholar]
- 18.Pal S, Peterson E M, de la Maza L M. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect Immun. 1996;64:5341–5348. doi: 10.1128/iai.64.12.5341-5348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal S, Theodor I, Peterson E M, de la Maza L M. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997;65:3361–3369. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patton D L, Kuo C-C. Histopathology of Chlamydia trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J Reprod Fertil. 1989;85:647–656. doi: 10.1530/jrf.0.0850647. [DOI] [PubMed] [Google Scholar]
- 21.Patton D L, Landers D V, Schachter J. Experimental Chlamydia trachomatis salpingitis in mice: initial studies on the characterization of the leukocyte response to chlamydial infection. J Infect Dis. 1989;159:1105–1110. doi: 10.1093/infdis/159.6.1105. [DOI] [PubMed] [Google Scholar]
- 22.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 23.Perry L L, Feilzer K, Portis J L, Caldwell H D. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J Immunol. 1998;160:2905–2914. [PubMed] [Google Scholar]
- 23a.Perry, L. L., S. Hughes, J. Carlson, and J. Igietseme. Unpublished results.
- 24.Picker L J, Martin R J, Trumble A, Newman L S, Collins P A, Bergstresser P R, Leung D Y M. Differential expression of lymphocyte homing receptors by human memory/effector T cells in pulmonary versus cutaneous immune effector sites. Eur J Immunol. 1994;24:1269–1277. doi: 10.1002/eji.1830240605. [DOI] [PubMed] [Google Scholar]
- 25.Pretolani M, Ruffie C, Lapa e Silva J R, Joseph D, Lobb R R, Vargaftig B B. Antibody to very late activation antigen 4 prevents antigen-induced bronchial hyperreactivity and cellular infiltration in the guinea pig airways. J Exp Med. 1994;180:795–805. doi: 10.1084/jem.180.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabb H A, Olivenstein R, Issekutz T B, Renzi P M, Martin J G. The role of the leukocyte adhesion molecules VLA-4, LFA-1, and Mac-1 in allergic airway responses in the rat. Am J Respir Crit Care Med. 1994;149:1186–1191. doi: 10.1164/ajrccm.149.5.8173758. [DOI] [PubMed] [Google Scholar]
- 27.Richards I M, Kolbasa K P, Hatfield C A, Winterrowd G E, Vonderfecht S L, Fidler S F, Griffin R L, Brashler J R, Krzesicki R F, Sly L M, Ready K A, Staite N D, Chin J E. Role of very late activation antigen-4 in the antigen-induced accumulation of eosinophils and lymphocytes in the lungs and airway lumen of sensitized brown Norway rats. Am J Respir Cell Mol Biol. 1996;15:172–183. doi: 10.1165/ajrcmb.15.2.8703473. [DOI] [PubMed] [Google Scholar]
- 28.Schachter J. Pathogenesis of chlamydial infections. Pathol Immunopathol Res. 1989;8:206–220. doi: 10.1159/000157149. [DOI] [PubMed] [Google Scholar]
- 29.Schachter J, Dawson C R. Is trachoma an ocular component of a more generalised chlamydial infection? Lancet. 1979;i:702–703. doi: 10.1016/s0140-6736(79)91151-6. [DOI] [PubMed] [Google Scholar]
- 30.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor H R, Whittum-Hudson J, Schachter J, Caldwell H D, Prendergast R A. Oral immunization with chlamydial major outer membrane protein (MOMP) Investig Ophthalmol Vis Sci. 1988;29:1847–1853. [PubMed] [Google Scholar]
- 32.Tuffrey M, Alexander F, Conlan W, Woods C, Ward M. Heterotypic protection of mice against chlamydial salpingitis and colonization of the lower genital tract with a human serovar F isolate of Chlamydia trachomatis by prior immunization with recombinant serovar L1 major outer-membrane protein. J Gen Microbiol. 1992;138:1707–1715. doi: 10.1099/00221287-138-8-1707. [DOI] [PubMed] [Google Scholar]
- 33.Tuffrey M, Alexander F, Taylor-Robinson D. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J Exp Pathol. 1990;71:403–410. [PMC free article] [PubMed] [Google Scholar]
- 34.Williams D M, Schachter J. Role of cell-mediated immunity in chlamydial infection: implications for ocular immunity. Rev Infect Dis. 1985;7:754–759. doi: 10.1093/clinids/7.6.754. [DOI] [PubMed] [Google Scholar]